5-ALA Attenuates the Palmitic Acid-Induced ER Stress and Apoptosis in Bovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
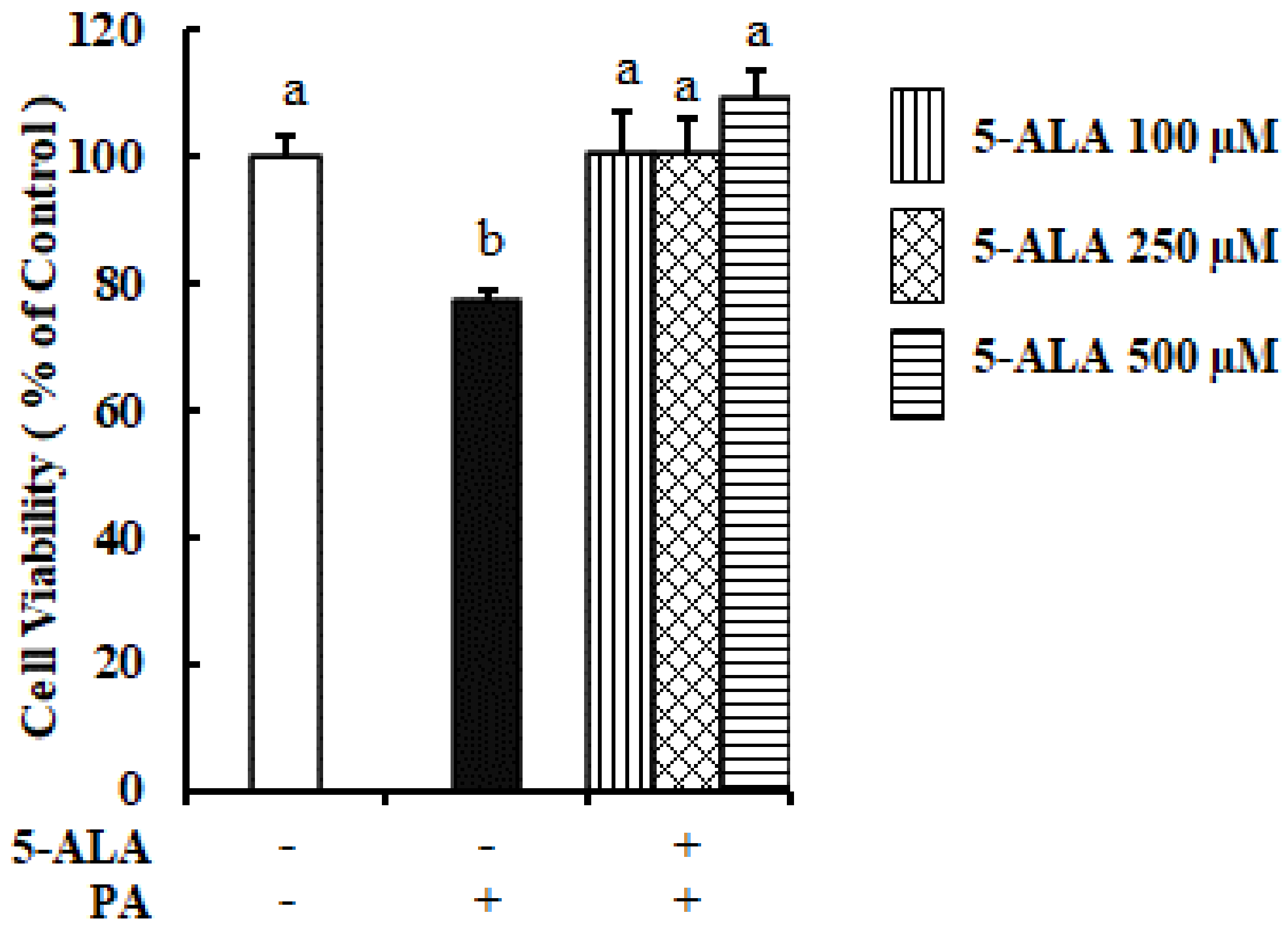
2.1. PA Reduced but 5-ALA Enhanced the MAC-T Cells Viability
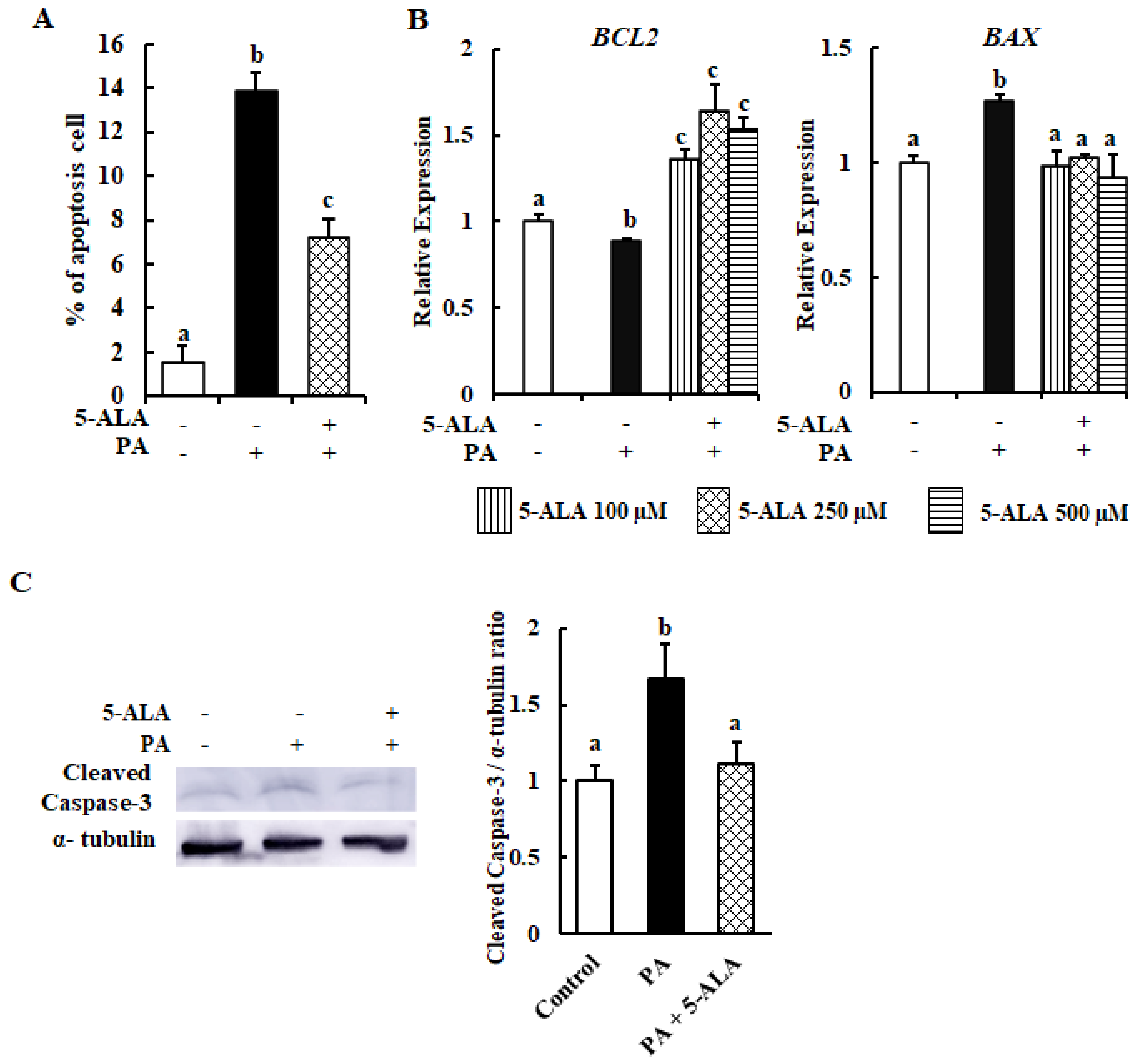
2.2. 5-ALA Reduced the Apoptosis Produced by PA in MAC-T Cells
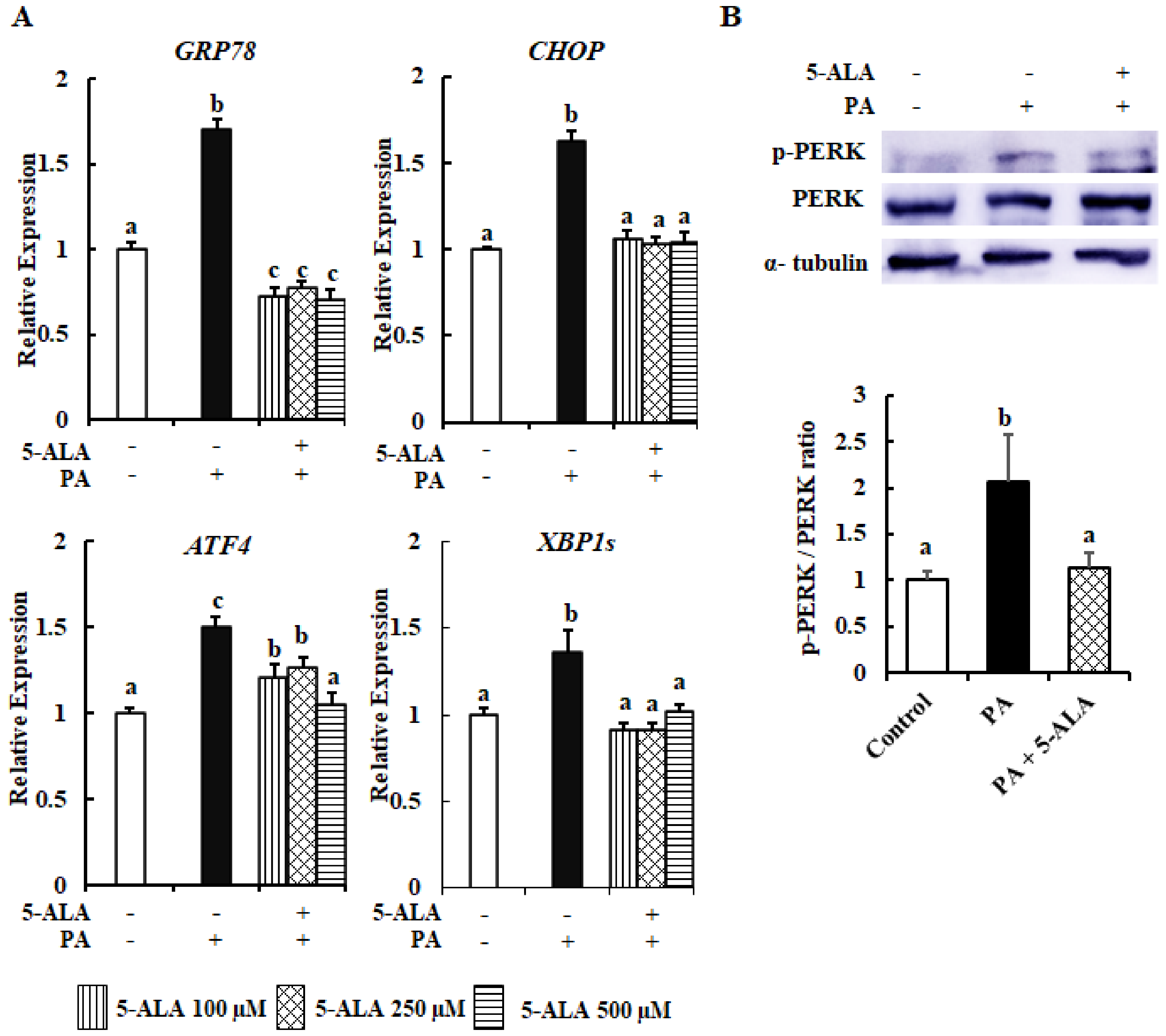
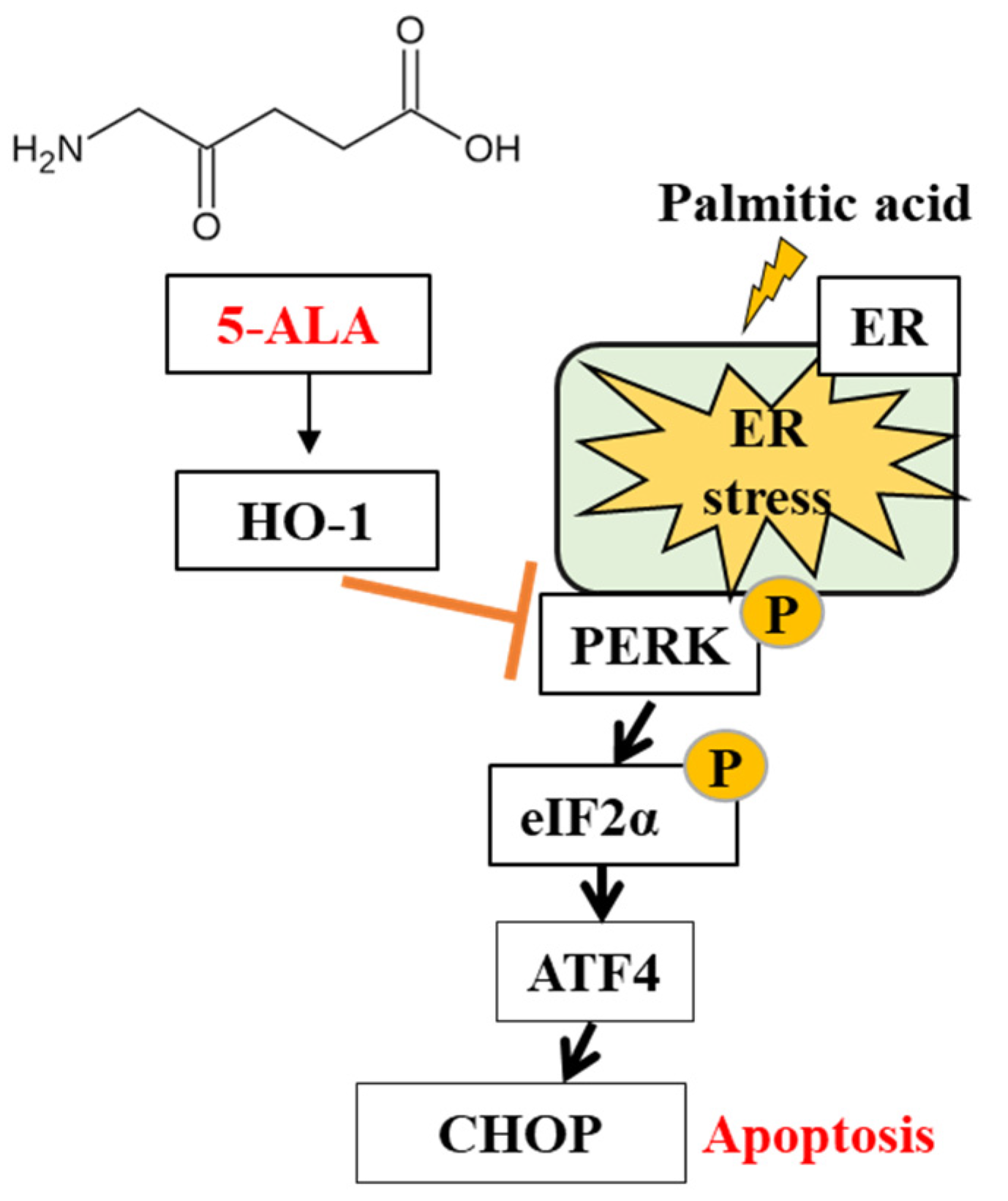
2.3. 5-ALA Ameliorated the PA-Induced ER Stress
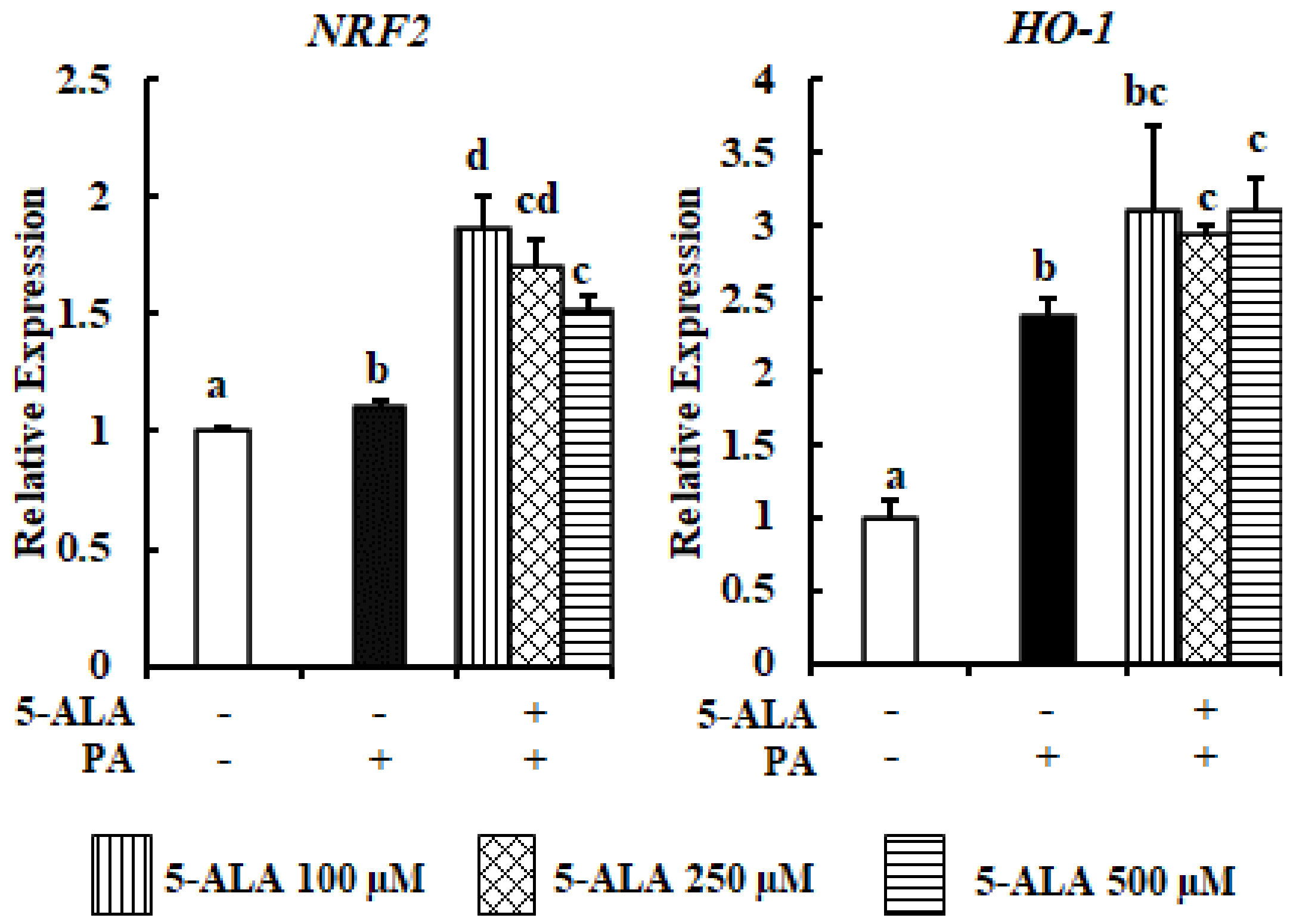
2.4. 5-ALA Culminated the PA-Induced OxS
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. Cell Viability Test
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Western Blot Analysis
4.6. Apoptosis Rate Analysis by Flow Cytometer
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Boutinaud, M.; Guinard-Flament, J. Hélènejammes The number and activity of mammary epithelial cells, determining factors for milk production. Reprod. Nutr. Dev. 2004, 44, 499–508. [Google Scholar] [CrossRef]
- Annen, E.; Fitzgerald, A.; Gentry, P.; McGuire, M.; Capuco, A.; Baumgard, L.; Collier, R. Effect of Continuous Milking and Bovine Somatotropin Supplementation on Mammary Epithelial Cell Turnover. J. Dairy Sci. 2007, 90, 165–183. [Google Scholar] [CrossRef]
- Capuco, A.V.; Ellis, S.E.; Hale, S.A.; Long, E.; Erdman, R.A.; Zhao, X.; Paape, M.J. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 2003, 81, 18–31. [Google Scholar] [CrossRef]
- Forbes, J.M. Interrelationships between physical and metabolic control of voluntary food intake in fattening, pregnant and lactating mature sheep: A model. Anim. Sci. 1977, 24, 91–101. [Google Scholar] [CrossRef]
- Grummer, R.R.; Bertics, S.J.; LaCount, D.W.; Snow, J.A.; Dentine, M.; Stauffacher, R.H. Estrogen Induction of Fatty Liver in Dairy Cattle. J. Dairy Sci. 1990, 73, 1537–1543. [Google Scholar] [CrossRef]
- Green, D.; Brink, D.; Bauer, M. Characterization of feed intake and estradiol-17 β during gestation and lactation in twin-bearing ewes. Small Rumin. Res. 1994, 13, 153–158. [Google Scholar] [CrossRef]
- Hayirli, A.; Grummer, R.R. Factors affecting dry matter intake prepartum in relationship to etiology of peripartum lipid-related metabolic disorders: A review. Can. J. Anim. Sci. 2004, 84, 337–347. [Google Scholar] [CrossRef]
- Janovick, N.; Boisclair, Y.; Drackley, J. Prepartum dietary energy intake affects metabolism and health during the periparturient period in primiparous and multiparous Holstein cows. J. Dairy Sci. 2011, 94, 1385–1400. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Andersen, J.B. Integration of Metabolism and Intake Regulation: A Review Focusing on Periparturient Animals. J. Dairy Sci. 2000, 83, 1573–1597. [Google Scholar] [CrossRef]
- Sharmin, M.M.; Mizusawa, M.; Hayashi, S.; Arai, W.; Sakata, S.; Yonekura, S. Effects of fatty acids on inducing endoplasmic reticulum stress in bovine mammary epithelial cells. J. Dairy Sci. 2020, 103, 8643–8654. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Hirabara, S.M.; Silveira, L.D.R.; Levada-Pires, A.C.; Curi, R.; Pithon-Curi, T.C. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J. Cell. Physiol. 2008, 216, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Abe, F.; Sano, Y.; Takahashi, K.; Inoue, K.; Nakajima, M.; Kohda, T.; Komatsu, N.; Ogura, S.-I.; Tanaka, T. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef]
- Islam, A.; Noguchi, Y.; Taniguchi, S.; Yonekura, S. Protective Effects of 5-Aminolevulinic Acid on Heat Stress in Bovine Mammary Epithelial Cells. Asian-Australasian J. Anim. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fujino, M.; Zhu, S.; Isaka, Y.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhu, P.; Li, X. 5-ALA/SFC enhances HO-1 expression through the MAPK/Nrf2 antioxidant pathway and attenuates murine tubular epithelial cell apoptosis. FEBS Open Bio 2019, 9, 1928–1938. [Google Scholar] [CrossRef]
- Uchida, A.; Kidokoro, K.; Sogawa, Y.; Itano, S.; Nagasu, H.; Satoh, M.; Sasaki, T.; Kashihara, N. 5-Aminolevulinic acid exerts renoprotective effect via Nrf2 activation in murine rhabdomyolysis-induced acute kidney injury. Nephrology 2017, 24, 28–38. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, P.; Fujino, M.; Isaka, Y.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhuang, J.; Li, X.-K. 5-aminolaevulinic acid (ALA), enhances heme oxygenase (HO)-1 expression and attenuates tubulointerstitial fibrosis and renal apoptosis in chronic cyclosporine nephropathy. Biochem. Biophys. Res. Commun. 2019, 508, 583–589. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, P.; Fujino, M.; Nishio, Y.; Chen, J.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhao, L.; et al. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem. Biophys. Res. Commun. 2016, 479, 663–669. [Google Scholar] [CrossRef]
- Sato, Y.; Fujimoto, S.; Mukai, E.; Sato, H.; Tahara, Y.; Ogura, K.; Yamano, G.; Ogura, M.; Nagashima, K.; Inagaki, N. Palmitate induces reactive oxygen species production and β-cell dysfunction by activating nicotinamide adenine dinucleotide phosphate oxidase through Src signaling. J. Diabetes Investig. 2014, 5, 19–26. [Google Scholar] [CrossRef]
- Barlow, J.; Affourtit, C. Novel insights into pancreatic β-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells. Biochem. J. 2013, 456, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Alam, M.R.; Waldeck-Weiermair, M.; Karsten, F.; Groschner, L.; Riederer, M.; Hallström, S.; Rockenfeller, P.; Konya, V.; Heinemann, A.; et al. Inhibition of Autophagy Rescues Palmitic Acid-induced Necroptosis of Endothelial Cells. J. Biol. Chem. 2012, 287, 21110–21120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-R.; Zhang, J.-A.; Zhang, Q.; Permatasari, F.; Xu, Y.; Wu, D.; Yin, Z.-Q.; Luo, D. Palmitic Acid Induces Production of Proinflammatory Cytokines Interleukin-6, Interleukin-1β, and Tumor Necrosis Factor-αvia a NF-κB-Dependent Mechanism in HaCaT Keratinocytes. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, S.; Wang, F.; Zhang, H.; Chen, Z.-J.; Wang, J.; Wang, Z.; Du, Z.; Ling, E.-A.; Liu, Q.; et al. Palmitic acid increases apoptosis of neural stem cells via activating c-Jun N-terminal kinase. Stem Cell Res. 2013, 10, 257–266. [Google Scholar] [CrossRef]
- Peterson, J.M.; Wang, Y.; Bryner, R.W.; Williamson, D.L.; Alway, S.E. Bax signaling regulates palmitate-mediated apoptosis in C2C12 myotubes. Am. J. Physiol. Metab. 2008, 295, E1307–E1314. [Google Scholar] [CrossRef]
- Zinszner, H.; Kuroda, M.; Wang, X.; Batchvarova, N.; Lightfoot, R.T.; Remotti, H.; Stevens, J.L.; Ron, D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998, 12, 982–995. [Google Scholar] [CrossRef]
- Yonekura, S.; Tsuchiya, M.; Tokutake, Y.; Mizusawa, M.; Nakano, M.; Miyaji, M.; Ishizaki, H.; Haga, S. The unfolded protein response is involved in both differentiation and apoptosis of bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 3568–3578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, L.; Zhang, H.; Wu, G.; Zhang, Z.; Lv, J. Chlorogenic acid against palmitic acid in endoplasmic reticulum stress-mediated apoptosis resulting in protective effect of primary rat hepatocytes. Lipids Heal. Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lei, L.; Wen, D.; Yang, L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J. Ovarian Res. 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, Y.; Sun, X.; Zhao, H.; Yao, M.; Hou, L.; Jiang, L. Up-regulation of HO-1 by Nrf2 activation protects against palmitic acid-induced ROS increase in human neuroblastoma BE(2)-M17 cells. Nutr. Res. 2018, 52, 80–86. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxidative Med. Cell. Longev. 2015, 2016, 1–14. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.-C.; El-Aty, A.A.; Jeong, J.H. Protectin DX suppresses hepatic gluconeogenesis through AMPK-HO-1-mediated inhibition of ER stress. Cell. Signal. 2017, 34, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Maamoun, H.; Zachariah, M.; McVey, J.H.; Green, F.R.; Agouni, A. Heme oxygenase (HO)-1 induction prevents Endoplasmic Reticulum stress-mediated endothelial cell death and impaired angiogenic capacity. Biochem. Pharmacol. 2017, 127, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, S.; Tang, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia–reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: Role of SIRT1 activation. Apoptosis 2017, 22, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zeng, X.; Wang, H.; Hou, Q.; Tan, C.; Xu, Q.; Wang, H. 14,15-Epoxyeicosatrienoic Acid Suppresses Cigarette Smoke Extract-Induced Apoptosis in Lung Epithelial Cells by Inhibiting Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2015, 36, 474–486. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Xie, X.; Chen, H.; Zhu, Q.; Ge, Z.; Wei, H.; Deng, J.; Xia, Z.; Lian, Q. Heme Oxygenase-1 Reduces Sepsis-Induced Endoplasmic Reticulum Stress and Acute Lung Injury. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmin, M.M.; Islam, M.A.; Yamamoto, I.; Taniguchi, S.; Yonekura, S. 5-ALA Attenuates the Palmitic Acid-Induced ER Stress and Apoptosis in Bovine Mammary Epithelial Cells. Molecules 2021, 26, 1183. https://doi.org/10.3390/molecules26041183
Sharmin MM, Islam MA, Yamamoto I, Taniguchi S, Yonekura S. 5-ALA Attenuates the Palmitic Acid-Induced ER Stress and Apoptosis in Bovine Mammary Epithelial Cells. Molecules. 2021; 26(4):1183. https://doi.org/10.3390/molecules26041183
Chicago/Turabian StyleSharmin, Mst Mamuna, Md Aminul Islam, Itsuki Yamamoto, Shin Taniguchi, and Shinichi Yonekura. 2021. "5-ALA Attenuates the Palmitic Acid-Induced ER Stress and Apoptosis in Bovine Mammary Epithelial Cells" Molecules 26, no. 4: 1183. https://doi.org/10.3390/molecules26041183
APA StyleSharmin, M. M., Islam, M. A., Yamamoto, I., Taniguchi, S., & Yonekura, S. (2021). 5-ALA Attenuates the Palmitic Acid-Induced ER Stress and Apoptosis in Bovine Mammary Epithelial Cells. Molecules, 26(4), 1183. https://doi.org/10.3390/molecules26041183




