A 60% Edible Ethanolic Extract of Ulmus davidiana Inhibits Vascular Endothelial Growth Factor-Induced Angiogenesis
Abstract
1. Introduction
2. Results
2.1. U60E Reduces NO Production by Suppressing eNOS Activity in Human Umbilical Vein Endothelial Cells (HUVECs)
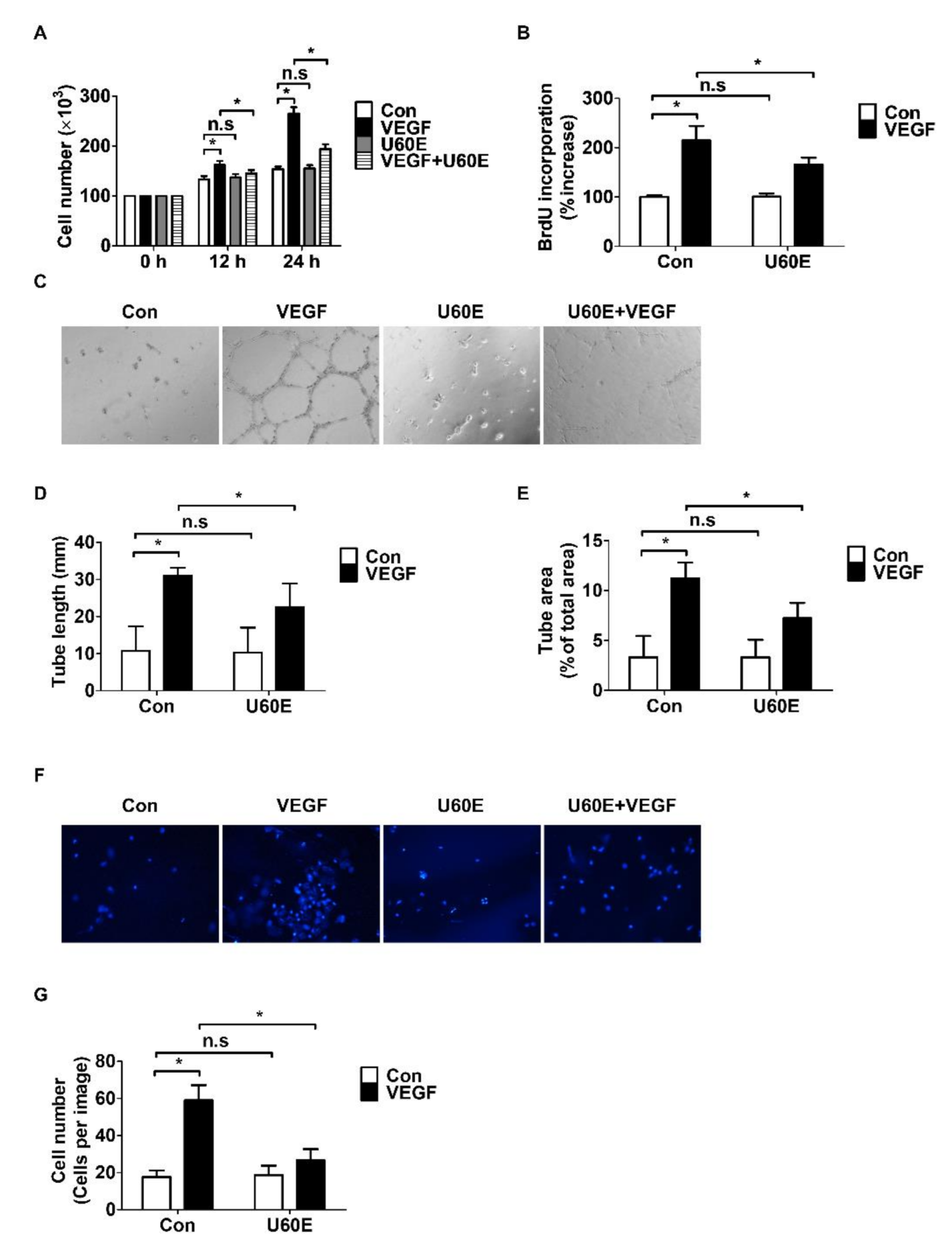
2.2. U60E Inhibits VEGF-Induced Angiogenesis in HUVECs
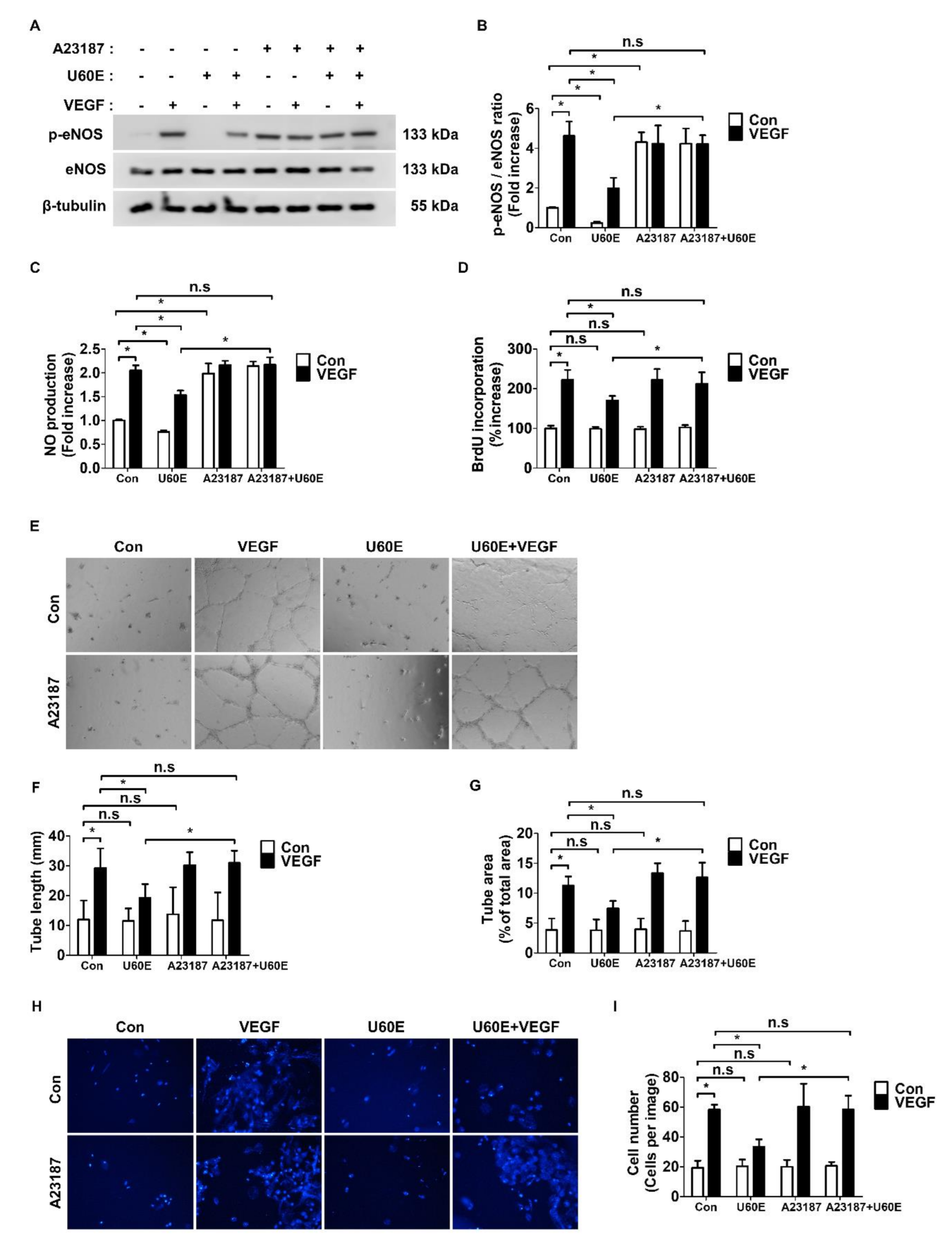
2.3. U60E Inhibits Angiogenesis by Suppressing VEGF-Mediated eNOS Activity
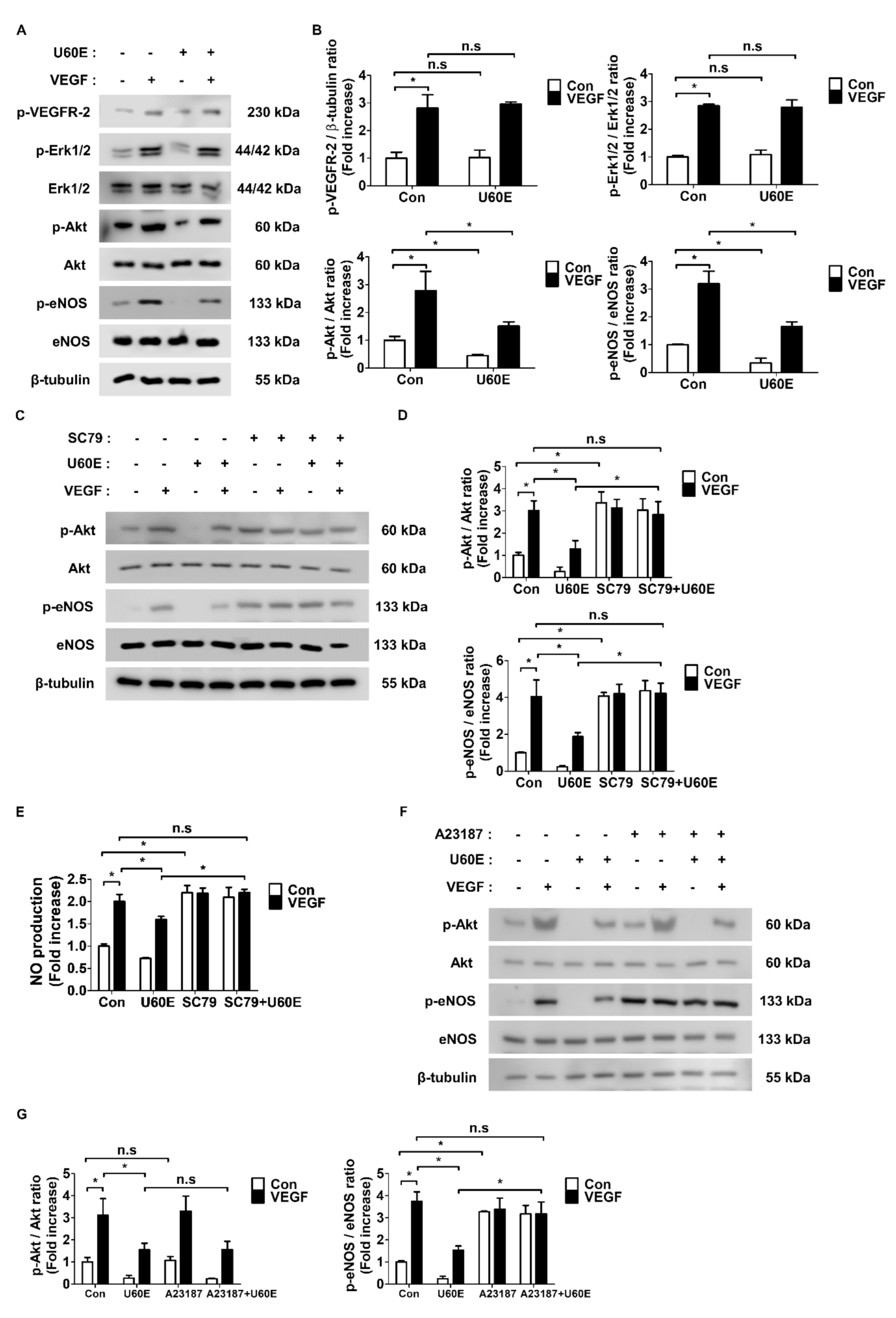
2.4. U60E Inhibits eNOS Activity and NO Production by Reducing Akt Activity
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Reagents and Antibodies
4.3. Preparation of U60E Extracts
4.4. Cell Viability Assay
4.5. Western Blot Analysis
4.6. BrdU Enzyme-Linked Immunosorbent Assay (ELISA) for Cell Proliferation Estimation
4.7. Cell Counting
4.8. Tube Formation
4.9. Transwell Cell Migration Assay
4.10. NO Production Measurement
4.11. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Aiello, L.P. Angiogenic pathways in diabetic retinopathy. N. Engl. J Med. 2005, 353, 839–841. [Google Scholar] [CrossRef]
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef]
- Matsumoto, T.; Claesson-Welsh, L. VEGF receptor signal transduction. Sci. STKE 2001, 2001, re21. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell. Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 2002, 90, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Karayiannakis, A.J.; Syrigos, K.N.; Polychronidis, A.; Zbar, A.; Kouraklis, G.; Simopoulos, C.; Karatzas, G. Circulating VEGF levels in the serum of gastric cancer patients: Correlation with pathological variables, patient survival, and tumor surgery. Ann. Surg. 2002, 236, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chang, Y.; Xu, J.; Zhang, Q. Predictive Significance of Serum Level of Vascular Endothelial Growth Factor in Gastric Cancer Patients. BioMed Res. Int. 2016, 2016, 8103019. [Google Scholar] [CrossRef] [PubMed]
- Linder, C.; Linder, S.; Munck-Wikland, E.; Auer, G.; Aspenblad, U.; Strander, H. Evaluation of tissue and serum VEGF in patients with head and neck carcinoma. Angiogenesis 1998, 2, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lutty, G.A.; McLeod, D.S.; Merges, C.; Diggs, A.; Plouet, J. Localization of vascular endothelial growth factor in human retina and choroid. Arch. Ophthalmol. 1996, 114, 971–977. [Google Scholar] [CrossRef]
- Zhou, Z.; Ju, H.; Sun, M.; Chen, H. Serum Vascular Endothelial Growth Factor Levels Correlate with Severity of Retinopathy in Diabetic Patients: A Systematic Review and Meta-Analysis. Dis. Markers 2019, 2019, 9401628. [Google Scholar] [CrossRef]
- Burgos, R.; Simo, R.; Audi, L.; Mateo, C.; Mesa, J.; Garcia-Ramirez, M.; Carrascosa, A. Vitreous levels of vascular endothelial growth factor are not influenced by its serum concentrations in diabetic retinopathy. Diabetologia 1997, 40, 1107–1109. [Google Scholar] [CrossRef]
- Sato, T.; Kusaka, S.; Shimojo, H.; Fujikado, T. Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology 2009, 116, 2165–2169. [Google Scholar] [CrossRef]
- Sonmez, K.; Drenser, K.A.; Capone, A., Jr.; Trese, M.T. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology 2008, 115, 1065–1070.e1. [Google Scholar] [CrossRef]
- Meadows, K.L.; Hurwitz, H.I. Anti-VEGF therapies in the clinic. Cold. Spring. Harb. Perspect. Med. 2012, 2, a006577. [Google Scholar] [CrossRef]
- Abcouwer, S.F. Angiogenic Factors and Cytokines in Diabetic Retinopathy. J. Clin. Cell. Immunol. 2013, 1–12. [Google Scholar] [CrossRef]
- Hartnett, M.E. Vascular endothelial growth factor antagonist therapy for retinopathy of prematurity. Clin. Perinatol. 2014, 41, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Afarid, M.; Sadegi Sarvestani, A.; Rahat, F.; Azimi, A. Intravitreal Injection of Bevacizumab: Review of our previous Experience. Iran. J. Pharm. Res. 2018, 17, 1093–1098. [Google Scholar] [PubMed]
- Lee, S.-E.; Kim, Y.-S.; Kim, J.-E.; Bang, J.-K.; Seong, N.-S. Antioxidant Activity of Ulmus davidiana var. japonica N. and Hemipteleae davidii P. Korean J. Med. Crop Sci. 2004, 12, 321–327. [Google Scholar]
- Choi, S.Y.; Lee, S.; Choi, W.H.; Lee, Y.; Jo, Y.O.; Ha, T.Y. Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica. J. Med. Food 2010, 13, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, J.S.; Yang, K.M. Ultrafine particles of Ulmus davidiana var. japonica induce apoptosis of gastric cancer cells via activation of caspase and endoplasmic reticulum stress. Arch. Pharm. Res. 2014, 37, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Korean Folk Medicine, Monographs Series No. 3; Publishing Center of Seoul National University: Seoul, Korea, 1996. [Google Scholar]
- Jung, H.J.; Jeon, H.J.; Lim, E.J.; Ahn, E.K.; Song, Y.S.; Lee, S.; Shin, K.H.; Lim, C.J.; Park, E.H. Anti-angiogenic activity of the methanol extract and its fractions of Ulmus davidiana var. japonica. J. Ethnopharmacol. 2007, 112, 406–409. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, C.W.; Jung, Y.J. Anti-inflammatory and immune-modulating effect of Ulmus davidiana var. japonica Nakai extract on a macrophage cell line and immune cells in the mouse small intestine. J. Ethnopharmacol. 2013, 146, 608–613. [Google Scholar] [CrossRef]
- Kim, Y.C.; Lee, M.K.; Sung, S.H.; Kim, S.H. Sesquiterpenes from Ulmus davidiana var. japonica with the inhibitory effects on lipopolysaccharide-induced nitric oxide production. Fitoterapia 2007, 78, 196–199. [Google Scholar] [CrossRef]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.J.; Kim, S.Y.; et al. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorg. Chem. 2019, 91, 103145. [Google Scholar] [CrossRef]
- Bernatchez, P.N.; Soker, S.; Sirois, M.G. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J. Biol. Chem. 1999, 274, 31047–31054. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—in control of vascular function. Nat. Rev. Mol. Cell. Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef]
- Osaadon, P.; Fagan, X.J.; Lifshitz, T.; Levy, J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 2014, 28, 510–520. [Google Scholar] [CrossRef]
- Keating, G.M. Bevacizumab: A review of its use in advanced cancer. Drugs 2014, 74, 1891–1925. [Google Scholar] [CrossRef]
- Shih, T.; Lindley, C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin. Ther. 2006, 28, 1779–1802. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Wu, L.; Sanchez, J.G.; Maia, M.; Saravia, M.J.; Fernandez, C.F.; Evans, T. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye 2009, 23, 117–123. [Google Scholar] [CrossRef]
- Rini, B.I. Sunitinib. Expert. Opin. Pharmacother. 2007, 8, 2359–2369. [Google Scholar] [CrossRef]
- Hao, Z.; Sadek, I. Sunitinib: The antiangiogenic effects and beyond. OncoTargets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef]
- Takimoto, C.H.; Awada, A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: A review of clinical trials. Cancer Chemother. Pharmacol. 2008, 61, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Heiduschka, P.; Plagemann, T.; Li, L.; Alex, A.F.; Eter, N. Different effects of various anti-angiogenic treatments in an experimental mouse model of retinopathy of prematurity. Clin. Exp. Ophthalmol. 2019, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tsujinaka, H.; Fu, J.; Shen, J.; Yu, Y.; Hafiz, Z.; Kays, J.; McKenzie, D.; Cardona, D.; Culp, D.; Peterson, W.; et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat. Commun. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.L.; Ren, B.; Gao, X.W.; Luo, Y.; Cai, Y.; Zhou, K.; Du, A.J.; Zhao, Y. Inhibition of retinopathy of prematurity in rat by intravitreal injection of sorafenib. Int. J. Ophthalmol. 2014, 7, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Crawshaw, A.A.; Griffiths, C.E.; Young, H.S. Investigational VEGF antagonists for psoriasis. Expert. Opin. Investig. Drugs 2012, 21, 33–43. [Google Scholar] [CrossRef]
- Hong, J. Role of natural product diversity in chemical biology. Curr. Opin. Chem. Biol. 2011, 15, 350–354. [Google Scholar] [CrossRef]
- Butler, M.S. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475–516. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Esumi, H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta. Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Lu, X.; Qian, C.; Zhang, J.; Li, P.; Shi, L.; Zhao, P.; Fu, Z.; Pu, P.; et al. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the avbeta3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur. J. Cell Biol. 2011, 90, 642–648. [Google Scholar] [CrossRef]
- Guo, J.Y.; Yang, T.; Sun, X.G.; Zhou, N.Y.; Li, F.S.; Long, D.; Lin, T.; Li, P.Y.; Feng, L. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J. Biomed. Sci. 2011, 18, 79. [Google Scholar] [CrossRef]
- Michell, B.J.; Griffiths, J.E.; Mitchelhill, K.I.; Rodriguez-Crespo, I.; Tiganis, T.; Bozinovski, S.; de Montellano, P.R.; Kemp, B.E.; Pearson, R.B. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr. Biol. 1999, 9, 845–848. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, H.-O.; Park, K.-H.; Wie, M.-B.; Choi, S.-E.; Yun, J.-H. A 60% Edible Ethanolic Extract of Ulmus davidiana Inhibits Vascular Endothelial Growth Factor-Induced Angiogenesis. Molecules 2021, 26, 781. https://doi.org/10.3390/molecules26040781
Park J, Kim H-O, Park K-H, Wie M-B, Choi S-E, Yun J-H. A 60% Edible Ethanolic Extract of Ulmus davidiana Inhibits Vascular Endothelial Growth Factor-Induced Angiogenesis. Molecules. 2021; 26(4):781. https://doi.org/10.3390/molecules26040781
Chicago/Turabian StylePark, Jeongho, Hyun-Ouk Kim, Kwang-Hyun Park, Myung-Bok Wie, Sun-Eun Choi, and Jang-Hyuk Yun. 2021. "A 60% Edible Ethanolic Extract of Ulmus davidiana Inhibits Vascular Endothelial Growth Factor-Induced Angiogenesis" Molecules 26, no. 4: 781. https://doi.org/10.3390/molecules26040781
APA StylePark, J., Kim, H.-O., Park, K.-H., Wie, M.-B., Choi, S.-E., & Yun, J.-H. (2021). A 60% Edible Ethanolic Extract of Ulmus davidiana Inhibits Vascular Endothelial Growth Factor-Induced Angiogenesis. Molecules, 26(4), 781. https://doi.org/10.3390/molecules26040781







