Abstract
The production of cyclic carbonates from CO2 cycloaddition to epoxides, using the C-scorpionate iron(II) complex [FeCl2{κ3-HC(pz)3}] (pz = 1H-pyrazol-1-yl) as a catalyst, is achieved in excellent yields (up to 98%) in a tailor-made ionic liquid (IL) medium under mild conditions (80 °C; 1–8 bar). A favorable synergistic catalytic effect was found in the [FeCl2{κ3-HC(pz)3}]/IL system. Notably, in addition to exhibiting remarkable activity, the catalyst is stable during ten consecutive cycles, the first decrease (11%) on the cyclic carbonate yield being observed during the 11th cycle. The use of C-scorpionate complexes in ionic liquids to afford cyclic carbonates is presented herein for the first time.
1. Introduction
Over the last few decades, it has become clear that sharply increasing anthropogenic CO2 emissions is affecting the climate stability of the biosphere [1]. Therefore, a realistic transition from fossil carbon usage to alternative raw materials and commodities based on recycled carbon (CO2) is imperative. Since current carbon capture and storage (CCS) technologies are able to capture up to 90% of the CO2 produced [2], rendering it available in vast quantities and with satisfactory purity, a sustainable solution would entail its conversion into useful value-added commodity chemicals [3,4,5,6,7,8,9,10,11]. Among the possible strategies to use captured CO2, its catalytic reaction with epoxides to produce cyclic carbonates is one of the most promising applications as a renewable carbon source. The interest in cyclic carbonates is driven by their wide range of chemical and technological applications. To date, a considerable number of catalytic systems have been developed (either metal or organocatalysts) for the cycloaddition of CO2 and epoxides [3,4,5,6,7,8,9,10,12,13,14,15,16,17,18,19,20,21,22,23,24]. However, further improvements are needed, in particular, in (i) controlling the selectivity (to impair polycarbonates formation); (ii) achieving suitable catalytic activities for less reactive substrates (e.g., sterically hindered and internal epoxides); (iii) searching for milder efficient reaction conditions (high temperatures and pressures are ultimately associated with additional, indirect CO2 emissions, limiting their value from a technological standpoint); and (iv) finding active and selective catalysts able to be recycled and reused in consecutive cycles.
Tripodal nitrogen poly(1H-pyrazol-1-yl)-methane scorpionate ligands, [R(4−n)C(R’pz)n] (pz = 1H-pyrazol-1-yl), also known as C-scorpionates (analogy with a scorpion; see Figure 1a), are one of the most versatile classes of ligands in coordination chemistry. They are able to (i) stabilize transition metals in a wide range of oxidation states, (ii) combine the tripodal architecture needed for efficient three-point biding with a chelating coordination site, leaving up to three sites to other co-ordinations; and (iii) provide a degree of steric bulk, avoiding, e.g., dimerization reactions. Variation of the substituents at different positions on the pyrazolyl rings, or at the at the methine carbon atom, leads to a range of steric and electronic effects, with this tunability being one of the most important advantages of poly(1H-pyrazol-1-yl)-methane ligands [25,26,27].
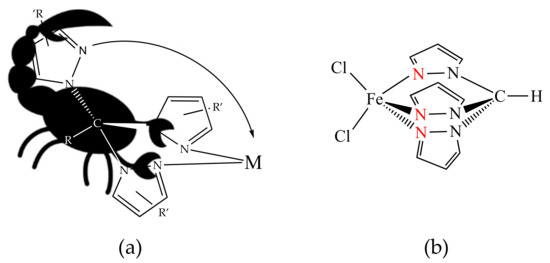
Figure 1.
(a) Analogy between a C-scorpionate coordination to a metal center (M) and the attack of a scorpion on its prey. (b) Structure of the dichloro hydrotris(1H-pyrazol-1-yl)-methane Fe(II) complex [FeCl2{κ3-HC(pz)3}] (pz = 1H-pyrazol-1-yl).
C-scorpionate complexes have found a wide range of applications in bioinorganic chemistry [26,28,29,30], in particular in metalloenzyme modelling studies mimicking histidine nitrogen coordination by pyrazole to the metal ion binding sites at Cu proteins (e.g., hemocyanin, ascorbate oxidase, and superoxide dismutase), Fe proteins (hemerythrin), or Mn proteins (superoxide dismutase) [31].
The ability of these ligands to readily modify their scaffold, either at the pyrazolyl rings or/and at the methinic carbon atom, to tailor electronic, steric, and coordination properties as desired for a particular application, could provide unique catalytic effects towards the application of CO2 and epoxides as raw materials for the sustainable synthesis of cyclic carbonates.
In this work, the above cycloaddition reaction issues, in particular iv), finding active and selective catalysts able to be recycled and reused in consecutive cycles, are addressed by using the bio-inspired C-homoscorpionate Fe(II) catalyst [FeCl2{κ3-HC(pz)3}] (pz = 1H-pyrazol-1-yl) [25,26,27] in tailor-made cheap ionic liquid media [32]. The selected [FeCl2{κ3-HC(pz)3}] complex (Figure 1b) exhibits a tetragonal pyramid coordination polyhedron with one of the donor N pyrazolyl atoms at the axial position and where the iron atom has one empty coordination site that could be easily occupied by a new substrate [33], such as an epoxide. Moreover, it is easy (one step) to synthesize [34], in water, at r.t., from the available and cheap iron salt and the simplest ligand of the tris(1H-pyrazol-1-yl)methane carbon scorpionate class, hydrotris(1H-pyrazol-1-yl)-methane, HC(pz)3. To our knowledge, to date, this class of compounds has not been tested as a catalyst for cyclic carbonate synthesis.
2. Results and Discussion
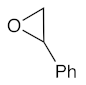
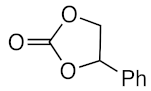
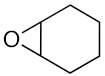
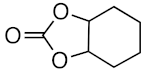
At first, the knowledge [9,14,15,16,17] that some types of ionic liquids are able to catalyze the cycloaddition of CO2 to epoxides prompted us to perform a series of experiments with the green, commercially available and affordable ionic liquids (IL) depicted in Figure 2 and selected model epoxides (Scheme 1). In addition to the typical propylene and styrene oxides (bearing an alkyl or phenyl group, respectively), the very challenging (internal epoxide) cyclohexene oxide was also chosen.
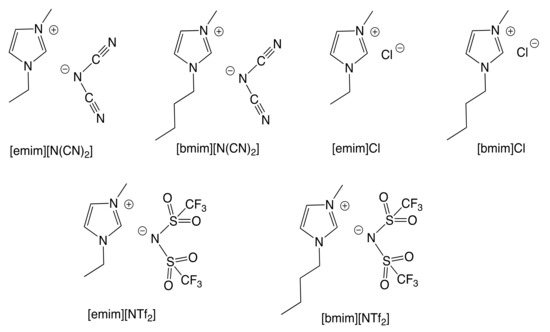
Figure 2.
Room-temperature ionic liquids used in this work.
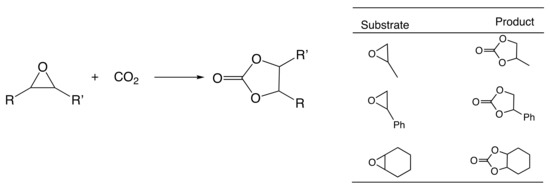
Scheme 1.
Selective cycloaddition of CO2 to epoxides.
The starting reaction conditions were selected in view of the reported [35] cycloaddition reaction to cyclohexene oxide substrate. Then, the reaction parameters (e.g., time, temperature, CO2 pressure, IL to substrate ratio) were optimized (see experimental section) but the obtained carbonate yields under such conditions (epoxide (5 mmol), [tBu4N]Br (0.3%mol vs. epoxide), IL (8.3–18.8 mol), CO2 (8 bar), 24 h, 80 °C) were very low (up to 5%, for [bmim][N(CN)2]), even in the presence of promoting [Bu4N]Br [36,37] (which apparently requires higher temperatures and CO2 pressures to operate as a catalyst). Note that the presence of the ionic liquid allowed us to use a lower amount of [tBu4N]Br than the level usually reported in the literature [6,38,39] as being required to achieve high epoxide conversions. Nevertheless, in all experiments, only a single reaction product, the corresponding carbonate, was identified, as shown in Scheme 1.
The IL [bmim][N(CN)2] is known to interact with the C-homoscorpionate iron(II) complex [FeCl2{κ3-HC(pz)3}] (pz = 1H-pyrazol-1-yl) and a favorable catalytic synergistic effect was reported [33] for other reactions. Iron catalysts in CO2 chemistry have been reported for CO2 reduction and hydroformylation reactions, but their use in CO2/epoxide chemistry has been less extensively explored. Therefore, herein the above system was tested as a catalyst for the synthesis of cyclic carbonates under the above optimal conditions. The obtained results are presented in Table 1. For propylene and styrene oxide substrates, excellent yields (up to 98.3%, in [bmim][N(CN)2]), entry 2, Table 1) were achieved for the corresponding cyclic carbonates concomitant with a selectivity of 100%. Even the less reactive cyclohexene oxide was successfully converted, attaining good carbonate yields (up to 72.7%, in [bmim][N(CN)2]), entry 14, Table 1). [FeCl2{κ3-HC(pz)3}] was added to the reaction medium within a 0.3–0.8% mol vs. epoxide range, exhibiting its best performance at a concentration of 0.5% mol vs. epoxide. The increase up to 0.8% mol vs. epoxide did not lead to a significant improvement in the products yields, and therefore the lowest 0.5% mol vs. epoxide was selected. The kinetic profiles are depicted in Figure 3.

Table 1.
Selected data a for the cycloaddition of CO2 to epoxides catalyzed by [FeCl2{κ3-HC(pz)3}] in different ionic liquid (IL) media.
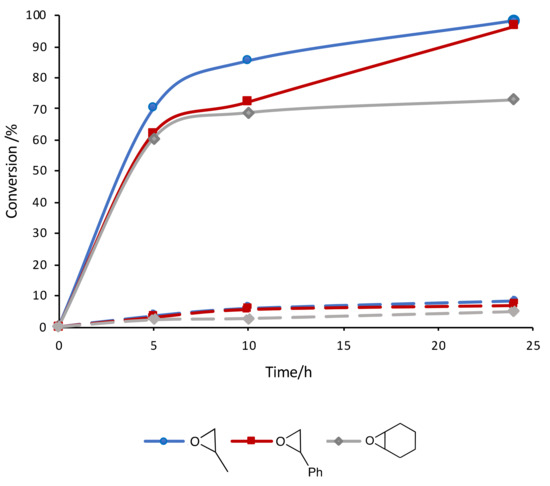
Figure 3.
Kinetic profile of the cycloaddition of CO2 to different epoxides catalyzed by the [FeCl2{κ3-HC(pz)3}]/IL system (solid lines, in [bmim][N(CN)2]; dashed lines, in THF).
All three substrates follow the trend of achieving conversions depending in the IL anions in the following order: N(CN)2 > Cl > NTf2. Ionic liquids with Cl− and [NTf2]− anions exhibit significantly higher viscosity ([emim]Cl, 44 cP; [bmim]Cl 55 cP; [emim][NTf2], 100 cP; [emim][NTf2], 190 cP [40]) than ILs bearing [N(CN)2]− counterions ([emim][N(CN)2], 15 cP; [emim][N(CN)2], 33 cP [40]) as a result of intra and intermolecular hydrogen bonding and van der Waals interactions [41]. Since a high viscosity directly affects the mass transfer of a reaction, and vice versa [42], the viscosity imparted by the anion of the ionic liquid appears to be an important parameter in the study of the catalytic performance of the C-scorpionate Fe(II) complex in such media. The observed catalyst activity could also be linked to the relative coordination ability of these anions to the metal center; however, this clearly needs further investigation.
The ionic liquids’ catalytic performance was also studied, and the results for the conversion of styrene epoxide into styrene carbonate are presented in Table 2. Under the reaction conditions used, the ionic liquids of this study, per se, did not exhibit catalytic activity for the cycloaddition of carbon dioxide and epoxides.

Table 2.
Cycloaddition a of CO2 to styrene epoxide in different ionic liquids.
It is worth mentioning that the [FeCl2{κ3-HC(pz)3}] complex by itself, although maintaining the selectivity found for the [FeCl2{κ3-HC(pz)3}]/IL system, exhibited a much worse catalytic performance, leading to carbonate yields of up to 6.5% (Table 3).

Table 3.
Selected data a for the cycloaddition of CO2 to epoxides catalyzed by [FeCl2{κ3-HC(pz)3}] in THF.
Thus, the synergistic catalysis found by combining [FeCl2{κ3-HC(pz)3}] with the appropriate IL (preferably [bmim][N(CN)2]) significantly improved the efficiency of the present carbonate synthetic process. Conversely, poor yields were observed in the molecular solvent THF, highlighting the benefits of conducting the process in an IL media.
The [FeCl2{κ3-HC(pz)3}]/IL catalytic system exhibits better performance and/or requires milder reaction conditions than several other catalysts, including organocatalysts and iron-based catalysts previously found in the literature [6,38,43,44,45,46]. Table 4 presents a comparison of some of these catalytic systems.

Table 4.
Cycloaddition of CO2 to epoxides catalyzed by different classes and types of homogeneous catalysts.
For example, Park et al., [43] used alkylmethylimidazolium-based ionic liquids, bearing the anions Cl−, [BF4]− or [PF6]–, as catalysts for the cycloaddition of CO2 (9.6 bar) to allyl glycidyl ether, which took 48 h at 100 °C to generate the five-membered cyclic carbonate in good yields (entry 1, Table 4). On the other hand, the binary catalytic system presented in entry 2 of Table 4, although operating at mild conditions (25 °C, 1.01 bar of CO2 for 24 h) led to moderate carbonate yields [44].
Jing et al. [45] reported an iron porphyrin complex as a catalyst for the coupling of propylene oxide and CO2, yielding a propylene oxide conversion of only 10% after 3 h (entry 3, Table 4). Recently, Jones et al., [46] used a series of air-stable iron(III) acetate complexes bearing salan, salen, or salalen ligands for the coupling of CO2 and the challenging cyclohexene oxide substrate in the presence of tetrabutylammonium chloride (TBAC) as co-catalyst and under solvent-free conditions, at 80 °C and 10 bar CO2. The cis-cyclohexene carbonate was selectively formed as the exclusive product (entry 4, Table 4). Thus, under quite similar conditions, our C-scorpionate Fe(II)/IL catalyst exhibited superior performance, leading to higher carbonate yields. However, although leading to significantly higher conversion values of propylene, styrene, and cyclohexene oxides (98.3, 94.6, and 72.7%, entries 2, 8, and 14 of Table 1, respectively) than those obtained with amino-bis(phenolate) iron (II) complexes (74, 31 and 9%, respectively) [10], the later catalytic system led to higher turnover number (TON) or turnover frequency (TOF) values (2960, 1240, and 364, respectively, for propylene, styrene, and cyclohexene oxides) in comparison to ours (197, 193 and 145, entries 2, 8, and 14 of Table 1 respectively). A similar behavior was found for thioether-tiophenolate bimetallic iron(III) complexes [9], leading to conversions of propylene oxide up to 50% and reaching the remarkable TOF value of 4990 h−1.
Notably, none of the above studies reported the recovery or reusability of the used iron catalysts [6,9,12,45,46].
Herein, the stability of the [FeCl2{κ3-HC(pz)3}]/IL catalytic system is a relevant advantage that allowed it to be recycled and reused at least for ten consecutive cycles without losing its initial activity (see Figure 4 for [bmim][N(CN)2]).
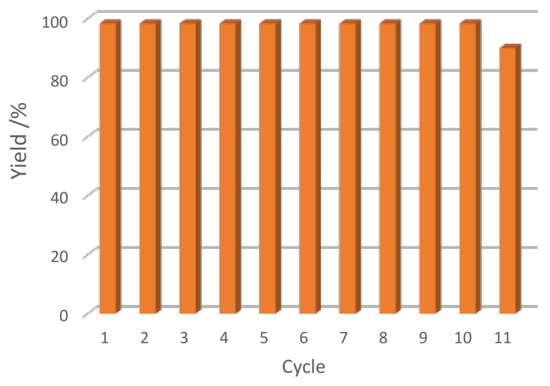
Figure 4.
Effect of the number of catalytic cycles on the yield of the cyclic carbonate obtained by cycloaddition of CO2 to propylene oxide catalyzed by [FeCl2{κ3-HC(pz)3}] in [bmim][N(CN)2].
Remarkably, our system of producing cyclic carbonates presents improvements relative to previously reported ones, that can overcome the undesirable low TON or TOF values. It can be considered sustainable and economical as its activity was almost constant after recycling at least ten times.
The IR spectra in the 4000–500 cm−1 range of FeCl2{κ3-HC(pz)3}]/IL before and after the four consecutive catalytic cycles matched (compare Figure 5a,b), proving the stability of the C-scorpionate complex in the reaction ionic liquid medium.
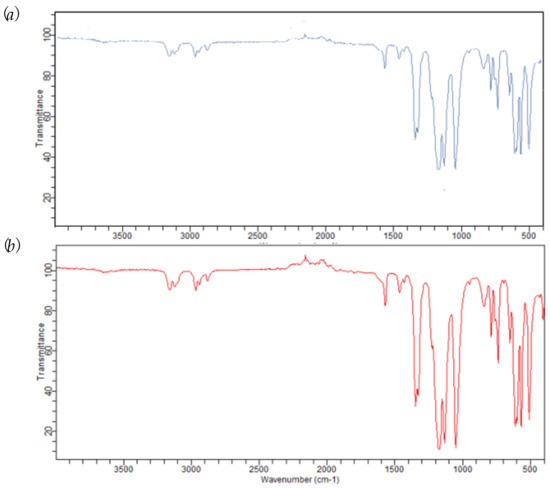
Figure 5.
Infrared spectra in the 500–4000 cm−1 range of (a) [FeCl2{κ3-HC(pz)3}]/[bmim][NTf2] and (b) FeCl2{κ3-HC(pz)3}]/[bmim][NTf2] after the 4th catalytic run.
Previous works on the synthesis of cyclic carbonates from CO2 and epoxides suggested the parallel requirement of both Lewis-base activation of the CO2 and Lewis-acid-activation of the epoxide [6,38]. Thus, it is likely that the C-scorpionate Fe(II) center could serve as a Lewis acid for epoxide coordination and activation (where the scorpionate ligand has a key role), whereas the ionic liquid would activate CO2 and combined with TBAB (added as cocatalyst) would promote bromide (and chlorine or DCA) anions to act as a nucleophile, thereby promoting the ring opening. This would be followed by the ring opening of the coordinated epoxide and consequent intramolecular ring closing, releasing the cyclic carbonate and recovering the catalytic system.
To best of our knowledge, reports regarding possible reaction mechanisms for the cycloaddition of CO2 to epoxides are scarce. DFT calculations for propylene oxide substrate in the absence and presence of alkylmethylimidazolium chloride ([Cnmim]Cl, n = 2, 4, or 6) ionic liquids performed by H. Sun et al. [47] suggested that cycloaddition is a multipath reaction that could proceed through two and five (or more) possible routes in the absence or presence of [Cnmim]Cl, respectively. In all the cases considered, the rate-determining steps involve the ring opening of the epoxide. The study also demonstrated that there are cooperative actions of the cation and anion of the ionic liquid which stabilize intermediates as well as transition states through hydrogen-bonding interaction, thus facilitating the ring opening of the epoxide.
3. Materials and Methods
The reagents were purchased from Aldrich (St. Louis, MO, USA) and used without further purification. Carbon dioxide gas of 99.99% purity was used. Hydrotris(1H-pyrazol-1-yl)methane, HC(pz)3, was synthesized according to the literature method [48,49]. The C-scorpionate complex [FeCl2{κ3-HC(pz)3}] was prepared according to the literature [34] and characterized by the conventional techniques.
The cycloaddition reactions of CO2 and epoxide were carried out in a stainless-steel autoclave (16.0 cm3) equipped with a stirrer and temperature control system. The selected epoxide (2.0–10.0 mmol), THF (2.5 cm3), or the chosen ionic liquid (8.3–18.8 mol), tetra-n-butylammonium bromide, [Bu4N]Br (TBAB, 0.3% mol vs. epoxide) and, when used, [FeCl2{κ3-HC(pz)3}] (0.3–0.8% mol vs. epoxide), were added to the autoclave. Then, the reactor was purged twice and the CO2 selected pressure (1–8 bar) was charged in the reactor at r.t. The reaction was carried out at temperature within the 30–100 °C range, under autogenous conditions for the desired time (up to 24 h) with continuous stirring (600 rpm). The autoclave was cooled to r.t., the excess of pressure released, and the product(s) analyzed by 1H-NMR spectroscopy (Bruker 400 UltraShieldTM spectrometers, Rheinstetten, Germany); 1H chemical shifts δ expressed in ppm relative to SiMe4) after extraction from the IL media. The product quantification was performed by applying the internal standard method using CDCl3 (400 μL, used both as solvent and internal standard).
Catalyst recyclability in the IL medium under the optimal experimental reaction conditions was investigated. Each cycle was initiated after the preceding one upon the addition of new typical portions of all other reagents. After the completion of each run, the organics were extracted for analysis (see above), and the IL which contained the (dissolved) catalyst was washed several times with ether and dried in vacuo overnight at 70 °C. The stability of the catalyst was verified by comparison of the FTIR-ATR spectra (in a Bruker Vertex 40 Raman/IR spectrometer, Rheinstetten, Germany); of the mixture ([FeCl2{κ3-HC(pz)3}]/IL) before and after each catalytic run.
4. Conclusions
In conclusion, the use of scorpionate-based complexes in an IL media can provide high selectivity to cyclic carbonates in moderate to high yields under mild conditions. In particular, the cycloaddition of carbon dioxide and an epoxide in the presence of the C-homoscorpionate Fe(II) catalyst [FeCl2{κ3-HC(pz)3}] (pz = 1H-pyrazol-1-yl) in tailor-made cheap ionic liquid media such as [bmim][N(CN)2] and under mild conditions (80 °C; 1–8 bar) selectively yields up to 98% of the corresponding cyclic carbonate. In addition to a remarkable activity, the catalyst exhibits superior stability during ten consecutive catalytic cycles, a clear advantage relative to previously reported catalytic systems.
Future work is currently ongoing to extend the range of substrates and investigate the accelerated yields observed in IL compared to a molecular solvent, as well as to establish the corresponding reactional mechanisms.
Author Contributions
Conceptualization, A.P.C.R. and P.G.; investigation, A.P.C.R.; writing—original draft preparation, A.P.C.R. and L.M.D.R.S.M.; A.P.C.R. and L.M.D.R.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT (Fundação para a Ciência e a Tecnologia, Portugal - project UIDB/00100/2020 of the Centro de Química Estrutural). APCR thanks Instituto Superior Técnico for the Scientific Employment contract IST-ID/119/201.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Energy Technology Perspectives 2015, Mobilising Innovation to Accelerate Climate Action, International Energy Agency. Available online: https://www.iea.org/publications/freepublications/publication/ (accessed on 20 January 2021).
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.S.; Kozak, C.M. Ring-opening polymerization of epoxides and ring-opening copolymerization of CO2 with epoxides by a zinc amino-bis(phenolate) catalyst. Eur. Polym. J. 2019, 120, 109237. [Google Scholar] [CrossRef]
- Patil, N.G.; Boopathi, S.K.; Alagi, P.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Carboxylate Salts as Ideal Initiators for the Metal-Free Copolymerization of CO2 with Epoxides: Synthesis of Well-Defined Polycarbonates Diols and Polyols. Macromolecules 2019, 52, 2431–2438. [Google Scholar] [CrossRef]
- Kozak, C.M.; Ambrose, K.; Anderson, T.S. Copolymerization of carbon dioxide and epoxides by metal coordination complexes. Coord. Chem. Rev. 2018, 376, 565–587. [Google Scholar] [CrossRef]
- Andrea, K.A.; Kerton, F.M. Iron-catalyzed reactions of CO2 and epoxides to yield cyclic and polycarbonates. Polym. J. 2021, 53, 29–46. [Google Scholar] [CrossRef]
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Montoya, C.A.; Gómez, C.F.; Paninho, A.B.; Nunes, A.V.M.; Mahmudov, K.T.; Najdanovic-Visak, V.; Martins, L.M.D.R.S.; Silva, M.F.C.G.D.; da Ponte, M.N.; Pombeiro, A.J.L. Cyclic carbonate synthesis from CO2 and epoxides using zinc(II) complexes of arylhydrazones of β-diketones. J. Catal. 2016, 335, 135–140. [Google Scholar] [CrossRef]
- Della Monica, F.; Vummaleti, S.V.C.; Buonerba, A.; De Nisi, A.; Monari, M.; Milione, S.; Grassi, A.; Cavallo, L.; Capacchione, C. Coupling of Carbon Dioxide with Epoxides Efficiently Catalyzed by Thioether-Triphenolate Bimetallic Iron(III) Complexes: Catalyst Structure–Reactivity Relationship and Mechanistic DFT Study. Adv. Synth. Catal. 2016, 358, 3231–3243. [Google Scholar] [CrossRef]
- Elkurtehi, A.I.; Kerton, F.M. Coupling Reactions of Carbon Dioxide with Epoxides Catalyzed by Vanadium Aminophenolate Complexes. ChemSusChem 2017, 22, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Carbon dioxide-to-methanol single-pot conversion using a C-scorpionate iron(II) catalyst. Green Chem. 2017, 19, 4801–4962. [Google Scholar] [CrossRef]
- Alhashmialameer, D.; Collins, J.; Hattenhauer, K.; Kerton, F.M. Iron amino-bis(phenolate) complexes for the formation of organic carbonates from CO2 and oxiranes. Catal. Sci. Technol. 2016, 6, 5364–5373. [Google Scholar] [CrossRef]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Li, Z.; Wang, H.; Fan, M.; Wang, J. Efficient Ionic-Liquid-Promoted Chemical Fixation of CO2 into α-Alkylidene Cyclic Carbonates. ChemSusChem 2017, 10, 1120–1127. [Google Scholar] [CrossRef]
- Jiang, X.; Gou, F.; Chen, F.; Jing, H. Cycloaddition of epoxides and CO2 catalyzed by bisimidazole-functionalized porphyrin cobalt(iii) complexes. Green Chem 2016, 18, 3567–3576. [Google Scholar] [CrossRef]
- Hu, J.; Ma, J.; Zhu, Q.; Zhang, Z.; Wu, C.; Han, B. Transformation of Atmospheric CO2 Catalyzed by Protic Ionic Liquids: Efficient Synthesis of 2-Oxazolidinones. Angew. Chem Int. Ed. 2015, 54, 5399–5403. [Google Scholar] [CrossRef]
- Goodrich, P.; Gunaratne, H.Q.N.; Jacquemin, J.; Jin, L.; Lei, Y.; Seddon, K.R. Sustainable Cyclic Carbonate Production, Utilizing Carbon Dioxide and Azolate Ionic Liquids. ACS Sust. Chem. Eng. 2017, 5, 5635–5641. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 17, 1966–1987. [Google Scholar] [CrossRef]
- Sopeña, S.; Martin, E.; Escudero-Adán, E.C.; Kleij, A.W. Pushing the Limits with Squaramide-Based Organocatalysts in Cyclic Carbonate Synthesis. ACS Catal. 2017, 7, 3532–3539. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, G.-X.; Zhang, W.-Z.; Lu, X.-B. CO2 Adducts of Phosphorus Ylides: Highly Active Organocatalysts for Carbon Dioxide Transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- Arayachukiat, S.; Kongtes, C.; Barthel, A.; Vummaleti, S.V.C.; Poater, A.; Wannakao, S.; Cavallo, L.; D’Elia, V. Ascorbic Acid as a Bifunctional Hydrogen Bond Donor for the Synthesis of Cyclic Carbonates from CO2 under Ambient Conditions. ACS Sustain. Chem. Eng. 2017, 5, 6392–6397. [Google Scholar] [CrossRef]
- Kumatabara, Y.; Okada, M.; Shirakawa, S. Triethylamine Hydroiodide as a Simple Yet Effective Bifunctional Catalyst for CO2 Fixation Reactions with Epoxides under Mild Conditions. ACS Sustain. Chem. Eng. 2017, 5, 7295–7301. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Martin, E.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. An Efficient Iron Catalyst for the Synthesis of Five- and Six-Membered Organic Carbonates under Mild Conditions. Adv. Synth. Catal. 2012, 354, 469–476. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxido vanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Cat. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-scorpionate complexes: Ever young catalytic tools, Coord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 2016, 2236–2252. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Silva, A.; Luís, D.; Santos, S.; Silva, J.; Mendo, A.S.; Coito, L.; Silva, T.F.S.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. Biological characterization of the antiproliferetive potential of Co(II) and Sn(IV) coordination compounds in human cancer cell lines: Role of comparative proteomics. Drug Metabol. Drug Interact. 2013, 28, 167–176. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.; Kuznetsov, M.L.; Fernandes, A.R.; Silva, A.; Santos, S.; Pan, C.-J.; Lee, J.-F.; Hwang, B.-J.; et al. Cobalt complexes with pyrazole ligands as catalysts for the peroxidative oxidation of cyclohexane. XAS studies and biological applications. Chem. Asian J. 2014, 9, 1132–1143. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Jesus, J.; Martins, P.; Figueiredo, S.; Rosa, D.; Martins, L.M.D.R.S.; Corvo, M.L.; Carvalheiro, M.C.; Costa, P.M.; Baptista, P.V. Multifunctional gold-nanoparticles: A nanovectorization tool for the targeted delivery of novel chemotherapeutic agents. J. Control. Release 2017, 245, 52–61. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Pombeiro, A.J.L. Synthesis and Biological Applications of Tris(pyrazol-1-yl)-Methane and -Borate Metal Complexes; Smolenski, P., Gawryszewska, P., Eds.; Ligands: Synthesis, Characterization and Role in Biotechnology; Nova Science Publishers Inc.: New York, NY, USA, 2014; Chapter 4; pp. 117–140. ISBN 978-1-63117-143-7. [Google Scholar]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic Oxidation of Cyclohexane with Hydrogen Peroxide and a Tetracopper(II) Complex in an Ionic Liquid. C. R. Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning cyclohexane oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organometallics 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate Vanadium, Iron and Copper Complexes as Selective Catalysts for the Peroxidative Oxidation of Cyclohexane under Mild Conditions. Adv. Synth. Cat. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Li, C.Y.; Su, Y.C.; Lin, C.H.; Huang, H.Y.; Tsai, C.Y.; Lee, T.Y.; Ko, B.T. Synthesis and characterization of trimetallic cobalt, zinc and nickel complexes containing amine-bis(benzotriazole phenolate) ligands: Efficient catalysts for coupling of carbon dioxide with epoxides. Dalton Trans. 2017, 461, 5399–15406. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Dong, K.; Cheng, W.-G.; Sun, J.; Zhang, S.-J. Insights into quaternary ammonium salts-catalyzed fixation carbon dioxide with epoxides. Catal. Sci. Technol. 2012, 2, 1480–1484. [Google Scholar] [CrossRef]
- Fuchs, M.A.; Zevaco, T.A.; Ember, E.; Walter, O.; Held, I.; Dinjus, E. Synthesis of cyclic carbonates from epoxides and carbon dioxide catalyzed by an easy-to-handle ionic iron(iii) complex. Dalton Trans. 2013, 42, 5322–5329. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Shukri, M.A.A.M.; Kiatkittipong, W.; Lim, J.W.; Show, P.L.; Lam, M.K.; Assabumrungrat, S. Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review. Processes 2020, 8, 548. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Silva, M.F.C.G.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Silva, T.F.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Org. Chem. 2011, 24, 764–773. [Google Scholar] [CrossRef]
- Jiang, S.; Hua, Y.; Wang, Y.; Wang, X. Viscosity of Typical Room-Temperature Ionic Liquids: A Critical Review. J. Phys. Chem. Ref. Data 2019, 48, 033101. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, M.J.; Aki, S.N.V.K.; Anderson, J.L.; Dixon, J.K.; Brennecke, J.F. Improving carbon dioxide solubility in ionic liquids. J. Phys. Chem. B 2007, 111, 9001–9009. [Google Scholar] [CrossRef]
- Park, D.W.; Mun, N.Y.; Kim, K.H.; Kim, I.; Park, S.W. Addition of carbon dioxide to allyl glycidyl ether using ionic liquids catalysts. Catal. Today 2006, 115, 130–133. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Kodama, K.; Hirose, T. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions. Green Chem. 2016, 18, 1229–1233. [Google Scholar] [CrossRef]
- Jin, L.; Jing, H.; Chang, T.; Bu, X.; Wang, L.; Liu, Z. Metal porphyrin/phenyltrimethylammonium tribromide: High efficient catalysts for coupling reaction of CO2 and epoxides. J. Mol. Catal. A Chem. 2007, 261, 262–266. [Google Scholar] [CrossRef]
- Driscoll, O.J.; Hafford-Tear, C.H.; McKeown, P.; Stewart, J.A.; KociokKöhn, G.; Mahon, M.F.; Jones, M.D. Highly efficient palladium-catalysed carbon dioxide hydrosilylation employing PMP ligand. Dalton Trans. 2019, 48, 15049–51058. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, D.J. Density Functional Theory Study on the Cycloaddition of Carbon Dioxide with Propylene Oxide Catalyzed by Alkylmethylimidazolium Chlorine Ionic Liquids. Phys. Chem. A 2007, 111, 8036–8043. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Grattan, T.C.; Brown, K.J.; Little, C.A.; Lamba, J.J.; Rheingold, A.L.; Sommer, R.D. Syntheses of tris (pyrazolyl) methane ligands and {[tris (pyrazolyl) methane] Mn (CO) 3} SO3CF3 complexes: Comparison of ligand donor properties. J. Organomet. Chem. 2000, 607, 120–128. [Google Scholar] [CrossRef]
- Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Syntheses and Properties of Re(III) Complexes Derived from Hydrotris(1-pyrazolyl)methanes. Molecular Structure of [ReCl2(HCpz3)(PPh3)][BF4]. J. Organomet. Chem. 2005, 690, 1947–1958. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).