Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection
Abstract
1. Introduction
2. Results
2.1. GC/MS Analysis
2.2. Parasitological Study
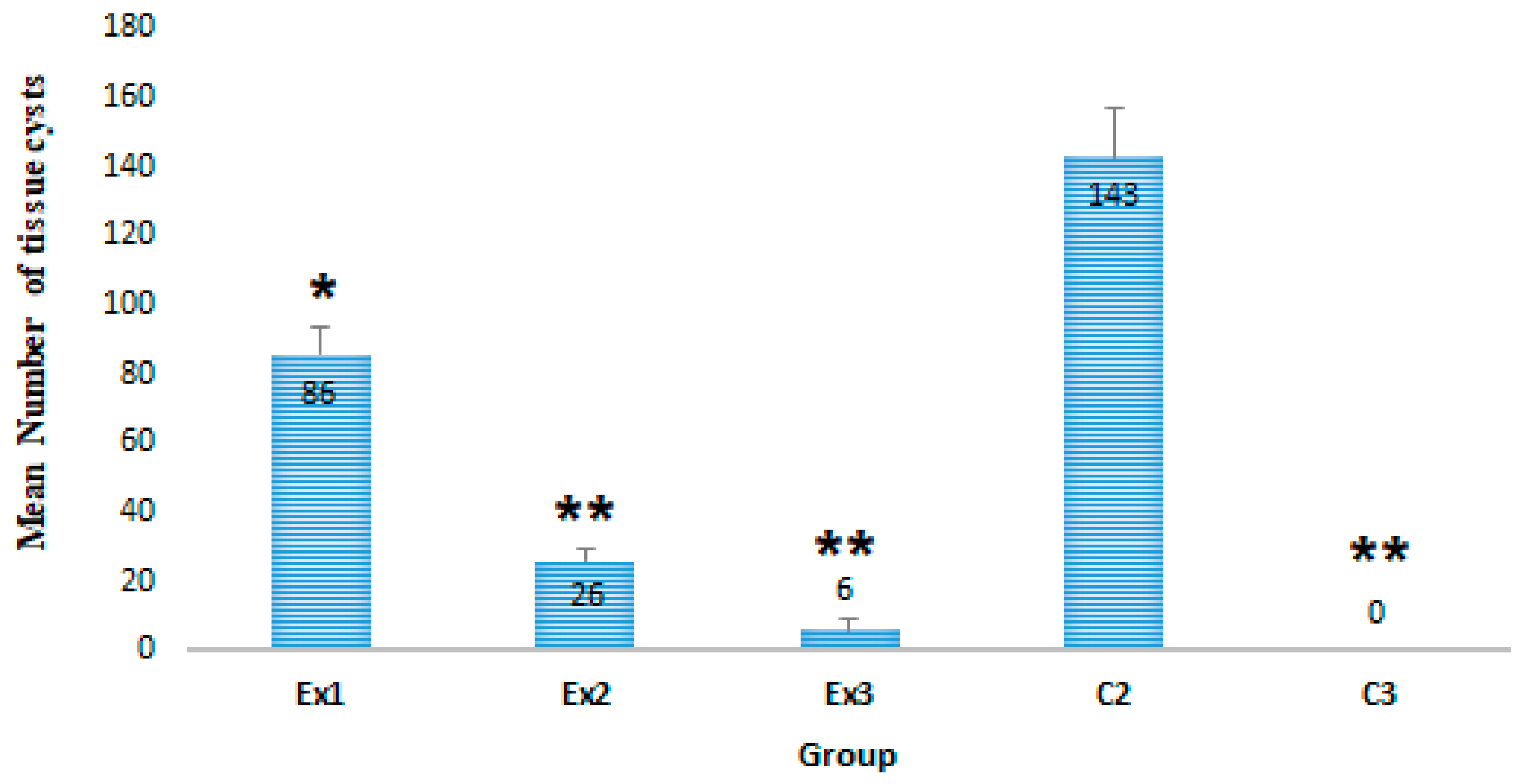
2.2.1. The Mean Number of T. gondii Tissue Cysts
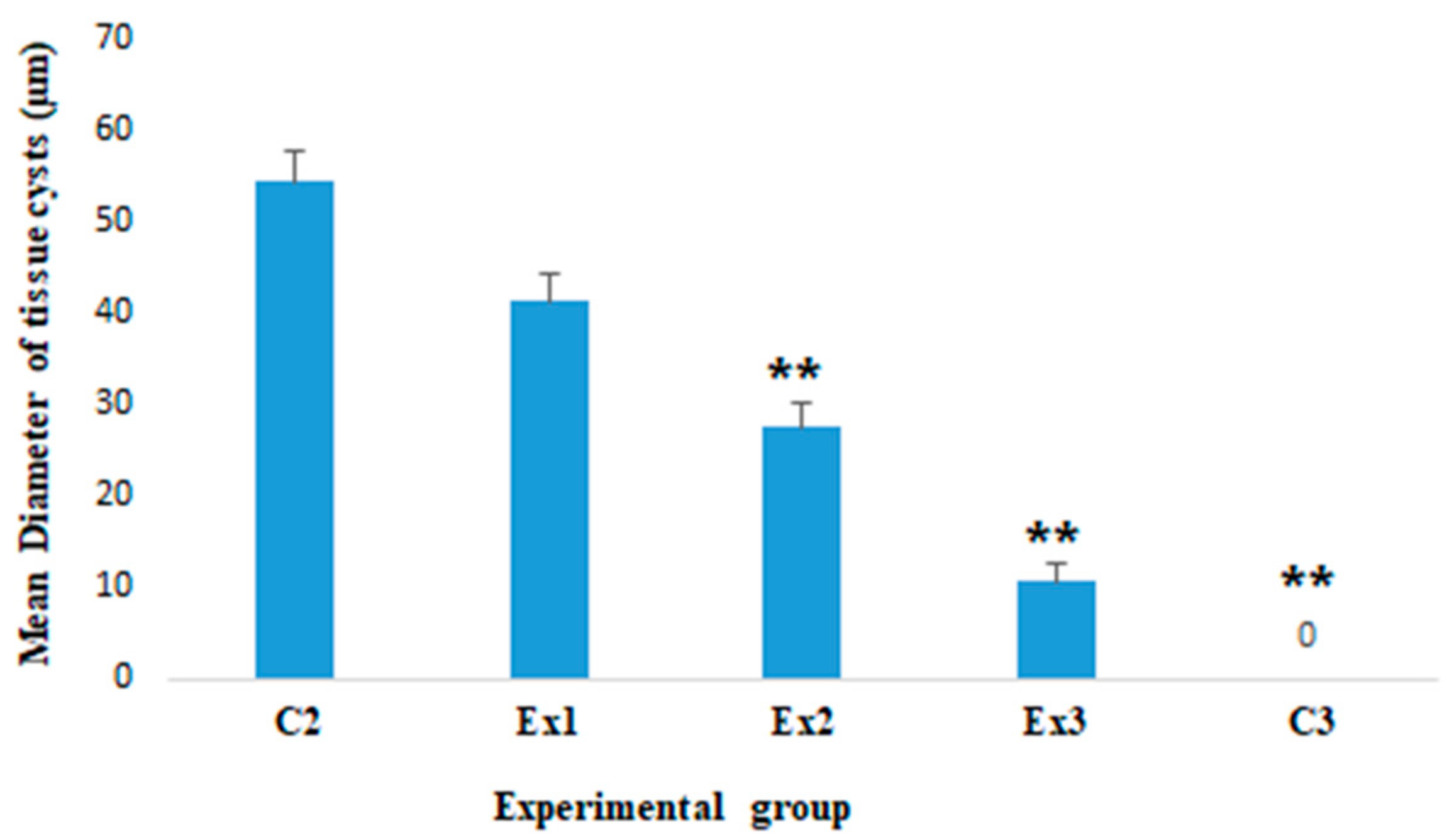
2.2.2. The Mean Diameter of T. gondii Tissue Cysts
2.3. Cytokine Expression by Real-Time PCR
3. Discussion
4. Materials and methods
4.1. Collecting the Plant Materials
4.2. Isolation of the Essential Oil
4.3. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis of Essential Oil
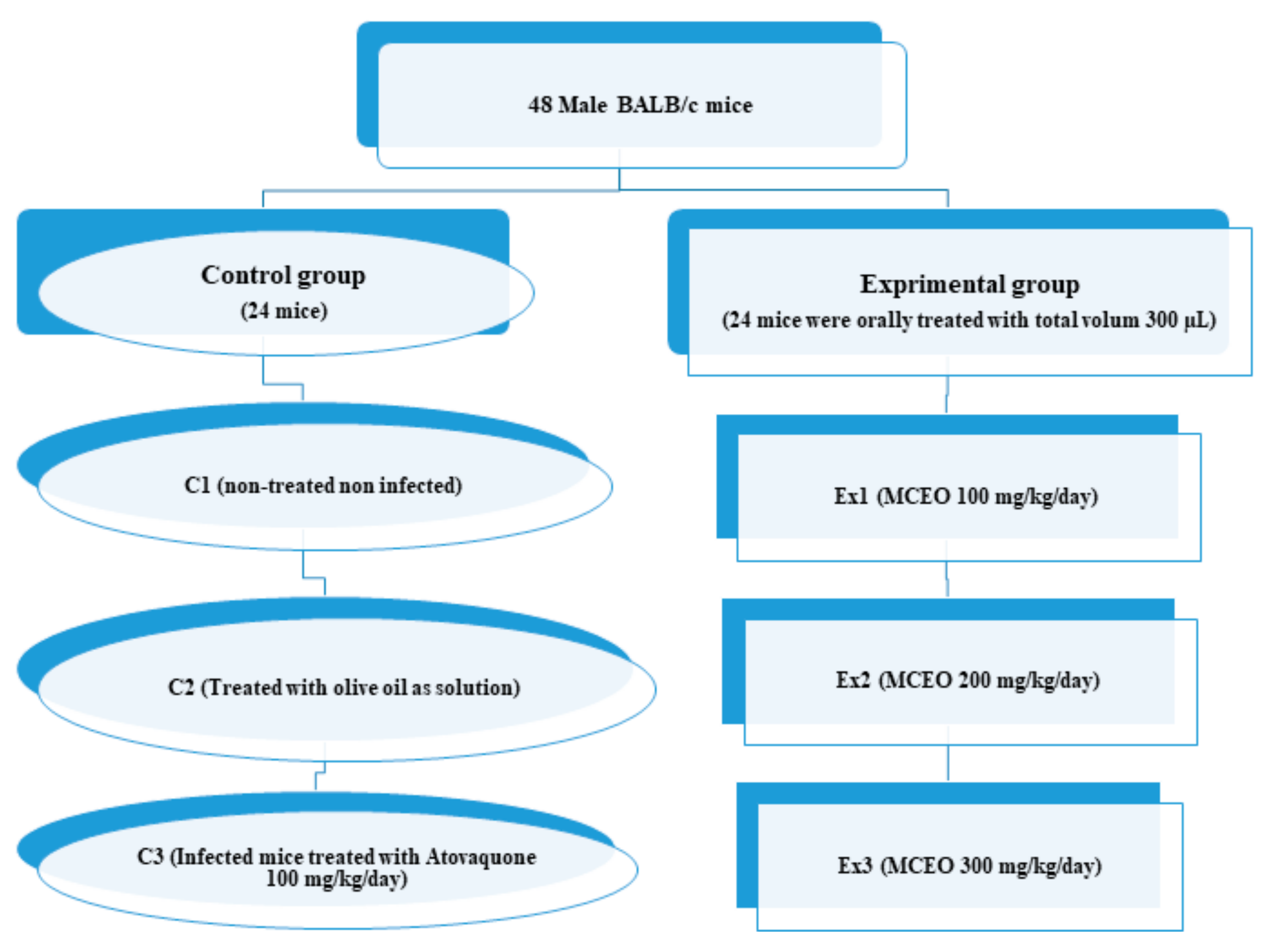
4.4. Experimental Design and Infection
4.4.1. Animals
4.4.2. Parasite
4.4.3. Animal Model of Chronic Toxoplasmosis
4.4.4. Design
4.5. Serological Tests
4.6. Sample Collection
4.7. Anti-Parasitic Activity
4.8. Induction of Innate Immune System
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mose, J.M.; Kagira, J.M.; Kamau, D.M.; Maina, N.; Ngotho, M.; Karanja, S.M. A Review on the Present Advances on Studies of Toxoplasmosis in Eastern Africa. Biomed. Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khryanin, A.; Reshetnikov, O.V.; Kuvshinova, I.N. Toxoplasmosis: Epidemiology, Diagnosis, Treatment. Antibiot. Chemoterapy 2015, 60, 16–21. [Google Scholar]
- Shaapan, R.M. The common zoonotic protozoal diseases causing abortion. J. Parasit. Dis. 2016, 40, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Elfadaly, H.A.; Hassanain, M.A.; Shaapan, R.M.; Barakat, A.M.; Toaleb, N.I. Serological and hormonal assays of murine materno-fetal Toxoplasma gondii infection with emphasis on virulent strains. World J. Med. Sci. 2012, 7, 248–254. [Google Scholar]
- Artimani, T.; Shabanian, S.; Heidari-Soureshjani, S.; Asadi-Samani, M.; Luther, T. A review of Iranian medicinal plants with teratogenic and abortion-inducing side effects. Int. J. Pharm. Sci. Res. 2017, 8, 2372–2377. [Google Scholar]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef]
- Azami, S.J.; Amani, A.; Keshavarz, H.; Najafi-Taher, R.; Mohebali, M.; Faramarzi, M.A.; Mahmoudi, M.; Shojaee, S. Nanoemulsion of atovaquone as a promising approach for treatment of acute and chronic toxoplasmosis. Eur. J. Pharm. Sci. 2018, 117, 138–146. [Google Scholar] [CrossRef]
- Toaleb, N.I.; Shaapan, R.M.; Hassan, S.E.; El Moghazy, F.M. High diagnostic efficiency of affinity isolated fraction in camel and cattle toxoplasmosis. World Med. Sci. J. 2013, 8, 61–66. [Google Scholar]
- Rezaei, F.; Sarvi, S.; Sharif, M.; Hejazi, S.H.; Pagheh, A.S.; Aghayan, S.A.; Daryani, A. A systematic review of Toxoplasma gondii antigens to find the best vaccine candidates for immunization. Microb. Pathog. 2019, 126, 172–184. [Google Scholar] [CrossRef]
- Mohammadipour, F.; Niazi, M.; Veiskaramian, A.; Mokhayeri, Y.; Moayyedkazemi, A.R. Toward nonalcoholic fatty liver treatment; a review on herbal medicine treatment. J. Crit. Rev. 2020, 7, 554–564. [Google Scholar]
- Delfani, S.; Mohammadrezaei-Khorramabadi, R.; Ghamari, S.; Boroujeni, R.K.; Khodabandeloo, N.; Khorzoughi, M.G.; Shahsavari, S. Systematic review for phytotherapy in Streptococcus Mutans. J. Pharm. Sci. Res. 2017, 9, 552. [Google Scholar]
- Masoori, L.; Yazdani, S.; Rezaei, F.; Amraei, M. Phytotherapy for Streptococcus viridans. J. Pharm. Sci. Res. 2017, 9, 1205–1208. [Google Scholar]
- Delfani, S.; Mohammadrezaei-Khorramabadi, R.; Abbaszadeh, S.; Naghdi, N.; Shahsavari, S. Phytotherapy for Streptococcus pyogenes. J. Pharm. Sci. Res. 2017, 9, 513. [Google Scholar]
- Niazi, M.; Saki, M.; Sepahvand, M.; Jahanbakhsh, S.; Khatami, M.; Beyranvand, M. In vitro and ex vivo scolicidal effects of Olea europaea L. to inactivate the protoscolecs during hydatid cyst surgery. Ann. Med. Surg. 2019, 42, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Abu El Ezz, N.M.T.; Khalil, F.A.M.; Shaapan, R.M. Therapeutic effect of onion (Allium cepa) and cinnamon (Cinnamomum zeylanicum) oils on cryptosporidiosis in experimentally infected mice. Glob. Vet. 2011, 7, 179–183. [Google Scholar]
- Aleksic, V.; Knežević, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef]
- Alipour, G.; Dashti, S.; Hosseinzadeh, H. Review of Pharmacological Effects of Myrtus communisL. and its Active Constituents. Phytother. Res. 2014, 28, 1125–1136. [Google Scholar] [CrossRef]
- Sisay, M.; Gashaw, T. Ethnobotanical, Ethnopharmacological, and Phytochemical Studies of Myrtus communis Linn: A Popular Herb in Unani System of Medicine. J. Evid. Based Integr. Med. 2017, 22, 1035–1043. [Google Scholar] [CrossRef]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Ezzatkhah, F.; Sharififar, F.; Sharifi, I.; Dezaki, E.S. Antileishmanial and Cytotoxic Effects of Essential Oil and Methanolic Extract of Myrtus communis L. Korean J. Parasitol. 2015, 53, 21–27. [Google Scholar] [CrossRef]
- Fadil, M.; Fikri-Benbrahim, K.; Rachiq, S.; Ihssane, B.; Lebrazi, S.; Chraibi, M.; Haloui, T.; Farah, A. Combined treatment of Thymus vulgaris L., Rosmarinus officinalis L. and Myrtus communis L. essential oils against Salmonella typhimurium: Optimization of antibacterial activity by mixture design methodology. Eur. J. Pharm. Biopharm. 2018, 126, 211–220. [Google Scholar] [CrossRef]
- Belmimoun, A.; Meddah, B.; Meddah, A.T.T.; Gabaldon, J.; Sonnet, P. Antifungal activity of Myrtus communis and Zygophyllum album extracts against human pathogenic fungi. Eur. J. Biol. Res. 2020, 10, 45–56. [Google Scholar]
- Kutlu, Z.; Gulaboglu, M.; Halıcı, Z.; Cınar, I.; Dıyarbakır, B. Biochemical Research of the Effects of Essential Oil Obtained from the Fruit of Myrtus communis L. on Cell Damage Associated with Lipopolysaccharide-Induced Endotoxemia in a Human Umbilical Cord Vein Endothelial Cells. Biochem. Genet. 2020, 1–20. [Google Scholar] [CrossRef]
- McLeod, R.; Lykins, J.; Noble, A.G.; Rabiah, P.K.; Swisher, C.N.; Heydemann, P.T.; McLone, D.G.; Frim, D.M.; Withers, S.; Clouser, F.; et al. Management of Congenital Toxoplasmosis. Curr. Pediatr. Rep. 2014, 2, 166–194. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine. 2019. Available online: https://apps.who.int/iris/handle/10665/312342 (accessed on 16 May 2019).
- Anand, U.; Jacobo-Herrera, N.J.; Altemimi, A.B.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, M.; Ziaiye, H.; Abdollahi, F.; Shabankhani, B. Effect of Methanolic essence and extract of Myrtus Communis on Trichomonas Vaginalis. J. Guilan Univ. Med. Sci. 2014, 12, 8–13. [Google Scholar]
- Mahmoudvand, H.; Fallahi, S.; Mahmoudvand, H.; Shakibaie, M.; Harandi, M.F.; Dezaki, E.S. Efficacy of Myrtus communis L. to Inactivate the Hydatid Cyst Protoscoleces. J. Investig. Surg. 2015, 29, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, F.; Esmaeili, S.; Abdullah, N.R.; Nateghpour, M.; Taghvai, M.; Kamkar, S.; Mosaddegh, M. In Vitro and In Vivo Antimalarial Evaluations of Myrtle Extract, a Plant Traditionally Used for Treatment of Parasitic Disorders. Biomed Res. Int. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Viuda-Martos, M.; Ruíz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Chemical composition of the essential oils obtained from some spices widely used in Mediterranean region. Acta Chim. Slov. 2007, 54, 921. [Google Scholar]
- Elfadaly, H.A.; Hassanain, N.A.; Shaapan, R.M.; Hassanain, M.A.; Barakat, A.M.A.; AbdelRahma, K.A. Molecular Detection and Genotyping of Toxoplasma gondii from Egyptian Isolates. Asian J. Epidemiol. 2016, 10, 37–44. [Google Scholar] [CrossRef]
- Oliveira, C.B.S.; Meurer, Y.S.R.; Medeiros, T.L.; Pohlit, A.M.; Silva, M.V.; Mineo, T.W.P.; Andrade-Neto, V.F. Anti-ToxoplasmaActivity of Estragole and Thymol in Murine Models of Congenital and Noncongenital Toxoplasmosis. J. Parasitol. 2016, 102, 369–376. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019, 2019. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Ziaali, N.; Ghazvini, H.; Shojaee, S.; Keshavarz, H.; Esmaeilpour, K.; Vahid, S. Toxoplasma gondii Infection Promotes Neuroinflammation Through Cytokine Networks and Induced Hyperalgesia in BALB/c Mice. Inflammation 2015, 39, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Shaapan, R.M.; Abo-ElMaaty, A.M.; El-Razik, K.A.A.; El-Hafez, S.M.A. PCR and serological assays for detection of Toxoplasma gondii infection in sport horses in Cairo, Egypt. Asian J. Anim. Vet. Adv. 2012, 7, 158–165. [Google Scholar] [CrossRef]
- Hassanain, M.; Elfadaly, H.; Shaapan, R.; Barakat, A. Biological Assay of Toxoplasma gondii Egyptian Mutton Isolates. Int. J. Zoo. Res. 2011, 7, 330–337. [Google Scholar] [CrossRef][Green Version]
- Ha, S.; Hamamura, M.J.; Nalcioglu, O.; Muftuler, L.T. A PIN diode controlled dual-tuned MRI RF coil and phased array for multi nuclear imaging. Phys. Med. Biol. 2010, 55, 2589–2600. [Google Scholar] [CrossRef]

| Amplicon | Primers | Sequence (5′–3′) |
|---|---|---|
| IL12 | F | ACGACATTCGTCAACTGCAA |
| R | TAAATTGGCACCCTGTAGGC | |
| IFN-γ | F | GATCGTGTCGTCACCAGAAAGG |
| R | TGCCTGGTAACGAGTTGTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaapan, R.M.; Al-Abodi, H.R.; Alanazi, A.D.; Abdel-Shafy, S.; Rashidipour, M.; Shater, A.F.; Mahmoudvand, H. Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. Molecules 2021, 26, 819. https://doi.org/10.3390/molecules26040819
Shaapan RM, Al-Abodi HR, Alanazi AD, Abdel-Shafy S, Rashidipour M, Shater AF, Mahmoudvand H. Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. Molecules. 2021; 26(4):819. https://doi.org/10.3390/molecules26040819
Chicago/Turabian StyleShaapan, Raafat M., Hiba Riyadh Al-Abodi, Abdullah D. Alanazi, Sobhy Abdel-Shafy, Marzieh Rashidipour, Abdullah F. Shater, and Hossein Mahmoudvand. 2021. "Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection" Molecules 26, no. 4: 819. https://doi.org/10.3390/molecules26040819
APA StyleShaapan, R. M., Al-Abodi, H. R., Alanazi, A. D., Abdel-Shafy, S., Rashidipour, M., Shater, A. F., & Mahmoudvand, H. (2021). Myrtus communis Essential Oil; Anti-Parasitic Effects and Induction of the Innate Immune System in Mice with Toxoplasma gondii Infection. Molecules, 26(4), 819. https://doi.org/10.3390/molecules26040819









