Synthesis and Characterization of ZnO-SiO2 Composite Using Oil Palm Empty Fruit Bunch as a Potential Silica Source
Abstract
1. Introduction
2. Results
2.1. Palm Waste Silica
2.1.1. XRF Analysis
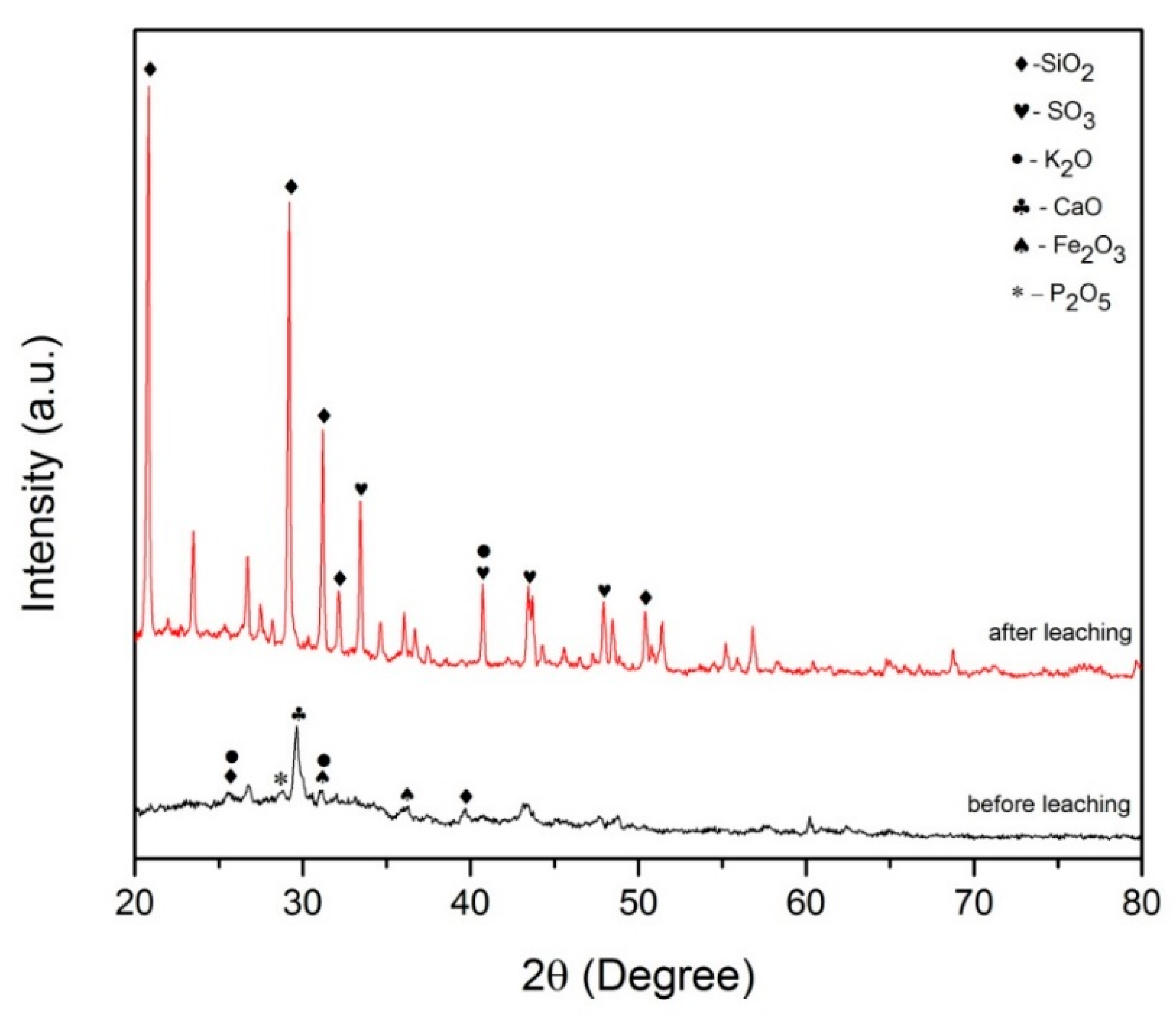
2.1.2. XRD Analysis
2.1.3. FESEM Analysis
2.2. ZnO-SiO2
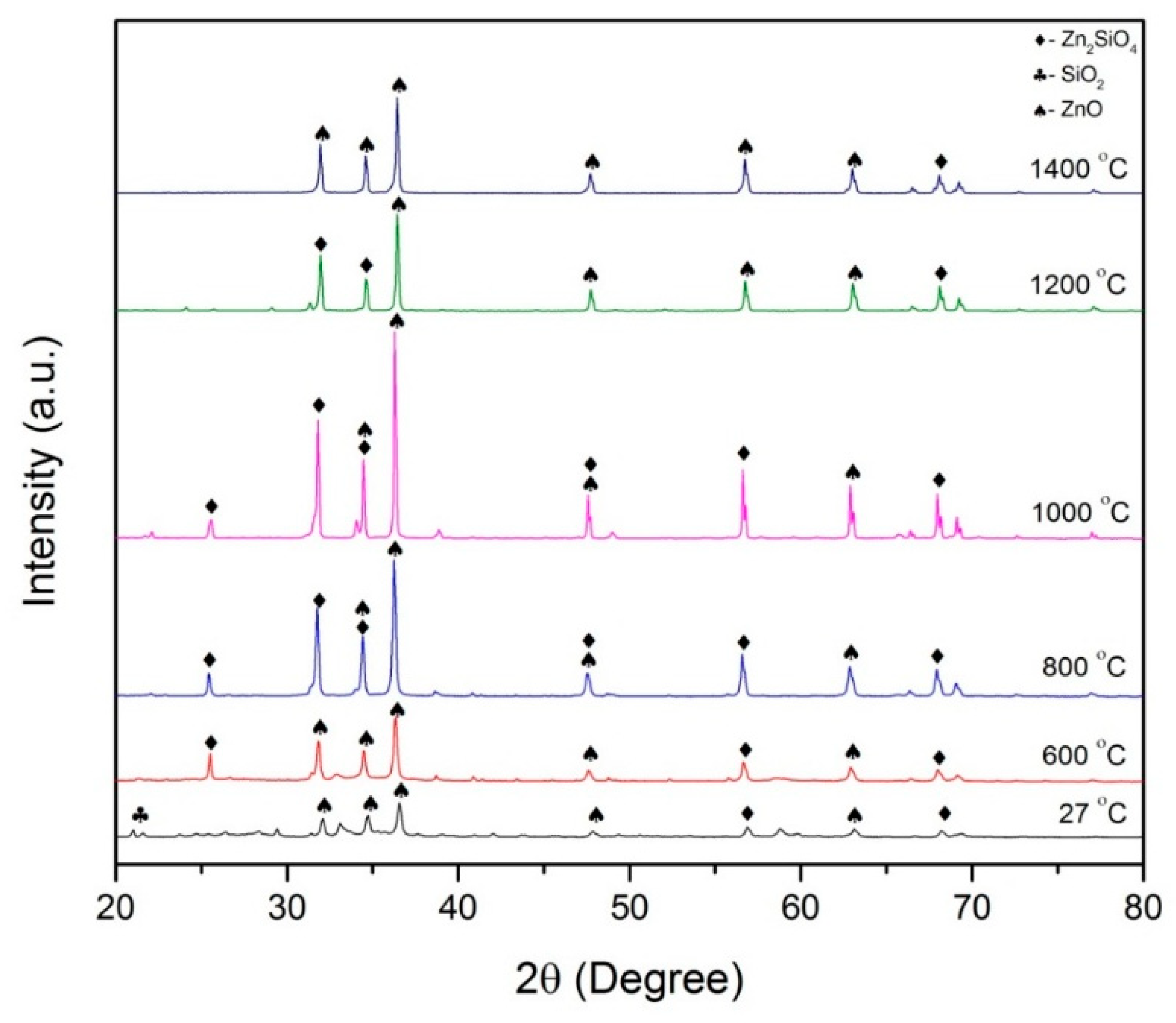
2.2.1. XRD Analysis
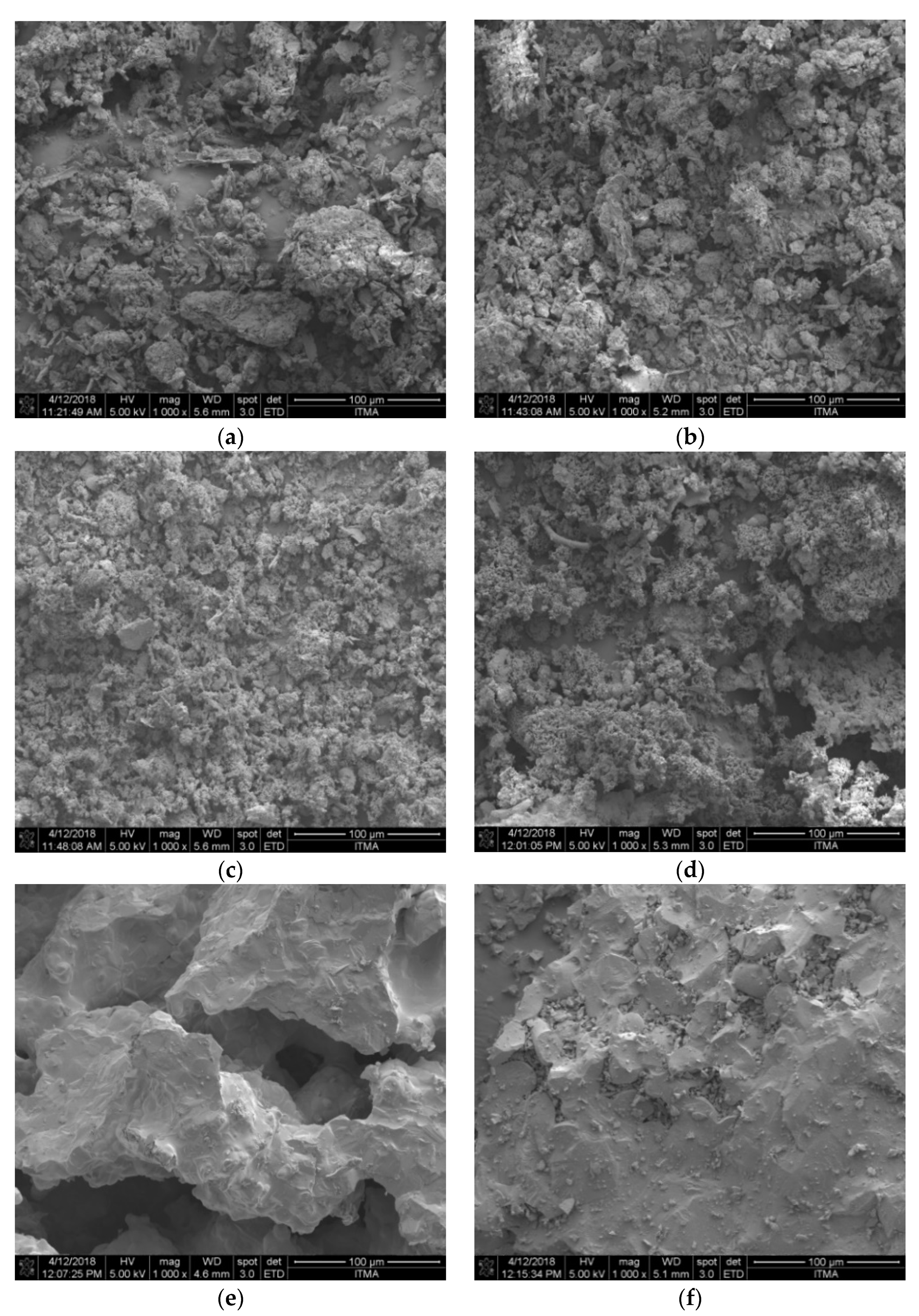
2.2.2. FESEM Analysis
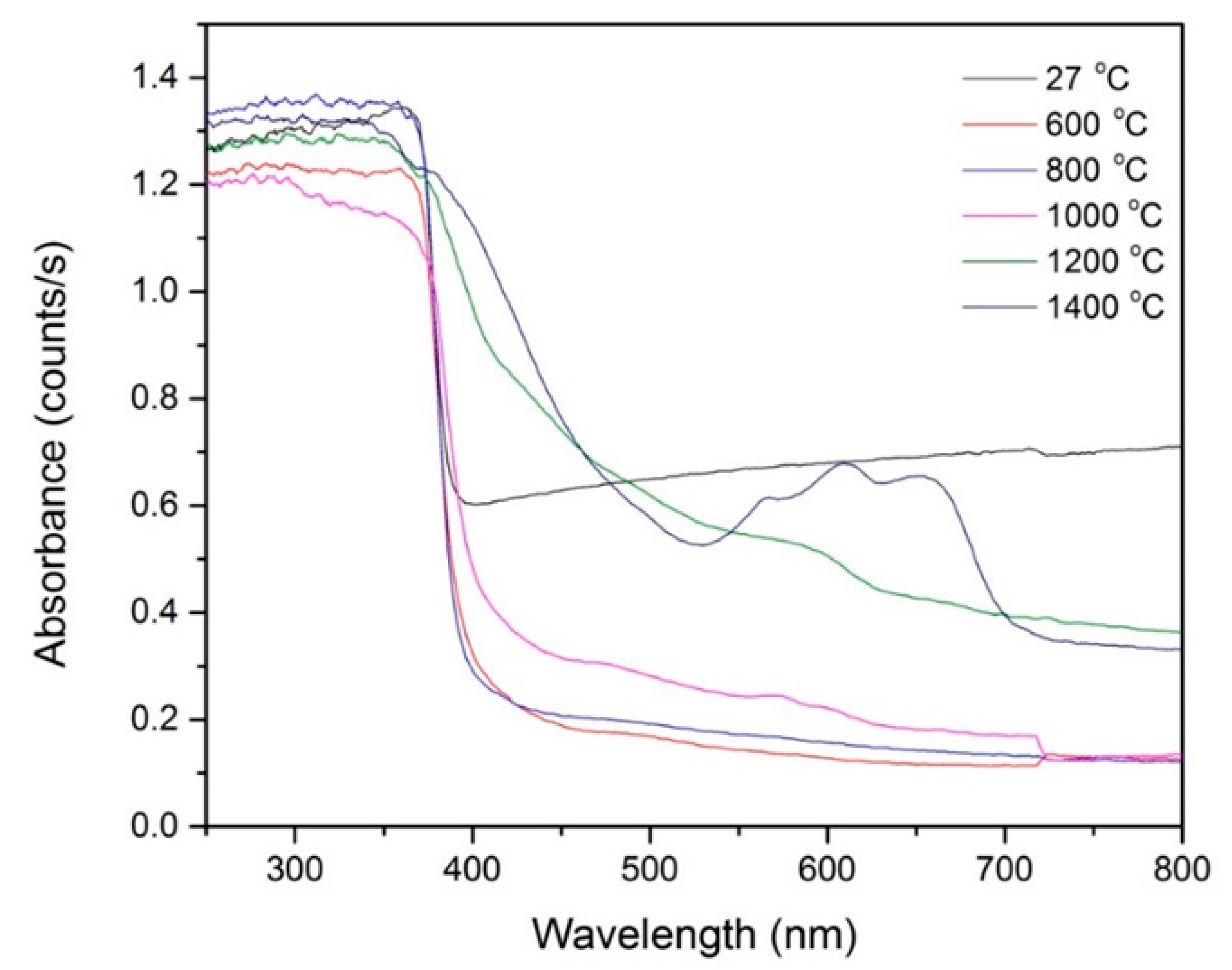
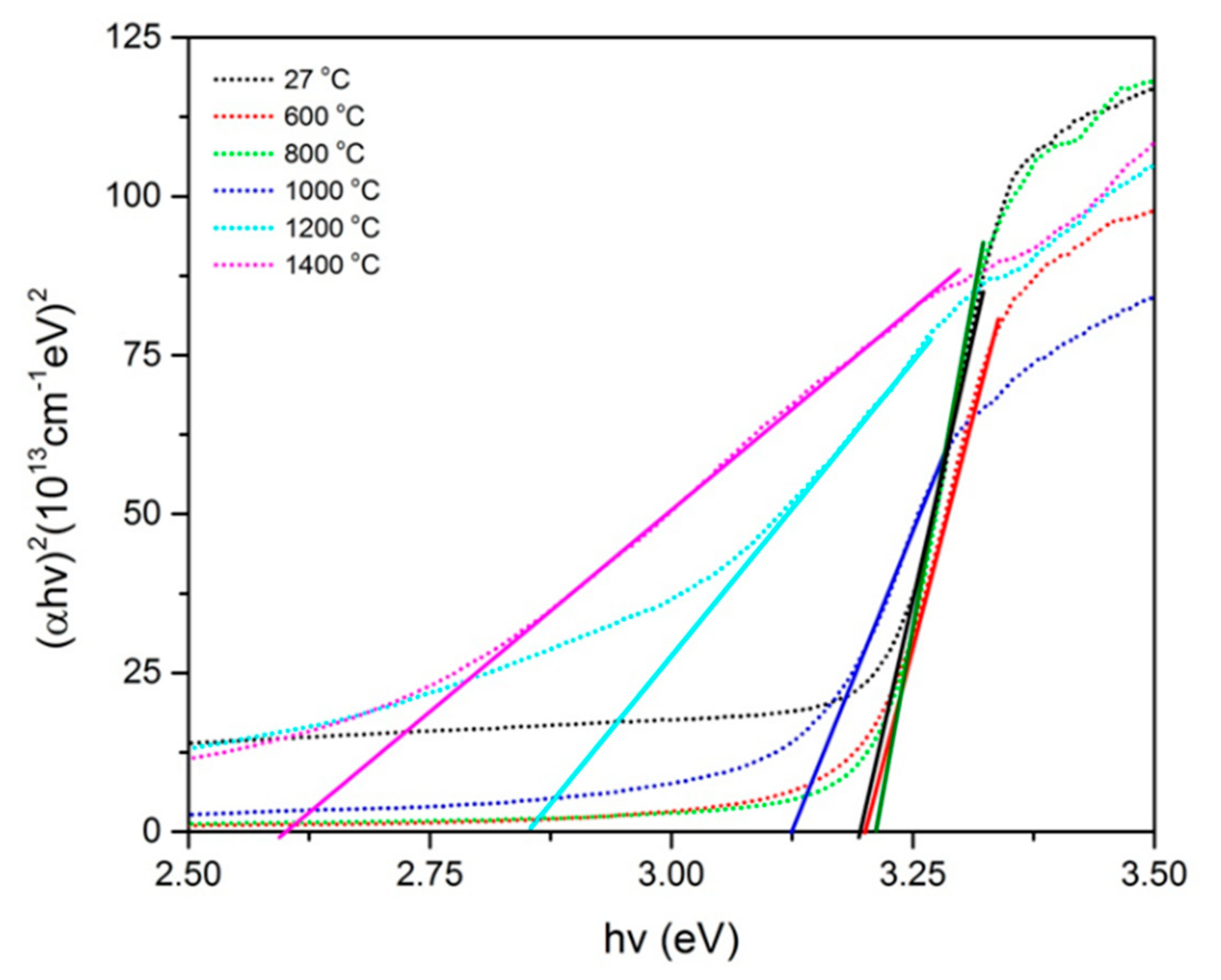
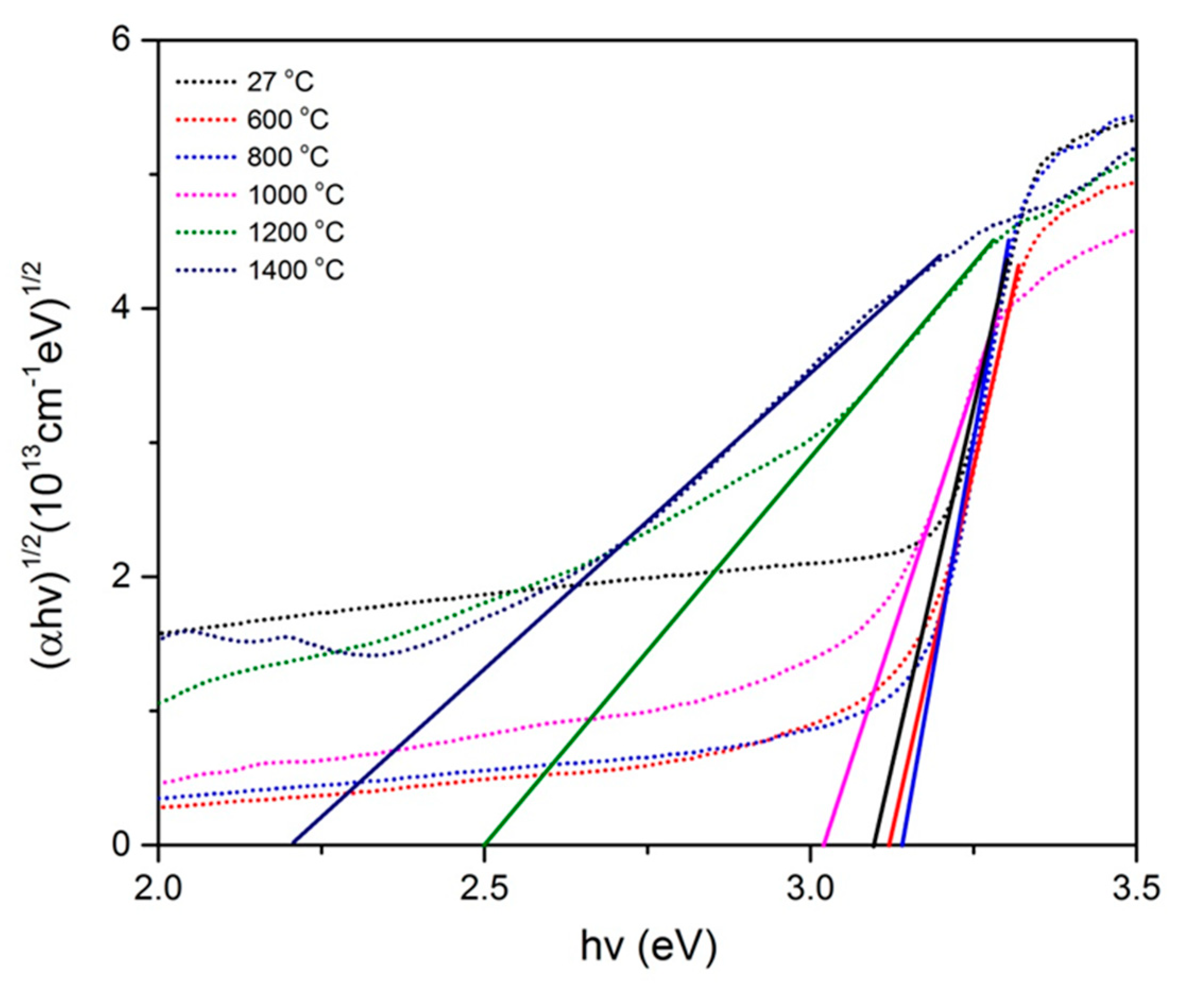
2.2.3. Optical Studies
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motaung, T.E.; Luyt, A.S. Effect of maleic anhydride grafting and the presence of oxidized wax on the thermal and mechanical behavior of LDPE/silica nanocomposites. J. Mater. Sci. Eng. A 2010, 527, 761–768. [Google Scholar] [CrossRef]
- Morpurgo, M.; Teoli, D.; Pignatto, M.; Attrezzi, M.; Spadaro, F.; Realdon, N. The effect of NaCO3, NaF and NH4OH on the stability and release behavior of sol-gel derived silica xerogels embedded with bioactive compounds. Acta Biomater. 2010, 6, 2246–2253. [Google Scholar] [CrossRef]
- Ge, J.; Huynh, T.; Hu, Y.; Yin, Y. Hierarchical magnetite/silica nanoassemblies as magnetically recoverable catalyst-supports. Nano Lett. 2008, 8, 931–934. [Google Scholar] [CrossRef]
- Affandi, S.; Setyawan, H.; Winardi, S.; Purwanto, A.; Balgis, R. A facile method for production of high-purity silica xerogels from bagasse ash. Adv. Powder Technol. 2009, 20, 468–472. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Lee, K.J.; Cho, M.; Lim, S.S.; Seo, S.K.; Cho, K.M.; Bang, K.S.; Oh, B.T. Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J. Ind. Eng. Chem. 2015, 29, 298–303. [Google Scholar] [CrossRef]
- Shim, J.; Velmurugan, P.; Oh, B.T. Extraction and physical characterization of amorphous silica made from corn cob ash at variable pH conditions via sol gel processing. J. Ind. Eng. Chem. 2015, 30, 249–253. [Google Scholar] [CrossRef]
- Sinyoung, S.; Kunchariyakun, K.; Asavapisit, S.; MacKenzie, K.J.D. Synthesis of belite cement from nano-silica extracted from two rice husk ashes. J. Environ. Manag. 2017, 190, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Mohd Zaid, M.H.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Abdul Wahab, S.A.; Khirel Azman, A.Z. Addition of ZnO nanoparticles on waste rice husk as potential host material for red-emitting phosphor. Mater. Sci. Semicond. Process. 2020, 106, 104774. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Pekov, I.V. Heterosilicates with tetrahedral-octahedral frameworks: Mineralogical and crystal-chemical aspects. Rev. Miner. Geochem. 2005, 57, 105–143. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite clinoptilolite: Therapeutic virtues of an ancient mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, B.; Fu, Z.; Yan, H.; Zhen, W.; Dong, W.; Xia, L.; Liu, W.; Jian, Z.; Li, F. Enhanced yellow-green photoluminescence from ZnO-SiO2 composite opal. J. Phys. Condens. Matter. 2004, 16, 7277–7286. [Google Scholar] [CrossRef]
- Takesue, M.; Hayashi, H.; Smith, R.L. Thermal and chemical methods for producing zinc silicate (willemite): A review. Prog. Cryst. Growth Charact. Mater. 2009, 55, 98–124. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Pita, K.; Kam, C.H. Sol-gel derived zinc silicate phosphor films for full-color display applications. J. Phys. Chem. Solids 2003, 64, 333–338. [Google Scholar] [CrossRef]
- Wahab, R.A.A.; Zaid, M.H.M.; Aziz, S.H.A.; Matori, K.A.; Fen, Y.W.; Yaakob, Y. Effects of sintering temperature variation on synthesis of glass-ceramic phosphor using rice husk ash as silica source. Materials 2020, 13, 5413. [Google Scholar] [CrossRef]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Wahab, Z.A.; Fen, Y.W.; Alibe, I.M. Synthesis and characterization of low cost willemite based glass–ceramic for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2016, 27, 11158–11167. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, N.; Tu, J.; Li, X.; Wang, R.; Zhang, T.; Shao, C. Preparation and humidity sensitive property of mesoporous ZnO-SiO2 composite. Sens. Actuators B Chem. 2010, 149, 413–419. [Google Scholar] [CrossRef]
- Tani, T.; Mädler, L.; Pratsinis, S.E. Synthesis of zinc oxide/silica composite nanoparticles by flame spray pyrolysis. J. Mater. Sci. 2002, 37, 4627–4632. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Chen, Z.; Zhao, X. Hydrothermal synthesis and optical properties of zinc silicate hierarchical superstructures. Mater. Lett. 2011, 65, 3030–3033. [Google Scholar] [CrossRef]
- Rasdi, N.M.; Fen, Y.W.; Azis, R.S.; Omar, N.A.S. Photoluminescence studies of cobalt (II) doped zinc silicate nanophosphors prepared via sol-gel method. Optik 2017, 149, 409–415. [Google Scholar] [CrossRef]
- Ouyang, X.; Kitai, A.H.; Xiao, T. Electroluminescence of the oxide thin film phosphors Zn2SiO4 and Y2SiO5. J. Appl. Phys. 1996, 79, 3229–3234. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Aziz, S.H.A.; Alassan, Z.N.; Samsudin, N.F. Development and characterization studies of Eu3+-doped Zn2SiO4 phosphors with waste silicate sources. Procedia Chem. 2016, 19, 21–29. [Google Scholar] [CrossRef]
- Samsudin, N.F.; Matori, K.A.; Wahab, Z.A.; Fen, Y.W.; Liew, J.Y.C.; Lim, W.F.; Zaid, M.H.M.; Omar, N.A.S. Manganese modified structural and optical properties of zinc soda lime silica glasses. Appl. Opt. 2016, 55, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Omar, N.A.S. Optical studies of crystalline ZnO–SiO2 developed from pyrolysis of coconut husk. Mater. Res. Express 2020, 7, 055901. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M. The physical and optical studies of crystalline silica derived from green synthesis of coconut husk ash. Appl. Sci. 2020, 10, 2128. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Exploring Eu3+-doped ZnO-SiO2 glass derived by recycling renewable source of waste rice husk for white-LEDs application. Results Phys. 2019, 15, 102596. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Omar, N.A.S.; Khaidir, R.E.M. Heat treatment effect on structural and optical properties of crystalline ZnO-SiO2 synthesized from coconut husk ash. Materials 2020, 13, 2555. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A. Europium doped low cost Zn2SiO4 based glass ceramics: A study on fabrication, structural, energy band gap and luminescence properties. Mater. Sci. Semicond. Process. 2017, 61, 27–34. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Norhafizah, M.R.; Nurzilla, M. Synthesis and optical properties of europium doped zinc silicate prepared using low-cost solid-state reaction method. J. Mater. Sci. Mater. Electron. 2016, 27, 1092–1099. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Pavitra, E.; Nagaraju, G.; Yu, J.S. Versatile properties of CaGd2ZnO5:Eu3+ nanophosphor: Its compatibility for lighting and optical display applications. Dalton Trans. 2015, 44, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Optical band gap and photoluminescence studies of Eu3+-doped zinc silicate derived from waste rice husks. Optik 2019, 182, 486–495. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A. Photoluminescence properties of Eu3+-doped low cost zinc silicate based glass ceramics. Optik 2016, 127, 3727–3729. [Google Scholar] [CrossRef]
- Rasdi, N.M.; Fen, Y.W.; Omar, N.A.S.; Azis, R.S.; Zaid, M.H.M. Effects of cobalt doping on structural, morphological, and optical properties of Zn2SiO4 nanophosphors prepared by sol-gel method. Results Phys. 2017, 7, 3820–3825. [Google Scholar] [CrossRef]

| Compounds | Percentage of Compositions | ||
|---|---|---|---|
| 600 °C | 700 °C | 800 °C | |
| CaO | 40.0 | 52.2 | 39.2 |
| K2O | 40.2 | 29.8 | 35.2 |
| SiO2 | 5.6 | 6.9 | 9.5 |
| P2O5 | 2.9 | 4.2 | 4.2 |
| Fe2O3 | 2.4 | 1.5 | 2.6 |
| Cl | 5.9 | 3.0 | 2.5 |
| SO3 | 1.7 | 1.3 | 2.1 |
| Compounds | Percentage of Compositions | |
|---|---|---|
| Before | After | |
| CaO | 39.2 | 17.8 |
| K2O | 35.2 | 16.4 |
| SiO2 | 9.5 | 45.6 |
| P2O5 | 4.2 | 0.0 |
| Fe2O3 | 2.6 | 3.3 |
| Cl | 2.5 | 0.0 |
| SO3 | 2.1 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmat, F.; Fen, Y.W.; Anuar, M.F.; Omar, N.A.S.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and Characterization of ZnO-SiO2 Composite Using Oil Palm Empty Fruit Bunch as a Potential Silica Source. Molecules 2021, 26, 1061. https://doi.org/10.3390/molecules26041061
Rahmat F, Fen YW, Anuar MF, Omar NAS, Zaid MHM, Matori KA, Khaidir REM. Synthesis and Characterization of ZnO-SiO2 Composite Using Oil Palm Empty Fruit Bunch as a Potential Silica Source. Molecules. 2021; 26(4):1061. https://doi.org/10.3390/molecules26041061
Chicago/Turabian StyleRahmat, Fida’i, Yap Wing Fen, Muhammad Fahmi Anuar, Nur Alia Sheh Omar, Mohd Hafiz Mohd Zaid, Khamirul Amin Matori, and Rahayu Emilia Mohamed Khaidir. 2021. "Synthesis and Characterization of ZnO-SiO2 Composite Using Oil Palm Empty Fruit Bunch as a Potential Silica Source" Molecules 26, no. 4: 1061. https://doi.org/10.3390/molecules26041061
APA StyleRahmat, F., Fen, Y. W., Anuar, M. F., Omar, N. A. S., Zaid, M. H. M., Matori, K. A., & Khaidir, R. E. M. (2021). Synthesis and Characterization of ZnO-SiO2 Composite Using Oil Palm Empty Fruit Bunch as a Potential Silica Source. Molecules, 26(4), 1061. https://doi.org/10.3390/molecules26041061







