Abstract
A series of novel S-, O- and Se-containing dispirooxindole derivatives has been synthesized using 1,3-dipolar cycloaddition reaction of azomethine ylide generated from isatines and sarcosine at the double C=C bond of 5-indolidene-2-chalcogen-imidazolones (chalcogen was oxygen, sulfur or selenium). The cytotoxicity of these dispiro derivatives was evaluated in vitro using different tumor cell lines. Several molecules have demonstrated a considerable cytotoxicity against the panel and showed good selectivity towards colorectal carcinoma HCT116 p53+/+ over HCT116 p53−/− cells. In particular, good results have been obtained for LNCaP prostate cell line. The performed in silico study has revealed MDM2/p53 interaction as one of the possible targets for the synthesized molecules. However, in contrast to selectivity revealed during the cell-based evaluation and the results obtained in computational study, no significant p53 activation using a reporter construction in p53wt A549 cell line was observed in a relevant concentration range.
1. Introduction
Design and development of novel potent anticancer therapeutics are the most important tasks of synthetic organic and medicinal chemistry. Among the compounds with antitumor action, an important place is occupied by the spiro and dispiro derivatives of indolinones, due to the conformational rigidity of spiro scaffold which allows the introduction into the molecules of functional groups necessary for interaction with biological targets in the required arrangement, and the indolinone fragment simulates the tryptophan moiety, in many cases involved in such interactions [1,2,3,4,5]. So, spiro-oxindole alkaloids, which were firstly derived from the families Apocynaceae and Rubiaceae [6] and latter were found in a wide range of complex natural products [7,8,9,10] have shown significant anticancer activity. These compounds contain the spiro ring fusion at position 3 of the indolinone core, with different substitutions around the pyrrolidine and indolinone moieties.
A promising direction in the treatment of cancer is the development of compounds that affect the interaction of p53–MDM2 proteins. The p53 protein, which is a tumor suppressor, is one of the potential targets of antitumor therapy. Tumor suppressor p53, not being complexed with its MDM2 inhibitor, can trigger cell apoptosis [11,12]. In more than 50% of tumor cell cultures, the p53 protein is mutated [12], and its activation or restoration of its function may be effective in anticancer therapy due to apoptosis initiating or arresting cell growth [13]. Note that some small molecules inhibit MDM2/p53 interaction and now are undergoing preclinical or clinical trials against different types of cancer [14,15,16,17,18]. Among these molecules, compound nutlin-3a is one of the most known inhibitors of the p53–MDM2 protein–protein interaction; this compound is able to bind to the p53–MDM2 pocket and inhibit this protein interaction in nanomolar concentrations [19]. Nutlin-3a induced nongenotoxic stabilization of p53 protein and subsequent activation of a p53 pathway [20]. The molecule of nutlin-3a, which definitely binds to the site 1 of MDM2 protein, may be a template for the design of new p53–MDM2 inhibitor molecules [21]. Since the indole ring of Trp23 residue of p53 is located deep inside a hydrophobic pocket of MDM2 and its NH group forms a hydrogen bond with the backbone carbonyl in MDM2, Trp23 appears to be most crucial for binding of p53 to MDM2. Previously [22], Wang’s group searched for chemical moieties that can mimic the Trp23 interaction with MDM2. In addition to the indole ring itself, they have found that oxindole can perfectly mimic the side chain of Trp23 for interaction with MDM2. These modeling studies also showed that compounds with a spiro-linked structure are capable of better binding to MDM2 by limiting the conformational mobility of the molecule (пpeдыдyщaя) and the spiro-(oxindole-3,3′-pyrrolidine) core structure may be used as the starting point for the design of a new class of MDM2 inhibitors. The oxindole can closely mimic the Trp23 side chain in p53 in both hydrogen bond formation and hydrophobic interactions with MDM2, and the spiro-pyrrolidine ring provides a rigid scaffold.
We have recently described a series of novel spiro-oxindoles containing thiohydantoin [23,24,25], selenohydantoin [26] or hydantoin [25,27] moieties, presumably having an anticancer effect by inhibiting the p53/MDM2 protein interaction; one such derivative has recently successfully completed preclinical trials as a drug for the treatment of colorectal cancer [28]. The most active compounds of this type have shown cytotoxicity in the 4–11 µm range on cancer cell lines HepG2, MCF-7, SiHa and HCT116, [23], and some p53 activation by Western blotting (see Supplementary Information, Figure S1).
In this paper, we present a series of novel compounds of the dispiro-indolinone series with a modified spiro-oxindole core (Figure 1) with promising anticancer activity. Most of the previously investigated thiohydantoin-based spiro-oxindoles [23,25,28] (Figure 1) had in their structures the nitrogen atom of the central pyrrolidine ring directly attached to the carbon atom at spiro-conjugation; in the series of compounds described in this article, nitrogen atom of pyrrolidine ring is in the central position of spiro-conjugated cycle, similar to the MI series compounds, which demonstrated a significant cytotoxic effect on the prostate cancer cell line LNCap, with IC50 = 86 nM, and on the colorectal cancer cell line HCTwt, with IC50 = 22 μM, and were recognized as a selective inhibitor of p53–MDM2 interaction due to theirability to induce cell apoptosis in tumor cells without affecting healthy ones [29].
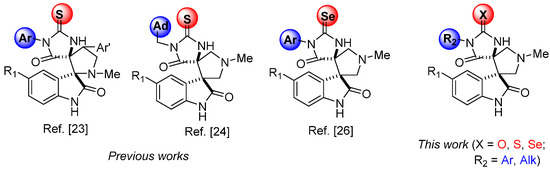
Figure 1.
Compounds synthesized earlier and in this work.
In contrast to the compounds of such structural type, described in [24,26], this article presents dispiro derivatives with aryl and non-carcass alkyl substituents at N(3) position and with different exocyclic chalcogen atoms (oxygen, sulfur or selenium) in imidazolone fragment. Some results of cytotoxic action mechanisms studying for synthesized compounds are also presented, as well as the molecular docking data to evaluate their possible binding affinity toward MDM2.
2. Resultsand Discussion
2.1. Chemistry
To obtain the target dispyro derivatives 4, 6, 9, the series of disubstituted thioureas 1a–j or thiohydantoins 2a, b (Scheme 1) was initially synthesized starting from aryl- or alkyl-amine and ethyl isothiocyanatoacetate. The reaction proceeded smoothly in ether at room temperature and furnished the desired intermediates with 61–98% yield.
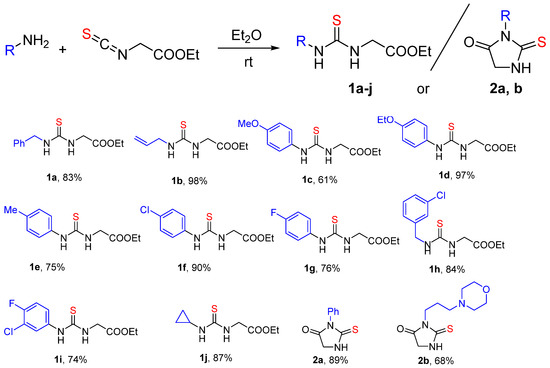
Scheme 1.
Synthesis of the compounds 1a–k, 2.
Compounds 1a–j and 2a, b were then treated with equimolar amount of isatin or 5-chloroisatin to obtain indolidene-thiohydantoins 3a–x (Scheme 2), analogously to previously described reactions of substituted thioureas with aromatic aldehydes [30]. Finally, compounds 3a–x were reacted with sarcosine and paraformaldehyde in toluene under reflux to obtain the desired substituted dispiroindolinones 4a–x in a moderate-to-high yield. The reaction, apparently, proceeds according to the mechanism of 1,3-dipolar cycloaddition of azomethine ylide generated from isatin and sarcosine at the C=C double bond of indolidenehydantoins 3 [23]. According to the-NMR spectroscopy data, the reactions in all cases proceed with the formation of single diastereomeric products 4a–x with the relative S*, R*-configuration, which was confirmed by the data of X-ray crystallographic analysis for the compound 4s (Figure 2).
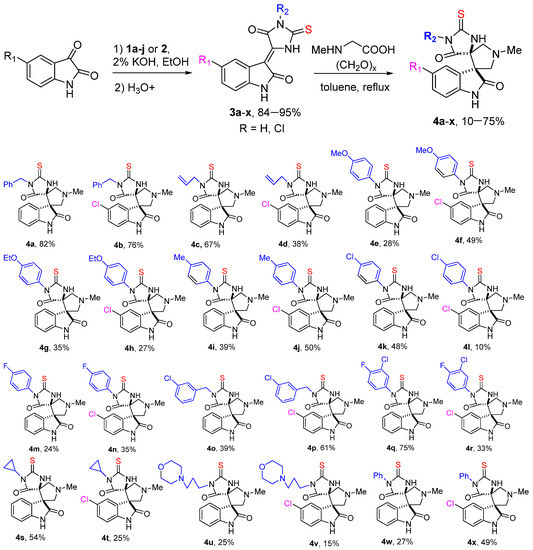
Scheme 2.
Synthesis of 3-(5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-ones 3a–x and dispiroindolinines 4a–x.
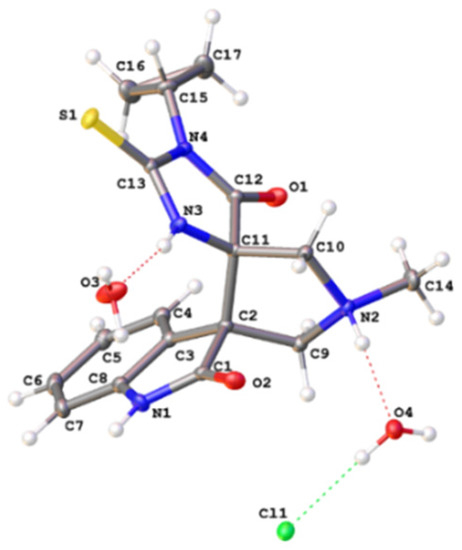
Figure 2.
Molecular structure of compound 4s (as a salt with HCl).
For comparison, we have synthesized some O- and Se-containing analogs of the spiro-hydantoins 4, namely for compounds 4g, 4h, 4q, 4r. Some hydantoin derivatives containing spiro-linked indolinone fragments showed significant in vitro cytotoxic activity [25]. The ability of organoselenium compounds to exhibit antioxidant properties [31,32] mimicking the action of the glutathione peroxidase enzyme [33] makes it possible to use them in anticancer therapy as auxiliary antioxidants to neutralize the oxidizing agents produced by certain anticancer drugs.
Selenohydantoin derivatives 6g, 6h, 6q, 6r may be readily obtained from sarcosine, paraformaldehyde and the corresponding indolidene-selenohydantoins 5g, 5h, 5q, 5r according to the modified method described in [26,34] (Scheme 3).
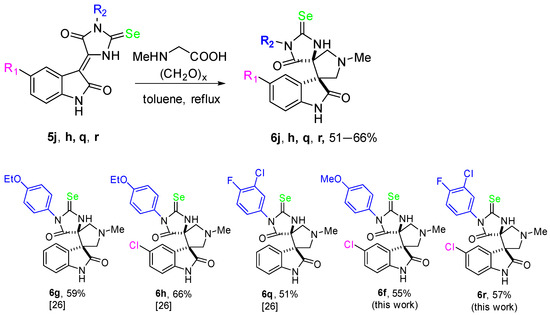
Scheme 3.
Synthesis of Se-containing analogue of the compounds 4.
O-containing derivatives 9 were synthesized from S-alkylated derivatives 7 of corresponding thiohydantoins 3 (Scheme 4). Thus, the starting compounds 3f, 3h, 3r were vigorously stirred with MeI in KOH/EtOH at room temperature for 30 min and then were treated with HCl/EtOH under reflux conditions to provide the corresponding hydantoins 8 in good yield (about 70%). The desired products 9f, 9h, 9r were obtained at the compounds 8 interaction with sarcosine and paraformaldehyde in 75% yields.
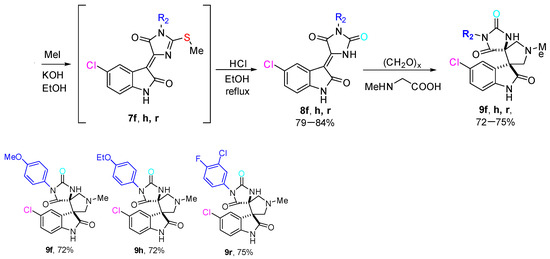
Scheme 4.
Synthesis of O-containing analogue of the compounds 4.
2.2. Biological Evaluation
2.2.1. Cytotoxicity
All the synthesized dispiro-oxindoles 4 and some of their selenium and oxygen analogues 6, 9 have subsequently been tested on their in vitro anticancer efficiency against a panel of different tumor cell lines, on the assumption that they, like the previously described compounds of dispiro-thiohydantoins type [23], may be able to inhibit p53/MDM protein interaction. The used models included human prostate cancer cell lines LNCaP and PC3, breast cancer cell line (MCF-7), human colon cancer cells (HCT116+/+, p53 positive, p53+/+ and HCT116−/−, p53 negative, p53−/−), human lung adenocarcinoma epithelial cell line (A549), SV40-transformed normal human lung fibroblast cells (VA13), as well as human embryonic kidney 293 cells (Hek293) stably expressing SV40 large T antigen (Hek293T). The cytotoxicity of the evaluated molecules was properly assessed using a MTT assay based on the modified approach reported by Ferrari and colleagues [35]. Nutlin-3 [19], known as P53/MDM2 interaction inhibitor, were also tested on some cell lines for comparison. The results of the cytotoxicity study are summarized in Table 1.

Table 1.
Cytotoxicity of dispiro-oxindoles 4, 6, 9 against different cell lines (MTT test).
As shown in Table 1, the most potent compounds from the 4a–x series exhibited a CC50 value in the range of 1.1–12.6 µM against the used cells’ panel. However, no relevant selectivity was observed among the cell types, although compound 4f, for example, exhibits a cytotoxic effect that exceeds the effect of the nutlinreference sample. Therefore, this compound demonstrated rather overt cytotoxic effect. Seven compounds (4a–f, 4u, 4f, 6r, 9r) were found to be selective on LNCaP cells over PC3 cells. Among them, the upper selectivity towards the remaining cells was observed for compounds 4e, 4f, 4u, 4w. Compounds 4e, 4f, 4u, 4w showed the best selectivity index (S value for cell line pairs is defined as ratio of its CC50 values) for LNCaP/PC3 cells (>30, 2.8, 15.7, 5.3, respectively). Compounds 4a, 4b, 4d, 4f showed moderate selectivity and efficiency. The negative response of PC3 cells to the treatment with the compounds having high S values possible may be associated with MDM2/p53 mode of action due to p53 tumor suppressor pathway in this cell type which is, in most cases, disrupted by human papilloma virus (HPV) [36]. The activated p53 induces the transcription of MDM2, which can directly interact with transactivation domain of p53 thereby inhibiting its transcription activity by targeting it for polyubiquitination and further proteasome-mediated degradation [37]. In many cancer cells, including HepG2, Hek and MCF-7, the overexpression of MDM2 gene is actually observed resulting in significant apoptosis attenuation. However, the obtained results do not allow to draw an unambiguous conclusion whether the studied compounds are actually involved in the direct activation of p53.
For instance, compound 4f inhibited the proliferation of LNCaP and PC3 cells with CC50 values of 4.5 ± 0.32 µM and 12.6 ± 2.3 µM, respectively, in contrast to its close structural analogue 4h with no activity at all (the difference is in p-position of the phenyl ring: compound 4f contains methoxy substituent while compound 4h ethoxy group). It can be primarily attributed to steric clashes; however, this hypothesis is under debate because of compounds 4i and 4j with methyl group in p-position weakly inhibited cell growth across the panel as compared to compound 4f. This may be partly explained by an additional hydrogen bond that can be provided by OMe group. To further elucidate the dominant mode of action, we used HCT116+/+ and HCT116−/− cells by analogy with the paper published by Shangary and colleagues [38]. This isogenic cell line is commonly applied to investigate the p53/MDM2-dependent mode of action. Compounds, 4p, 4u and 4w showed a significant selectivity against HCT116+/+ over HCT116−/−, with absolute S values which are >2.9, >1.8 and >4.8, respectively. For the control sample, etoposide (a topoisomerase poison [39]), the selectivity index was 1.97, therefore compounds with S > 2 should be rather classified as having poor selectivity. Under the same conditions, nutlin-3, the knownp53/MDM2-interaction inhibitor [19] was found to be also active and selective against HCT116+/+ cells over HCT116−/− cells showing CC50 values of 3.3 ± 0.13 µM and 35.12 ± 2.65 µM, respectively, and S = 10.6. Summarizing, based on the assay performed, only three thiohydantoine derivatives, 4p, 4u and 4w, can be reasonably regarded as the most promising candidates for further evaluation and optimization.
Interestingly, although compound 4r was absolutely inactive against LNCaP and PC3 cells, both their analogues, as 6r (selenohydantoin derivative) and as 9r (hydantoin derivative) showed good activity vs. LNCaP line over PC3 cells.
2.2.2. Molecular Docking Study
To further investigate the possibility of the obtained spiro derivatives to inhibit the interaction of P53 and MDM2 proteins, we studied them using the molecular docking. Initially, we have collected a database of some analogues of the compounds 4 scaffold (I–VI, Figure 3) [3,4,14,40,41] and speculated that small-molecule MDM2 inhibitors are the most similar in structure to our series. All compounds selected for comparison contained an oxindole fragment spiro-conjugated with the pyrrolidine ring. For instance, compound II has shown an IC50 value of 22 µM (mitogenesis inhibition, dye assay, WST-8) against PC3 human prostate adenocarcinoma cells (p53-null) [41], while under the same conditions towards LNCaP cells (androgen-dependent), it has demonstrated IC50 = 18 ± 13 nM [41].
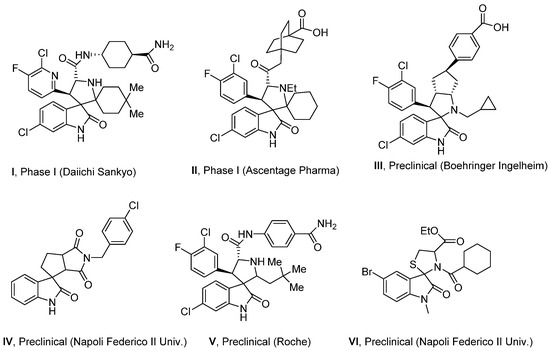
Figure 3.
Examples of small-molecule MDM2 inhibitors with spiro-oxindole fragments in the molecules [40,41,42,43,44].
Cytotoxicity of the compound II has been evaluated also against HCT116+/+ and HCT116−/− human colon carcinoma cells [41]. It has shown 80-fold selectivity towards p53-positive cells with IC50 values of 0.1 µM (vs. HCT116+/+) and 8 µM (vs. HCT116−/−), respectively. Compound IV demonstrated a CC50 value of 0.44 µM against HEK293 cells (24 h cytotoxicity, MTT assay) [14]. Compound V was reported to inhibit the growth and progression of HCT116 cells with an IC50 value of 90 nM (MTT assay) [4]. Molecule VI was evaluated against MCF7 cells (hormone-dependent) and PC3 cell line [42]. As a result, it showed high cytotoxicity and provided IC50 values of 40 nM and 0.41 µM, respectively. These reference activities are comparable with that observed for the most active compounds disclosed in the current work. The protein–protein interaction between MDM2 and p53 is observed via the first ∼120 N-terminal amino acid (AA) residues of MDM2 and the first 30 N-terminal AAs of p53 [43]. Twenty years ago, the first high-resolution co-crystal structure of MDM2 with a p53 peptide (residues 15–29, PDB code 1YCR) was reported by Kussie and co-workers [44]. Since, more than 50 crystallographic complexes have been published for a variety of small-molecule MDM2/p53 inhibitors belonging to different classes, including spiro-oxindoles.
The analysis of MDM2/p53 binding interface revealed that MDM2-bound p53 peptide adopts an α-helical conformation and interacts with MDM2 primarily through the hydrophobic triad of Phe19, Trp23 and Leu26. These “trident” of i, i + 4, i + 7 binds tightly into a medium-sized pocket in the structure of MDM2. This compact and well-defined binding site has been used to design many small-molecule high-affinity MDM2 inhibitors which effectively block the MDM2/p53 interaction thereby blocking tumor growth and progression. To elucidate the possible binding affinity of the evaluated compounds toward MDM2, a static 3D molecular docking study was performed in ICM-Pro software [45] based on several available X-ray data, including 4JVR, 4MDQ, 4JWR as well as 5C5A, 5HMH and 4ZYF. The binding site for 3D-molecular docking study was constructed following the standard procedure of binding site preparation in ICM-Pro with default settings. The procedure included the following steps: converting PDB-file to ICM-Pro object, optimizing hydrogens, excluding water molecules, moving template ligand out from pocket, and constructing receptor maps also with default settings in ICM-Pro.
The binding site for 3D-molecular docking study was constructed following the standard procedure of binding site preparation in ICM-Pro with default settings. The procedure included the following steps: converting PDB-file to ICM-Pro object, optimizing hydrogens, excluding water molecules, moving template ligand out from pocket, and constructing receptor maps also with default settings in ICM-Pro. The binding site was then compared with the binding mode revealed recently for recombinant p53 binding domain (residues 17–125) [21].
The validation of the constructed docking model was performed using the reference compounds as they were found in PDB-files. For the 4JVR-based model particularly, we have provided the results of molecular docking study as compared to original X-ray data (Figure 4A, compound V docked into the binding site in the same conformation with RMSD = 0.23). The reference compounds were then docked into the constructed model starting from 2D or 3D structures with or without stereo assignment. The obtained results (Figure 4A) were well correlated with the published RSA data. The most promising dispiro compound 4u, described in this work, was then docked into the static pocket using an extensive range of key force-fields, particularly describing hydrophobic interactions. As shown in Figure 4B, the selected molecule has a very similar to the reference compound binding mode. However, the predicted active conformation is distinct from that published for other MDM2 inhibitors. Thus, methyl group of compound 4u is located in a deep cavity by analogy to most of the reported 5-halogen substituted oxindoles.
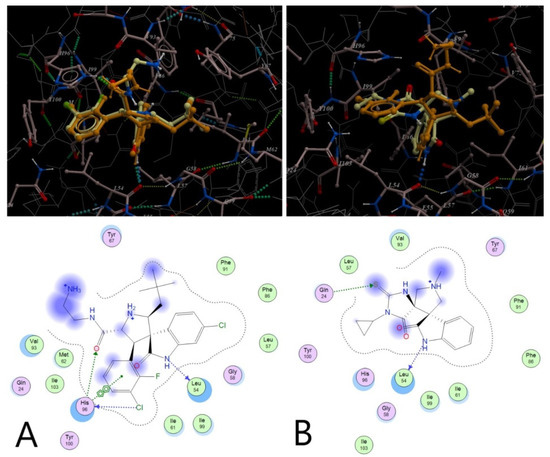
Figure 4.
The results of moleculardocking study: (A) MDM2 inhibitor V (see Figure 3) bound in the target pocket (4JVR)—RSA data (orange), the predicted active conformation (yellow, Eb = −88 kcal/mol), RMSD = 0.23; (B) compound 4u (orange, Eb = −53 kcal/mol) and the reference compound V (yellow) docked into the same binding site (the best conformations are shown).
Thus, although the mode of binding for the compound 4u areambiguous, its scaffold has the 3D-pharmacophore elements critical for binding to the pre-defined MDM2 pocket as compared to the reported MDM2/p53 inhibitors, including other spiro-oxindoles.
2.2.3. P53 Activation
In an effort to further elucidate the underlying mechanism of action of the compounds 4–6 we have performed cell-based assay with the p53 reporter construction [46] particularly sensitive to MDM2 inhibitors. In general, the obtained results demonstrated that the activation of p53 was observed upon the treatment with high concentrations of all synthesized compounds (>100 μM). This concentration is close to highly cytotoxic range (only 7–10% of cells stayed alive). In the same concentrations, 2.1-fold p53 activation was observed for compound 9r; under the same conditions, nutlin-3a showed from 3.6- and up to 5.1-fold increase in the p53 activation [47]. The effect of compounds 4 was slightly higher than the threshold value and could presumably be attributed to p53 activation primarily due to cell death and not vice versa.
Thus, although nutlin, chosen as a reference molecule, is comparable to the compound 4f in terms of cytotoxicity, unlike nutlin, the MDM2 protein is apparently not the main target of the compounds described in this work
3. Materials and Methods
3.1. General Information
All common reagents were purchased from commercial suppliers and used as received. The melting points are uncorrected.1H-NMR spectra were recorded on Bruker Avance 400 and Agilent MR-400 spectrometers at 400 MHz in CDCl3 or DMSO-d6. Chemical shifts were measured relative to solvent residual signals and referenced in part per million to TMS. Chemical shifts are reported in parts per million relative to TMS. High resolution mass spectra (HRMS) were recorded on an OrbitrapElite (Thermo Scientific) mass spectrometer with electrospray ionization (ESI) and orbital trap. To inject solutions with a concentration of 0.1 to 9 mg/mL (in 1% formic acid in acetonitrile), direct injection into the ion source using a syringe pump (5 mL/min) was used. The spray voltage was ±3.5 kV, the temperature of the capillary was 275 °C.
Compound 2a was synthesized as described in [48,49].
X-ray study was performed on diffractometer Bruker APEX DUO (MoKα-radiation, graphite monochromator, φ-scan). The X-ray structure was solved by direct methods and refined using full-matrix anisotropic approximation for F2hkl. The location of the hydrogen atoms was predicted geometrically, their positions were well-adjusted using the “rider” model Uiso(H) = 1.5Ueq(C) formethyl groups and 1.2 Ueq(X) for the remaining H-atoms. All calculations were performed in SHELX software version 2015 [50] and OLEX-2 [51].
CCDC 2120852 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (Embargoed Date 8 September 2022), or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Thioureas (1) and Thiohydantoins (2)
Amine (1 equiv) was added to a solution of ethyl isothiocyanatoacetate (1 equiv) in ether. The resulting mixture was stirred for 1 hour at room temperature. After the reaction was completed (TLC control), the solvent was evaporated in vacuo and the formed precipitate was filtered off, washed with cold diethyl ether and dried in air.
Ethyl 2-(3-benzylthioureido)acetate (1a)
From 0.54 g (5.0 mmol) of benzylamine and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1a (1.0 g, 83%) was obtained as a white solid.1H-NMR (400 MHz, CDCl3) δ: 8.25 (bs, 1H, NH), 7.40 (d, J = 8.7 Hz, 2H), 7.26 (d, J = 8.7 Hz, 2H), 6.70 (bs, 1H, NH), 4.42 (s, 2H), 4.22 (q, J = 9.2 Hz, 2H), 1.29 (t, J = 9.2 Hz, 3H). HRMS (ESI+) m/z calcd. for (C12H16N2O2S, M + H): 253.1005, found: (M + H): 253.1015.
Ethyl 2-(3-allylthioureido)acetate (1b)
From 0.29 g (5.0 mmol) of allylamine and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1b (0.93 g, 98%) was obtained as a yellow oil. 1H-NMR (400 MHz, CDCl3) δ: 6.82 (bs, 1H, NH), 6.74 (s, 1H, NH), 5.85 (m, 1H), 5.28 (d, J = 17.1 Hz, 1H), 5.20 (d, J = 10.2 Hz, 1H), 4.38 (d, J = 4.9 Hz, 1H), 4.21 (q, J = 7.2 Hz, 2H), 4.05 (s, 2H), 1.28 (t, J = 7.2 Hz, 3H). HRMS (ESI+) m/z calcd. for (C8H15N2O2S, M + H): 203.0849, found: (M + H): 203.0857.
Ethyl 2-(3-(4-methoxyphenyl)thioureido)acetate (1c)
From 0.62 g (5.0 mmol) of 4-methoxyaniline and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1c (0.77 g, 61%) was obtained as a light yellow solid. M.p. 94–96 °C. 1H-NMR (400 MHz, CDCl3) δ: 8.12 (bs, 1H, NH), 7.23 (d, J = 8.1 Hz, 2H), 7.16 (d, J = 8.3 Hz, 2H), 6.60 (bs, 1H, NH), 4.41 (s, 2H), 4.20 (q, J = 7.2 Hz, 2H), 2.36 (s, 3H), 1.27 (t, J = 7.2 Hz, 3H). HRMS (ESI+) m/z calcd. for (C12H16N2O3S, M + H): 269.0954, found: (M + H): 269.0958.
Ethyl 2-(3-(4-ethoxyphenyl)thioureido)acetate (1d)
From 0.69 g (5.0 mmol) of 4-ethoxyaniline and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1d (1.30 g, 97%) was obtained as a purple solid. M.p. 124–126 °C. 1H-NMR (400 MHz, CDCl3) δ: 7.90 (bs, 1H, NH), 7.19 (d, J = 9.1 Hz, 2H), 6.93 (d, J = 8.9 Hz, 2H), 6.44 (bs, 1H, NH), 4.41 (s, 2H), 4.20 (q, J = 7.2 Hz, 2H), 4.04 (q, J = 7.0 Hz, 2H), 1.42 (t, J = 7.0 Hz, 3H), 1.27 (t, J = 7.2 Hz, 3H). HRMS (ESI+) m/z calcd. for (C13H18N2O3S, M + H): 283.1111, found: (M + H): 283.1106.
Ethyl 2-(3-(p-tolyl)thioureido)acetate (1e)
From 0.54 g (5.0 mmol) of 4-methylaniline and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1e (0.89 g, 75%) was obtained as a plum solid. M.p. 132–134 °C. 1H-NMR (400 MHz, CDCl3) δ: 7.91 (bs, 1H, NH), 7.21 (d, J = 8.9 Hz, 2H), 6.95 (d, J = 8.9 Hz, 2H), 6.45 (bs, 1H, NH), 4.41 (s, 2H), 4.20 (q, J = 7.2 Hz, 2H), 3.82 (s, 3H), 1.28 (t, J = 7.2 Hz, 3H).
Ethyl 2-(3-(4-chlorophenyl)thioureido)acetate (1f)
From 0.64 g (5.0 mmol) of 4-chloroaniline and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1f (1.16 g, 90%) was obtained as a white solid. M.p. 154–156 °C. 1H-NMR (400 MHz, CDCl3) δ: 8.25 (bs, 1H, NH), 7.40 (d, J = 8.7 Hz, 2H), 7.25 (d, J = 8.7 Hz, 2H), 6.70 (bs, 1H, NH), 4.42 (s, 2H), 4.22 (q, J = 7.2 Hz, 2H), 1.29 (t, J = 7.2 Hz, 3H). HRMS (ESI+) m/z calcd. for (C11H13ClN2O2S, M + H): 273.0459, found: (M + H): 273.0468.
Ethyl 2-(3-(4-fluorophenyl)thioureido)acetate (1g)
From 0.56 g (5.0 mmol) of 4-fluoroaniline and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1g (0.97 g, 76%) was obtained as a white solid. M.p. 119–120 °C. 1H-NMR (400 MHz, CDCl3) δ: 8.15 (bs, 1H, NH), 7.33–7.25 (m, 2H), 7.14 (t, J = 8.5 Hz, 2H), 6.56 (bs, 1H, NH), 4.42 (s, 2H), 4.21 (q, J = 7.1 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H). HRMS (ESI+) m/z calcd. for (C11H13FN2O2S, M + H): 257.0755, found: (M + H): 257.0766.
Ethyl 2-(3-(3-chlorobenzyl)thioureido)acetate (1h)
From 0.74 g (5.0 mmol) of e-chlorobenzylamine and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate. compound 1h (1.12 g, 84%) was obtained as a white solid. M.p. 122–123 °C. 1H-NMR(400 MHz, CDCl3) δ: 7.39–7.19 (m, 5H), 4.67 (bs, 2H), 4.38 (s, 2H), 4.18 (q, J = 7.1 Hz, 2H), 1.27 (t, J = 7.1 Hz, 3H). HRMS (ESI+) m/z calcd. for (C12H15ClN2O2S, M + H): 287.0616, found: (M + H): 287.0628.
Ethyl 2-(3-(3-chloro-4-fluorophenyl)thioureido)acetate (1i)
From 0.73 g (5.0 mmol) of 4-fluoro,3-chloroaniline and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1i (1.08 g, 74%) was obtained as a white solid. M.p. 111–112 °C. 1H-NMR (400 MHz, CDCl3) δ: 8.26 (bs, 1H, NH), 7.42 (dd, J1 = 2.4 Hz, J2 = 6.4 Hz, 1H), 7.26–7.17 (m, 2H), 6.71 (bs, 1H, NH), 4.42 (s, 2H), 4.22 (q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.2 Hz, 3H). HRMS (ESI+) m/z calcd. for (C11H12ClFN2O2S, M + H): 291.0365, found: (M + H): 291.0377.
Ethyl 2-(3-cyclopropylthioureido)acetate (1j)
From 0.29 g (5.0 mmol) of cyclopropylamine and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1j (0.8 g, 87%) was obtained as a white solid. M.p. 129–130 °C. 1H-NMR (400 MHz, CDCl3) δ: 6.86 (bs, 1H, NH), 6.56 (bs, 1H, NH), 4.45 (d, J = 4.5 Hz, 2H), 4.27 (q, J = 7.0 Hz, 2H), 2.54 (bs, 1H), 1.32 (t, J = 7.2 Hz, 1H), 0.92–0.85 (m, 2H), 0.76–0.69 (m, 2H).
3-(3-Morpholinopropyl)-2-thioxoimidazolidin-4-one (2b)
From 0.72 g (5.0 mmol) of 3-(N-morpholino)propylamine and 0.73 g (5.0 mmol) of ethyl isothiocyanatoacetate, compound 1k (0.82 g, 68%) was obtained as a pink solid. M.p. 149–151 °C. 1H-NMR (400 MHz, CDCl3) δ: 8.33 (bs, 1H, NH), 4.08 (s, 2H), 3.89 (t, J = 6.9 Hz, 2H), 3.82–3.74 (m, 4H), 2.72–2.55 (m, 6H), 2.04–1.93 (m, 2H).
3.2.2. General Procedure for the Synthesis of 5-Substituted-2-thiohydantoins 3a–x
Thioureidoacetate 1 or 2-thioxoimidazolidine 2 (1 equiv) was dissolved in 2% KOH/EtOH; then isatin or 5-chloroisatin (1 equiv) was added. The resulting mixture was stirred for 30 min. After the reaction was completed (TLC control), the mixture was poured into water and neutralized with HCl. The formed precipitate was filtered off, washed with cold water, then washed with cold diethyl ether and dried in air.
(Z)-3-(1-Benzyl-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3a)
From 1a (0.36 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3a (0.48 g, 94%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.56 (s, 1H, NH), 11.10 (s, 1H, NH)8.53 (d, J = 7.8 Hz, 1H), 7.43–7.26 (m, 6H), 7.03 (td, J1 = 1.0 Hz, J2 = 7.7 Hz, 1H), 6.93 (d, J = 7.8 Hz, 1H), 5.06 (s, 2H). HRMS (ESI+) m/z calcd. for (C18H13N3O2S, M + H): 336.0801, found: (M + H): 336.0797.
(Z)-3-(1-Benzyl-5-oxo-2-thioxoimidazolidin-4-ylidene)-5-chloroindolin-2-one (3b)
From 1a (0.36 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3b (0.51 g, 92%) was obtained as a dark red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.63 (s, 1H, NH), 11.18 (s, 1H, NH),8.55 (m, 1H), 7.43–7.25 (m, 6H), 6.92 (d, J = 8.3 Hz, 1H), 5.05 (s, 2H). HRMS (ESI+) m/z calcd. for (C18H12ClN3O2S, M + H): 370.0411, found: (M + H): 370.0411.
(Z)-3-(1-Allyl-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3c)
From 1b (0.28 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol,) compound 3c (0.36 g, 83%) was obtained as a red solid. M.p. 257–259 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.50 (bs, 1H, NH), 11.07 (s, 1H, NH),8.52 (d, J = 7.8 Hz, 1H), 7.32 (td, J1 = 1.0 Hz, J2 = 7.7 Hz, 1H), 7.04 (td, J1 = 0.7 Hz, J2 = 7.7 Hz, 1H), 6.92 (d, J = 7.8 Hz, 1H), 5.86 (m, 1H), 5.20 (dd, J1 = 1.0 Hz, J2 = 9.7 Hz, 1H), 5.17 (m, 1H), 4.45 (d, J = 5.0 Hz, 2H). HRMS (ESI+) m/z calcd. for (C14H11N3O2S, M + H): 286.0644, found: (M + H): 286.0643.
(Z)-3-(1-Allyl-5-oxo-2-thioxoimidazolidin-4-ylidene)-5-chloroindolin-2-one (3d)
From 1b (0.28 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3d (0.40 g, 83%) was obtained as a dark red solid. M.p. 258–260 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.56 (bs, 1H, NH), 11.19 (s, 1H, NH),8.55 (d, J = 2.0 Hz, 1H), 7.36 (dd, J1 = 2.2 Hz, J2 = 8.3 Hz, 1H), 6.93 (d, J = 8.31 Hz, 1H), 5.86 (m, 1H), 5.25–5.16 (m, 2H), 4.45 (d, J = 5.1 Hz, 2H). HRMS (ESI+) m/z calcd. for (C14H10ClN3O2S, M + H): 320.0255, found: (M + H): 320.0252.
(Z)-3-(1-(4-Methoxyphenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3e)
From 1c (0.38 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3e (0.49 g, 93%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.60 (bs, 1H, NH), 10.95 (bs, 1H, NH), 8.56 (d, J = 7.70 Hz, 1H), 7.32 (d, J = 8.1 Hz, 2H), 7.29–7.22 (m, 3H), 6.97 (t, J = 7.6 Hz, 1H),6.90 (d, J = 7.8 Hz, 1H), 2.38 (s, 3H). HRMS (ESI+) m/z calcd. for (C18H13N3O3S, M + H): 352.0750, found: (M + H): 352.0771.
(Z)-5-Chloro-3-(1-(4-methoxyphenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3f)
From 1c (0.38 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3f (0.54 g, 94%) was obtained as a dark red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.69 (bs, 1H, NH), 11.24 (bs, 1H, NH), 8.54 (d, J = 2.2 Hz, 1H), 7.36–7.29 (m, 5H), 6.96 (dd, J1 = 2.2 Hz, J2 = 8.3 Hz, 1H), 2.39 (s, 3H). HRMS (ESI+) m/z calcd. for (C18H12ClN3O3S, M + H): 386.0360, found: (M + H): 386.0387.
(Z)-3-(1-(4-Ethoxyphenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3g)
From 1d (0.40 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3g (0.49 g, 89%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.62 (bs, 1H, NH), 11.14 (s, 1H, NH), 8.50 (d, J = 7.6 Hz, 1H), 7.37–7.29 (m, 3H), 7.06 (d, J = 8.9 Hz, 2H), 7.02 (t, J = 7.7 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 4.09 (q, J = 6.9 Hz, 2H), 1.36 (t, J = 6.9 Hz, 3H). HRMS (ESI+) m/z calcd. for (C19H15N3O3S, M + H): 366.0906, found: (M + H): 366.0895.
(Z)-5-Chloro-3-(1-(4-ethoxyphenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3h)
From 1d (0.40 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3h (0.52 g, 88%) was obtained as a dark red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.66 (bs, 1H, NH), 11.23 (s, 1H, NH),8.55 (s, 1H), 7.37 (dd, J1 = 2.2 Hz, J2 = 8.4 Hz, 1H), 7.33 (d, J = 8.7 Hz, 2H), 7.07 (d, J = 8.6 Hz, 2H), 6.96 (d, J = 8.3 Hz, 1H), 4.09 (q, J = 6.8 Hz, 2H), 1.36 (t, J = 6.8 Hz, 3H). HRMS (ESI+) m/z calcd. for (C19H15ClN3O3S, M + H): 400.0517, found: (M + H): 400.0496.
(Z)-3-(5-oxo-2-thioxo-1-(p-tolyl)imidazolidin-4-ylidene)indolin-2-one (3i)
From 1e (0.36 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3i (0.48 g, 95%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.62 (s, 1H, NH), 11.14 (s, 1H, NH), 8.50 (d, J = 7.8 Hz, 1H), 7.38–7.30 (m, 3H), 7.09 (d, J = 8.8 Hz, 2H), 7.03 (t, J = 7.6 Hz, 1H), 6.95 (d, J = 7.7 Hz, 1H), 3.83 (s, 3H). HRMS (ESI+) m/z calcd. for (C18H13N3O2S, M + H): 336.0801, found: (M + H): 336.0796.
(Z)-5-Chloro-3-(5-oxo-2-thioxo-1-(p-tolyl)imidazolidin-4-ylidene)indolin-2-one (3j)
From 1e (0.36 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3j (0.51 g, 92%) was obtained as a dark red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.67 (bs, 1H, NH), 11.25 (s, 1H, NH),8.55 (d, J = 1.4 Hz, 1H), 7.40–7.32 (m, 3H), 7.09 (d, J = 8.8 Hz, 2H), 6.96 (d, J = 8.3 Hz, 1H), 3.83 (s, 3H). HRMS (ESI+) m/z calcd. for (C18H12ClN3O2S, M + H): 370.0411, found: (M + H): 370.0414.
(Z)-3-(1-(4-Chlorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3k)
From 1f (0.39 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3k (0.45 g, 84%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.69 (bs, 1H, NH), 11.14 (bs, 1H, NH),8.48 (d, J = 7.7 Hz, 1H), 7.63 (d, J = 8.4 Hz, 2H), 7.48 (d, J = 8.4 Hz, 2H), 7.32 (t, J = 7.7 Hz, 1H), 7.02 (t, J = 7.7 Hz, 1H), 6.94 (d, J = 7.7 Hz, 1H). HRMS (ESI+) m/z calcd. for (C17H10ClN3O2S, M + H): 356.0255, found: (M + H): 356.0257.
(Z)-5-Chloro-3-(1-(4-chlorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3l)
From 1f (0.39 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3l (0.51 g, 86%) was obtained as a dark red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.76 (bs, 1H, NH), 11.24 (s, 1H, NH), 8.53 (d, J = 2.0 Hz, 1H), 7.65 (d, J = 8.6 Hz, 2H), 7.49 (d, J = 8.6 Hz, 2H), 7.38 (dd, J1 = 2.1 Hz, J2 = 8.3 Hz, 1H), 6.96 (d, J = 8.3 Hz, 1H). HRMS (ESI+) m/z calcd. for (C17H9Cl2N3O2S, M + H): 389.9865, found: (M + H): 389.9846.
(Z)-3-(1-(4-Fluorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3m)
From 1g (0.38 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3m (0.45 g, 89%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.68 (s, 1H, NH), 11.13 (s, 1H, NH), 8.50 (d, J = 7.0 Hz, 1H), 7.58–7.46 (m, 2H), 7.40 (t, J = 8.0 Hz, 2H), 7.33 (t, 7.5 Hz, 1H), 7.03 (t, J = 7.0 Hz, 1H), 6.95 (d, J = 7.0 Hz, 1H). HRMS (ESI-) m/z calcd. for (C17H10FN3O2S, M-H): 338.0394, found: (M-H): 338.0405.
(Z)-5-Chloro-3-(1-(4-fluorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3n)
From 1g (0.38 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3n (0.51 g, 91%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.75 (s, 1H, NH), 11.24 (s, 1H, NH), 8.56–8.52 (m, 1H), 7.55–7.48 (m, 2H), 7.46–7.35 (m, 3H), 6.96 (dd, J1 = 3.6 Hz, J2 = 8.3 Hz, 1H). HRMS (ESI-) m/z calcd. for (C17H9ClFN3O2S, M-H): 372.0004, found: (M-H): 372.0017.
(Z)-3-(1-(3-Chlorobenzyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3o)
From 1h (0.43 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3o (0.48 g, 87%) was obtained as a red solid. M.p. 296–298 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.59 (bs, 1H, NH), 11.09 (s, 1H, NH), 8.52 (d, J = 7.7 Hz, 1H), 7.46 (s, 1H), 7.40–7.29 (m, 4H), 7.03 (t, J = 7.6 Hz, 1H), 6.93 (d, J = 7.8 Hz, 1H), 5.05 (s, 2H). HRMS (ESI-) m/z calcd. for (C18H12ClN3O2S, M-H): 368.0266, found: (M-H): 368.0255.
(Z)-5-Chloro-3-(1-(3-chlorobenzyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3p)
From 1h (0.43 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3p (0.53 g, 87%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.66 (bs, 1H, NH), 11.18 (s, 1H, NH), 8.56 (d, J = 1.7 Hz, 1H), 7.46 (s, 1H), 7.41–7.32 (m, 4H), 6.94 (d, J = 8.3 Hz, 1H), 5.05 (s, 2H). HRMS (ESI-) m/z calcd. for (C18H11Cl2N3O2S, M-H): 401.9865, found: (M-H): 401.9879.
(Z)-3-(1-(3-Chloro-4-fluorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3q)
From 1i (0.44 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3q (0.47 g, 84%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.75 (bs, 1H, NH), 10.92 (s, 1H, NH), 8.56 (d, J = 7.8 Hz, 1H), 7.73 (dd, J1 = 1.88 Hz, J = 6.5 Hz, 1H), 7.61 (t, J = 9.0 Hz, 1H), 7.48 (m, 1H), 7.27 (t, J = 7.3 Hz, 1H), 6.98 (t, J = 7.6 Hz, 1H), 6.90 (d, J = 7.6 Hz, 1H). HRMS (ESI-) m/z calcd. for (C17H9ClFN3O2S, M-H): 372.0004, found: (M-H): 372.0017.
(Z)-5-Chloro-3-(1-(3-chloro-4-fluorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3r)
From 1i (0.44 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3r (0.53 g, 86%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.81 (bs, 1H, NH), 11.24 (s, 1H, NH), 8.51 (d, J = 1.8 Hz, 1H), 7.77 (dd, J1 = 2.3 Hz, J2 = 6.7 Hz, 1H), 7.65 (t, J = 8.99 Hz, 1H), 7.52 (m, 1H), 7.37 (dd, J1 = 2.02 Hz, J2 = 8.38 Hz, 1H), 6.95 (d, J = 8.3 Hz, 1H). HRMS (ESI-) m/z calcd. for (C17H8Cl2FN3O2S, M-H): 405.9615, found: (M-H): 405.9628.
(Z)-3-(1-Cyclopropyl-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3s)
From 1j (0.30 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3s (0.26 g, 92%) was obtained as a red solid. M.p. 277–279 °C (decomp.). 1H-NMR (400 MHz, DMSO-d6) δ: 11.35 (bs, 1H, NH), 11.08 (s, 1H, NH), 8.52 (d, J = 7.8 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.04 (t, J = 7.6 Hz, 1H), 6.92 (d, J = 7.8 Hz, 1H), 2.80 (m, 1H), 1.04–0.98 (m, 4H). HRMS (ESI-) m/z calcd. for (C14H11N3O2S, M-H): 284.0488, found: (M-H): 284.0499.
(Z)-5-Chloro-3-(1-cyclopropyl-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3t)
From 1j (0.30 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3t (0.29 g, 92%) was obtained as a red solid. M.p. 286–288 °C (decomp.). 1H-NMR (400 MHz, DMSO-d6) δ: 11.40 (s, 1H, NH), 11.19 (s, 1H, NH), 8.56 (m, 1H), 7.36 (d, J = 8.3 Hz, 1H), 6.94 (dd, J1 = 2.1 Hz, J = 8.3 Hz, 1H), 2.80 (m, 1H), 1.04–0.99 (m, 4H). HRMS (ESI-) m/z calcd. for (C14H10ClN3O2S, M-H): 318.0099, found: (M-H): 318.0112.
(Z)-3-(1-(3-Morpholinopropyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one hydrochloride (3u)
From 2a (0.37 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3u (0.57 g, 93%) was obtained as a red solid. M.p. 270–272 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.54 (s, 1H), 11.18 (s, 1H), 11.07 (bs, 1H), 8.54 (d, J = 8.0 Hz, 1H), 7.33 (t, J = 7.7 Hz, 1H), 7.05 (t, J = 7.7 Hz, 1H), 6.95 (d, J = 7.7 Hz, 1H), 3.96–3.88 (m, 4H), 3.78 (t, J = 11.8 Hz, 2H), 3.36 (d, J = 11.8 Hz, 2H), 3.22–3.13 (m, 2H), 3.07–2.95 (m, 2H), 2.19–2.09 (m, 2H). HRMS (ESI+) m/z calcd. for (C18H20N4O3S, M + H): 373.1328, found: (M + H): 373.1322.
(Z)-5-Chloro -3-(1-(3-morpholinopropyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one hydrochloride (3v)
From 2a (0.37 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound 3v (0.63 g, 95%) was obtained as a dark red solid. M.p. 249–251 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.61 (s, 1H), 11.31 (s, 1H), 11.09 (bs, 1H), 8.58 (d, J = 2.0 Hz, 1H), 7.37 (dd, J1 = 2.2 Hz, J2 = 8.4 Hz, 1H), 6.97 (d, J = 8.3 Hz, 1H), 3.96–3.88 (m, 4H), 3.85–3.75 (m, 4H), 3.35 (d, J = 11.6 Hz, 2H), 3.22–3.13 (m, 2H), 3.07–2.95 (m, 2H), 2.20–2.10 (m, 2H). HRMS (ESI+) m/z calcd. for (C18H19Cl1N4O3S, M + H): 407.0939, found: (M + H): 407.0917.
(Z)-3-(5-Oxo-1-phenyl-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3w)
From 2b (0.29 g, 1.5 mmol) and isatin (0.22 g, 1.5 mmol), compound 3w (0.45 g, 93%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.57 (s, 1H, NH), 10.87 (s, 1H, NH), 8.46 (d, J = 7.8 Hz, 1H), 7.52–7.41 (m, 3H), 7.37–7.33 (m, 2H), 7.21 (td, J1 = 1.1 Hz, J2 = 7.7 Hz, 1H), 6.91 (td, J1 = 1.0 Hz, J2 = 7.7 Hz, 1H), 6.86 (d, J = 7.7 Hz, 1H). HRMS (ESI-) m/z calcd. for (C17H11N3O2S, M-H): 320.0488, found: (M-H): 320.0506.
(Z)-5-Chloro-3-(5-oxo-1-phenyl-2-thioxoimidazolidin-4-ylidene)indolin-2-one (3x)
From 2b (0.29 g, 1.5 mmol) and 5-chloroisatin (0.27 g, 1.5 mmol), compound three times (0.50 g, 94%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.74 (bs, 1H, NH), 11.25 (s, 1H, NH), 8.55 (m, 1H), 7.59–7.49 (m, 3H), 7.47–7.42 (m, 2H), 7.37 (m, 1H), 6.96 (d, J = 8.4 Hz, 1H). HRMS (ESI-) m/z calcd. for (C17H10ClN3O2S, M-H): 354.0099, found: (M-H): 354.0113.
3.2.3. General Procedure for the Synthesis of Dispiroindolinones 4a–x
Corresponding 5-indolidene-2-thioxoimidazolidin 3 (1equiv) and sarcosine (4 equiv) were dissolved in toluene and the mixture heated to a boiling point. After that, paraformaldehyde (4 equiv) was added. The resulting mixture was refluxed for 5–8 hours (TLC control). After the reaction was completed, the solvent was evaporated in vacuo. The product was then purified using column chromatography (silica gel 60, 0.04–0.063 mm/230–400 mesh, CHCl3:MeOH/50:1) to afford products as a yellow or pink solid. This solid was washed with acetone to yield corresponding dispirooxindole as white crystalline solid.
1′-Methyl-1-benzyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4a)
From 3a (0.22 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4a (0.21 g, 82%) was obtained as a white solid. M.p. 189–190 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.42 (bs, 1H, NH), 7.26 (t, J = 7.6 Hz, 1H), 7.23–7.10 (m, 4H), 7.08 (d, J = 7.7 Hz, 1H), 6.86–6.74 (m, 3H), 4.74 (d, J = 15.3 Hz, 1H), 4.66 (d, J = 15.3 Hz, 1H), 4.38 (d, J = 12.6 Hz, 1H), 4.23 (d, J = 12.6 Hz, 1H), 3.40–3.30 (m, 3H), 3.25 (dd, J1 = 6.7 Hz, J2 = 9.4 Hz, 2H), 3.06 (d, J = 9.9 Hz, 1H), 2.44 (s, 3H), 2.17 (s, 6H). HRMS (ESI+) m/z calcd. for (C21H20N4O2S, M + H): 393.1379, found: (M + H): 393.1364.
5″-Chloro-1′-methyl-1-benzyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4b)
From 3b (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde, (0.08 g, 2.6 mmol) compound 4b (0.21 g, 76%) was obtained as a white solid. M.p. 152–153 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.54 (bs, 1H, NH), 7.34 (dd, J1 = 2.0 Hz, J2 = 8.3 Hz, 1H), 7.29 (d, J = 2.0 Hz, 1H), 7.25 (t, J = 7.5 Hz, 1H), 7.20–7.12 (m, 4H), 7.09 (d, J = 8.6 Hz, 1H), 6.75 (d, J = 6.5 Hz, 2H), 4.74 (d, J = 15.5 Hz, 1H), 4.67 (d, J = 15.5 Hz, 1H), 4.37 (d, J = 12.7 Hz, 1H), 4.20 (d, J = 12.7 Hz, 1H), 3.40–3.30 (m, 3H), 3.19 (t, J = 10.8 Hz, 2H), 3.07 (d, J = 9.7 Hz, 1H), 2.43 (s, 3H), 2.12 (s, 6H). HRMS (ESI+) m/z calcd. for (C21H19ClN4O2S, M + H): 427.0990, found: (M + H): 427.0976.
1′-Methyl-1-allyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4c)
From 3c (0.19 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4c (0.15 g, 67%) was obtained as a white solid. M.p. 281–283 °C(decomp.). 1H-NMR (400 MHz, DMSO-d6) δ: 10.57 (s, 1H, NH), 10.44 (s, 1H, NH), 7.19 (d, J = 7.7 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 6.86 (t, J = 7.6 Hz, 1H), 6.79 (d, J = 7.7 Hz, 1H), 5.47 (m, 1H), 4.89 (d, J = 10.4 Hz, 1H), 4.59 (d, J = 17.4 Hz, 1H), 4.18–4.03 (m, 2H), 3.40 (d, J = 9.8 Hz, 1H), 3.32 (d, J = 9.8 Hz, 1H), 3.16 (d, J = 9.9 Hz, 1H), 3.05 (d, J = 10.0 Hz, 1H), 2.45 (c, 3H). HRMS (ESI+) m/z calcd. for (C17H18N4O2S, M + H): 343.1223, found: (M + H): 343.1207.
5″-Chloro-1′-methyl-1-allyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4d)
From 3d (0.21 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4d (0.09 g, 38%) was obtained as a white solid. M.p. 141–142 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.49 (bs, 1H, NH), 7.33 (dd, J1 = 1.9 Hz, J2 = 8.4 Hz, 1H), 7.26 (d, J = 1.7 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 5.47 (m, 1H), 4.88 (d, J = 10.2 Hz, 1H), 4.55 (d, J = 17.2 Hz, 1H), 4.38 (d, J = 12.6 Hz, 1H), 4.22 (d, J = 12.6 Hz, 1H), 4.20–4.02 (m, 2H), 3.30–3.21 (m, 3H), 3.04 (d, J = 10.0 Hz, 1H), 2.44 (s, 3H), 2.19 (s, 6H). HRMS (ESI+) m/z calcd. for (C17H17ClN4O2S, M + H): 377.0833, found: (M + H): 377.0822.
1′-Methyl-1-(4-methoxyphenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4e)
From 3e (0.23 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4e (0.08 g, 28%) was obtained as a white solid. M.p. 155–156 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.67–10.57 (m, 2H, NH), 7.26 (t, J = 7.5 Hz, 1H), 7.20 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 7.5 Hz, 1H), 6.93 (t, J = 7.5 Hz, 1H), 6.86 (d, J = 7.7 Hz, 1H), 6.71 (d, J = 8.1 Hz, 2H), 3.49 (d, J = 9.9 Hz, 1H), 3.38 (m, 1H), 3.32 (m, 1H), 3.07 (d, J = 10.2 Hz, 1H), 2.47 (s, 3H), 2.31 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H20N4O3S, M + H): 409.1328, found: (M + H): 409.1323.
5″-Cchloro-1′-methyl-1-(4-methoxyphenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4f)
From 3f (0.25 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4f (0.14 g, 49%) was obtained as a white solid. M.p. 289–290 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.79 (s, 1H, NH), 10.74 (s, 1H, NH), 7.34 (dd, J1 = 2.0 Hz, J2 = 8.3 Hz, 1H), 7.23 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 1.8 Hz, 1H), 6.88 (d, J = 8.3 Hz, 1H), 6.74 (d, J = 8.1 Hz, 2H), 3.45–3.30 (m, 3H), 3.09 (d, J = 10.2 Hz, 1H), 2.47 (s, 3H), 2.32 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H19ClN4O3S, M + H): 443.0939, found: (M + H): 443.0940.
1′-Methyl-1-(4-ethoxyphenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4g)
From 3g (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4g (0.10 g, 35%) was obtained as a white solid. M.p. 240–241 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.63 (s, 1H, NH), 10.60 (s, 1H, NH), 7.26 (t, J = 7.7 Hz, 1H), 7.13 (d, J = 7.5 Hz, 1H), 6.98–6.89 (m, 3H), 6.68 (d, J = 7.7 Hz, 1H),6.73 (d, J = 8.6 Hz, 2H), 4.03 (q, J = 7.0 Hz, 2H), 3.49 (d, J = 10.0 Hz, 1H), 3.40–3.28 (m, 2H),3.07 (d, J = 10.0 Hz, 1H), 2.47 (s, 3H), 1.32 (t, J = 7.0 Hz, 3H). HRMS (ESI+) m/z calcd. for (C22H22N4O3S, M + H): 423.1485, found: (M + H): 423.1480.
5″-Chloro-1′-methyl-1-(4-ethoxyphenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4h)
From 3h (0.26 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4h (0.08 g, 27%) was obtained as a white solid. M.p. 268–271 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.80–10.61 (bs, 2H, NH), 7.33 (dd, J1 = 1.6 Hz, J2 = 8.2 Hz, 1H), 7.13 (d, J = 1.6 Hz, 1H), 6.95 (d, J = 8.8 Hz, 2H),6.87 (d, J = 8.3 Hz, 1H), 6.76 (d, J = 8.4 Hz, 2H), 4.04 (q, J = 7.0 Hz, 2H), 3.45–3.34 (m, 3H),3.40–3.28 (m, 2H), 3.09 (d, J = 10.3 Hz, 1H), 2.47 (s, 3H), 1.32 (t, J = 7.0 Hz, 3H). HRMS (ESI+) m/z calcd. for (C22H21ClN4O3S, M + H): 457.1095, found: (M + H): 457.1090.
1′-Methyl-1-(p-tolyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4i)
From 3i (0.22 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4i (0.10 g, 39%) was obtained as a white solid. M.p. 155–157 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.61 (bs, 1H, NH), 10.57 (bs, 1H, NH), 7.26 (dd, J1 = 1.0 Hz, J2 = 7.7 Hz, 1H), 7.14 (d, J = 7.2 Hz, 1H), 6.97–6.91 (m, 3H), 6.86 (d, J = 7.7 Hz, 1H), 6.75 (d, J = 8.8 Hz, 2H), 3.76 (s, 3H), 3.49 (d, J = 10.1 Hz, 1H), 3.37 (d, J = 10.1 Hz, 1H), 3.32 (m, 1H), 3.07 (d, J = 10.1 Hz, 1H), 2.47 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H20N4O2S, M + H): 393.1379, found: (M + H): 393.1384.
5″-Chloro-1′-methyl-1-(p-tolyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4j)
From 3j (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4j (0.14 g, 50%) was obtained as a white solid. M.p. 159–160 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.76 (s, 1H, NH), 10.69 (bs, 1H, NH), 7.33 (dd, J1 = 2.2 Hz, J2 = 8.3 Hz, 1H), 7.14 (d, J = 2.2 Hz, 1H), 6.99–6.94 (m, 2H), 6.87 (d, J = 8.3 Hz, 1H), 6.80–6.75 (m, 2H), 3.78 (s, 3H), 3.44–3.32 (m, 3H), 3.09 (d, J = 10.2 Hz, 1H), 2.47 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H19ClN4O2S, M + H): 427.0990, found: (M + H): 427.0987.
5″-Chloro-1′-methyl-1-(4-chlorophenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4k)
From 3k (0.23 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4k (0.13 g, 48%) was obtained as a white solid. M.p. 163–164 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.72 (bs, 1H, NH), 10.62 (s, 1H, NH), 7.53–7.48 (m, 2H), 7.26 (td, J1 = 1.2 Hz, J2 = 7.7 Hz, 1H), 7.12 (d, J = 7.4 Hz, 1H), 6.93 (td, J1 = 0.9 Hz, J2 = 7.7 Hz, 1H), 6.91–6.87 (m, 2H), 6.86 (d, J = 7.9 Hz, 1H), 3.49 (d, J = 10.1 Hz, 1H), 3.39–3.33 (m, 2H), 3.08 (d, J = 10.0 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H17ClN4O2S, M + H): 413.0833, found: (M + H): 413.0829.
5″-Chloro-1′-methyl-1-(4-chlorophenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4l)
From 3l (0.25 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4l (0.03 g, 10%) was obtained as a white solid. M.p. 239–240 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.84 (bs, 1H, NH), 10.77 (s, 1H, NH), 7.55–7.50 (m, 2H), 7.26 (dd, J1 = 2.2 Hz, J2 = 8.3 Hz, 1H), 7.12 (d, J = 2.1 Hz, 1H), 6.94–6.89 (m, 2H), 6.87 (d, J = 8.3 Hz, 1H), 3.49 (d, J = 10.1 Hz, 1H), 3.44–3.38 (m, 2H), 3.33 (m, 1H), 3.10 (d, J = 10.4 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H16Cl2N4O2S, M + H): 447.0443, found: (M + H): 447.0433.
1′-Methyl-1-(4-fluorophenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4m)
From 3m (0.22 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4m (0.06 g, 24%) was obtained as a white solid. M.p. 273–274 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.91 (bs, 1H, NH), 10.79 (s, 1H, NH), 7.55 (t, J = 9.0 Hz, 1H), 7.43 (dd, J1 = 2.1 Hz, J2 = 8.3 Hz, 1H), 7.13–7.07 (m, 2H), 6.94 (m, 1H), 6.89 (d, J = 8.3 Hz, 1H), 3.45–3.37 (m, 2H), 3.32 (d, J = 10.2 Hz, 1H), 3.10 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H17FN4O2S, M + H): 397.1129, found: (M + H): 397.1115
5″-Chloro-1′-methyl-1-(4-fluorophenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4n)
From 3n (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4n (0.10 g, 35%) was obtained as a white solid. M.p. 273–274 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.82 (bs, 1H, NH), 10.78 (bs, 1H, NH), 7.37–7.26 (m, 3H), 7.12 (s, 1H), 6.97–6.85 (m, 3H), 3.43–3.38 (m, 2H), 3.32 (d, J = 10.2 Hz, 1H), 3.10 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H16FClN4O2S, M + H): 431.0739, found: (M + H): 431.0760.
1′-Methyl-1-(3-chlorobenzyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4o)
From 3o (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4o (0.24 g, 87%) was obtained as a white solid. M.p. 273–274 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.59 (bs, 1H, NH), 10.57 (bs, 1H, NH), 7.28 (d, J = 7.6 Hz, 1H), 7.23–7.11 (m, 2H), 7.05 (s, 1H), 7.02 (d, J = 7.6 Hz, 1H), 6.78 (d, J = 7.7 Hz, 1H), 6.74 (d, J = 7.6 Hz, 1H), 6.67 (t, J = 7.6 Hz, 1H), 4.76 (d, J = 15.5 Hz, 1H), 4.66 (d, J = 15.5 Hz, 1H), 3.39 (d, J = 10.1 Hz, 1H), 3.20 (d, J = 10.0 Hz, 1H), 3.05 (d, J = 10.0 Hz, 1H), 2.44 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H19ClN4O2S, M + H): 427.0995, found: (M + H): 427.0981.
5″-Chloro-1′-methyl-1-(3-chlorobenzyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4p)
From 3p (0.26 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4p (0.18 g, 61%) was obtained as a white solid. M.p. 261–262 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.71 (s, 1H, NH), 10.68 (bs, 1H, NH), 7.29–7.20 (m, 2H), 7.18 (t, J = 7.9 Hz, 1H), 7.12 (s, 1H), 6.98 (s, 1H), 6.80 (d, J = 8.3 Hz, 1H), 6.67 (d, J = 7.3 Hz, 1H), 4.77 (d, J = 15.7 Hz, 1H), 4.68 (d, J = 15.7 Hz, 1H), 3.30 (d, J = 11.0 Hz, 1H), 3.24 (d, J = 8.8 Hz, 2H), 3.07 (d, J = 9.9 Hz, 1H), 2.44 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H18Cl2N4O2S, M + H): 461.0600, found: (M + H): 461.0610.
1′-Methyl-1-(3-chloro-4-fluorophenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione(4q)
From 3q (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4q (0.21 g, 75%) was obtained as a white solid. M.p. 252–254 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.81 (s, 1H, NH), 10.64 (s, 1H, NH), 7.51 (t, J = 8.9 Hz, 1H), 7.27 (t, J = 7.6 Hz, 1H), 7.12 (d, J = 7.3 Hz, 2H), 6.93 (t, J = 7.5 Hz, 1H), 6.89–6.81 (m, 3H), 3.47 (d, J = 10.2 Hz, 1H), 3.37 (d, J = 10.8 Hz, 2H), 3.08 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H16ClFN4O2S, M + H): 431.0739, found: (M + H): 431.0721.
5″-Chloro-1′-methyl-1-(3-chloro-4-fluorophenyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4r)
From 3r (0.27 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4r (0.10 g, 33%) was obtained as a white solid. M.p. 177–180 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.91 (bs, 1H, NH), 10.79 (s, 1H, NH), 7.55 (t, J = 9.0 Hz, 1H), 7.34 (dd, J1 = 2.1 Hz, J2 = 8.3 Hz, 1H), 7.13–7.07 (m, 2H), 6.94 (m, 1H), 6.89 (d, J = 8.3 Hz,1H), 3.45–3.37 (m, 2H), 3.32 (d, J = 10.2 Hz, 1H), 3.10 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H15Cl2FN4O2S, M + H): 465.0350, found: (M + H): 465.0341.
1′-Methyl-1-cyclopropyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4s)
From 3s (0.19 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4s (0.12 g, 54%) was obtained as a white solid. M.p. 272–273 °C (decomp.). 1H-NMR (400 MHz, DMSO-d6) δ: 10.50 (s, 1H, NH), 10.28 (s, 1H, NH), 7.19 (t, J = 7.7 Hz, 1H), 7.11 (d, J = 7.5 Hz, 1H), 6.89 (t, J = 7.6 Hz, 1H), 6.77 (d, J = 7.7 Hz, 1H), 3.30 (d, J = 10.0 Hz, 1H), 3.25 (d, J = 10.0 Hz, 1H), 3.14 (d, J = 10.0 Hz, 1H), 3.01 (d, J = 10.0 Hz, 1H), 2.45 (m, 1H), 2.43 (s, 3H), 0.82–0.70 (m, 2H), 0.62 (m, 1H), 0.11 (m, 1H). HRMS (ESI+) m/z calcd. for (C17H18N4O2S, M + H): 343.1223, found: (M + H): 343.1241.
5″-Chloro-1′-methyl-1-cyclopropyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4t)
From 3t (0.21 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4t (0.06 g, 25%) was obtained as a white solid. M.p. 275–276 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H, NH), 10.41 (s, 1H, NH), 7.26 (m, 1H), 7.10 (m, 1H), 6.79 (dd, J1 = 2.1 Hz, J2 = 8.3 Hz, 1H), 3.26–3.16 (m, 3H), 3.02 (d, J = 10.0 Hz, 1H), 2.43 (s, 3H), 0.89–0.74 (m, 2H), 0.59 (m, 1H), 0.11 (m, 1H). HRMS (ESI+) m/z calcd. for (C17H17ClN4O2S, M + H): 377.0834, found: (M + H): 377.0851.
1′-Methyl-1-(3-morpholinopropyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4u)
From 3u (0.24 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4u (0.08 g, 25%) was obtained as a white solid. M.p. 216–217 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.55 (s, 1H, NH), 10.39 (s, 1H, NH), 7.22–7.14 (m, 2H), 6.88 (t, J = 7.6 Hz, 1H), 6.78 (d, J = 7.8 Hz, 1H), 3.61–3.43 (m, 6H), 3.38 (d, J = 7.1 Hz, 1H), 3.31 (d, J = 10.6 Hz, 1H), 3.15 (d, J = 10.0 Hz, 1H), 3.04 (d, J = 10.0 Hz, 1H), 2.44 (s, 3H), 2.28–2.18 (m, 4H), 2.05 (t, J = 6.7 Hz, 2H), 1.42–1.33 (m, 2H). HRMS (ESI+) m/z calcd. for (C21H27N5O3S, M + H): 430.1907, found: (M + H): 430.1904.
5″-Chloro-1′-methyl-1-(3-morpholinopropyl)-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4v)
From 3v (0.26 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4v (0.05 g, 15%) was obtained as a white solid. M.p. 224–225 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.69 (s, 1H, NH), 10.49 (s, 1H, NH), 7.25 (dd, J1 = 1.8 Hz, J2 = 8.5 Hz, 1H), 7.19 (s, 1H), 6.80 (d, J = 8.1 Hz, 1H), 3.64–3.46 (m, 6H), 3.30–3.18 (m, 3H), 3.06 (d, J = 9.9 Hz, 1H), 2.43 (s, 3H), 2.28–2.19 (m, 4H), 2.10–2.03 (m, 2H), 1.44–1.28 (m, 2H). HRMS (ESI+) m/z calcd. for (C21H26ClN5O3S, M + H): 464.1517, found: (M + H): 464.1519.
1′-Methyl-1-phenyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4w)
From 3w (0.21 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 4w (0.07 g, 27%) was obtained as a white solid. M.p. 273–274 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.65 (bs, 1H, NH), 10.63 (s, 1H, NH), 7.45–7.36 (m, 3H), 7.27 (t, J = 7.7 Hz, 1H), 7.15 (d, J = 7.5 Hz, 1H), 6.94 (t, J = 7.6 Hz, 1H), 6.89–6.82 (m, 3H), 3.51 (d, J = 10.0 Hz, 1H), 3.37 (d, J = 10.0 Hz, 2H), 3.08 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H18N4O2S, M + H): 379.1223, found: (M + H): 379.1236.
5″-Chloro-1′-methyl-1-phenyl-2-thioxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (4x)
From three times (0.23 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound four times (0.14 g, 49%) was obtained as a white solid. M.p. 268–269 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.79 (bs, 1H, NH), 10.78 (bs, 1H, NH), 7.47–7.39 (m, 3H), 7.34 (dd, J1 = 1.6 Hz, J2 = 8.3 Hz, 1H), 7.14 (s, 1H), 6.91–6.85 (m, 3H), 3.43 (d, J = 10.2 Hz, 1H), 3.39 (d, J = 10.2 Hz, 1H), 3.34 (d, J = 10.2 Hz, 1H), 3.10 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H17ClN4O2S, M + H): 413.0834, found: (M + H): 413.0850.
3.2.4. General Procedure for the Synthesis of Dispiroindolinones 6
Corresponding 5-indolidene-2-selenoxoimidazolidin 5 (1 equiv) and sarcosine (4 equiv) were dissolved in toluene and the mixture heated to a boiling point. After that paraformaldehyde (4 equiv) was added. The resulting mixture was refluxed for 5–8 hours (TLC control). After the reaction was completed, the solvent was evaporated in vacuo. The product was then purified using column chromatography (silica gel 60, 0.04–0.063 mm/230–400 mesh, CHCl3:MeOH/50:1) to afford products as a white solid. This solid was washed with cold methanol to yield corresponding dispirooxindole as light brown crystalline solid.
5″-Chloro-1-(3-chloro-4-fluorophenyl)-1′-methyl-2-selenoxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (6r)
From 5r (0.30 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 6r (0.19 g, 57%) was obtained as light brown solid. M.p. 193–194 °C (decomp.). 1H-NMR (400 MHz, DMSO-d6) δ: 11.62 (s, 1H, NH), 10.81 (s, 1H, NH), 7.57 (t, J = 9.0 Hz, 1H), 7.36 (dd, J1 = 1.8 Hz, J2 = 8.3 Hz, 1H), 7.12 (m, 1H), 7.10 (m, 1H), 6.96 (m, 1H), 6.90 (d, J = 8.3 Hz, 1H), 3.43 (s, 2H), 3.32 (m, 1H), 3.12 (d, J = 10.2 Hz, 1H), 2.49 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H17ClN4O2S, M + H): 512.9800, found: (M + H): 513.0050.
5″-Chloro-1-(4-methoxyphenyl)-1′-methyl-2-selenoxodispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2″,5-dione (6f)
From 5f (0.28 g, 0.65 mmol), sarcosine (0.23 g, 2.6 mmol) and paraformaldehyde (0.08 g, 2.6 mmol), compound 6f (0.19 g, 71%) was obtained as a white solid. M.p. 284–285 °C (decomp.).1H-NMR (400 MHz, DMSO-d6) δ: 11.41 (bs, 1H, NH), 10.79 (s, 1H, NH), 7.33 (dd, J1 = 1.9 Hz, J2 = 8.3 Hz, 1H), 7.13 (d, J = 1.9 Hz, 1H), 6.96 (d, J = 8.7 Hz, 2H), 6.87 (d, J = 8.3 Hz, 1H), 6.78 (d, J = 8.6 Hz, 2H), 3.77 (s, 3H), 3.45–3.37 (m, 2H), 3.31 (m, 1H), 3.10 (d, J = 10.2 Hz, 1H), 2.48 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H20ClN4O3Se, M + H): 491.0384, found: (M + H): 491.0385.
3.2.5. General Procedure for the Synthesis of 5-Substituted Hydantoins 8
Corresponding 5-substituted-2-thiohydantoin 4 (1 equiv) was added to solution of the potassium hydroxide (1.05 equiv) in EtOH at room temperature (~4 mL EtOH for 100 mg of 3). After that, MeI (1.5 eq) was added and the reaction mixture was stirred at room temperature overnight. Then EtOH:HCl conc.(1:1) was added to the reaction (~4 mL EtOH for 100 mg of 3) and refluxed for 2 hours. Further, the reaction cooled to room temperature and formed precipitate was filtered off, washed with ethanol and dried in air. All compounds were obtained as red crystalline powders.
(Z)-5-Chloro-3-(1-(4-methoxyphenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (8f)
From 3f (0.116 g, 0.30 mmol), KOH (0.018 g, 0.32 mmol) and MeI (0.064 g, 0.45 mmol), compound 8f (0.089 g, 80%) was obtained as a red solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.08 (s, 1H, NH), 11.06 (s, 1H, NH), 8.59 (d, J = 2.0 Hz, 1H), 7.95 (s, 1H, Ar), 7.39 (d, J = 8.8 Hz, 2H), 7.33 (dd, J1= 2.2 Hz, J2 = 8.4 Hz, 1H), 7.08 (d, J = 9.0 Hz, 2H), 6.94 (d, J = 8.4 Hz, 1H), 3.82 (s, 3H). HRMS (ESI+) m/z calcd. for (C18H12ClN3O4 M + H): 370.0595, found: (M + H): 370.0588.
(Z)-5-Chloro-3-(1-(4-ethoxyphenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (8h)
From 3h (0.120 g, 0.30 mmol), KOH (0.018 g, 0.32 mmol) and MeI (0.064 g, 0.45 mmol), compound 8h (0.097 g, 84%) was obtained as a white solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.66 (s, 1H, NH), 11.24 (s, 1H, NH), 8.54 (m, 1H), 7.39–7.70 (m, 3H), 7.09–7.04 (m, 2H), 6.94 (m, 1H) 4.08 (q, J = 6.9 Hz, 2H), 1.36 (t, J = 6.9 Hz, 3H). HRMS (ESI+) m/z calcd. for (C19H14ClN3O4, M + H): 084.0751, found: (M + H): 413.0781.
(Z)-5-Chloro-3-(1-(3-chloro-4-fluorophenyl)-5-oxo-2-thioxoimidazolidin-4-ylidene)indolin-2-one (8r)
From 3r (0.122 g, 0.30 mmol), KOH (0.018 g, 0.32 mmol) and MeI (0.064 g, 0.45 mmol), compound 8r (0.093 g, 79%) was obtained as a white solid. M.p. > 300 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 11.22 (s, 1H, NH), 11.10 (s, 1H, NH), 8.54 (m, 1H), 7.77 (dd, J1 = 2.2 Hz, J2 = 6.7 Hz, 1H), 7.64 (m, 1H), 7.54 (m, 1H), 7.36 (m, 1H), 6.95 (t, J = 9.0 Hz, 1H). HRMS (ESI+) m/z calcd. for (C17H9Cl2FN3O3, M + H): 392.0005, found: (M + H): 491.0998.
3.2.6. General Procedure for the Synthesis of Dispiroindolinones 9
Corresponding imidazolidin 8 (1 equiv) and sarcosine (4 equiv) were dissolved in toluene and the mixture heated to a boiling point. After that, paraformaldehyde (4 equiv) was added. The resulting mixture was refluxed for 5–8 hours (TLC control). After the reaction was completed, the solvent was evaporated in vacuo. The product was then purified using column chromatography (silica gel 60, 0.04–0.063 mm/230–400 mesh, CHCl3:MeOH/50:1) to afford products as a white solid. This solid was washed with acetone to yield corresponding dispirooxindole as a white crystalline solid.
5″-Chloro-1-(4-methoxyphenyl)-1′-methyldispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2,2″,5-trione (9f)
From 8f (0.074 g, 0.20 mmol), sarcosine (0.071 g, 0.80 mmol) and paraformaldehyde (0.026 g, 0.80 mmol), compound 9f (0.061 g, 72%) was obtained as a white solid. M.p. 295–296 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.74 (s, 1H NH), 8.80 (s, 1H, NH), 7.33 (dd, J1 = 2.2 Hz, J2 = 8.3 Hz, 1H), 7.17 (d, J = 2.0 Hz, 1H), 6.98 (d, J = 9.0 Hz, 2H), 6.90 (d, J = 8.9 Hz, 2H), 6.87 (m, 1H), 3.44–3.36 (m, 2H), 3.31 (d, J = 10.2 Hz, 1H), 3.08 (d, J = 10.3 Hz, 1H), 2.47 (s, 3H). HRMS (ESI+) m/z calcd. for (C21H19ClN4O4, M + H): 427.1173, found: (M + H): 427.1177.
5″-Chloro-1-(4-ethoxyphenyl)-1′-methyldispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2,2″,5-trione (9f)
From 8h (0.076 g, 0.20 mmol), sarcosine (0.071 g, 0.80 mmol) and paraformaldehyde (0.026 g, 0.80 mmol), compound 9h (0.055 g, 72%) was obtained as a white solid. M.p. 273–274 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.74 (s, 1H, NH), 8.80 (s, 1H, NH), 7.32 (m, 1H), 7.17 (s, 1H), 7.00–6.92 (m, 2H), 6.92–6.82 (m, 3H), 4.08–3.98 (m, 2H), 3.45–3.36 (m, 2H), 3.29 (m, 1H), 3.07 (m, 1H), 2.46 (s, 3H), 1.37–1.27 (m, 3H). HRMS (ESI+) m/z calcd. for (C22H21ClN4O4 M + H): 441.1330, found: (M + H): 441.1295.
5″-Chloro-1-(3-chloro-4-fluorophenyl)-1′-methyldispiro[imidazolidine-4,3′-pyrrolidine-4′,3″-indoline]-2,2″,5-trione (9r)
From 8r (0.078 g, 0.20 mmol), sarcosine (0.071 g, 0.80 mmol) and paraformaldehyde (0.026 g, 0.80 mmol), compound 9r (0.067 g, 75%) was obtained as a white solid. M.p. 197–198 °C. 1H-NMR (400 MHz, DMSO-d6) δ: 10.75 (s, 1H, NH), 8.99 (s, 1H, NH), 7.55 (t, J = 9.0 Hz, 1H), 7.33 (dd, J1 = 2.2 Hz, J2 = 8.3 Hz, 1H), 7.24 (dd, J1 = 2.5 Hz, J2 = 6.7 Hz, 1H), 7.15 (d, J = 2.0 Hz, 1H), 7.06 (m, 1H), 6.87 (d, J = 8.3 Hz, 1H), 3.41–3.34 (m, 3H), 3.08 (d, J = 10.2 Hz, 1H), 2.46 (s, 3H). HRMS (ESI+) m/z calcd. for (C20H16Cl2FN4O3, M + H): 449.0578, found: (M + H): 449.0589.
3.3. Biological Evaluation
MTT test. The MTT assay was carried out according to [35] with few modifications; 3000 Cells (for HEK293T, A549 and MCF7cell lines) or 4000 cells (for VA13 cell line) were seeded in each well of a 96-well plate. After 20 h incubation, the tested compounds diluted in culture medium were added to the cells and incubated 72 h at 37 °C under CO2 (5%) atmosphere. Assays were performed in triplicates. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl-tetrazolium bromide) reagent was then added to the cells up to final concentration of 0.5 g/L (10X stock solution in PBS was used) and incubated for 2 h at 37 °C (5% CO2). The MTT solution was then discarded and 140 µL of DMSO was added. The plates were swayed on a shaker (60 rpm) to solubilize the formazan. The absorbance was measured using a microplate reader at a wavelength of 565 nm. The analysis of cytotoxicity and the estimation of IC50 values were carried out with the built-in functions in the GraphPad Prism program (GraphPad Software, Inc., San Diego, CA) P53 activation.
The β-galactosidase reporter construction equipped with the p53 promotor frame [46] was used to assess the p53 expression level in p53wt A549 cell line. The compounds were tested in the concentration range of 0.5–120 μM with triple dilution steps. The incubation time was 24 h. To take into account the toxic effect of the molecules, the output signal was normalized considering the number of the cells estimated by MTT test with the same incubation tome (24 h). The output was statistically significant if the background signal was exceeded two or more times.
4. Conclusions
In the present study, a series of novel dispiro-oxindole derivatives of 2-chaicogen-imidazol-4-ones has been described. The synthesized molecules have key 3D-pharmacophore features essential for binding into the major MDM2 pocket as it has been predicted during the molecular docking study. However, these compounds have an alternative binding mode in contrast to other MDM2 inhibitors, therefore, they should be cautiously regarded as having this mechanism of action. MTT test with different cell lines, including p53 positive and negative, has not provided unambiguous results on the mechanism of action for this series although some signs of p53 activation have been observed. Nevertheless, the most active compounds from this series show fairly good cytotoxicity values (2.2–9.8 μM) on various cell lines in the MTT test, which makes them promising for further optimization and research.
Supplementary Materials
The following are available online. Figure S1: NMR spectra of synthesized compounds.
Author Contributions
Conceptualization, M.K. and Y.I.; Data curation, D.S.; Funding acquisition, D.S., Y.I. and E.B.; Investigation, V.N., V.F. and R.S.; Methodology, M.K. and V.N.; Project administration, N.Z.; Resources, D.S., Y.I. and E.B.; Supervision, E.B.; Visualization, A.M. (Anna Moiseeva) and M.V.; Writing—original draft, Y.I. and M.V.; Writing—review and editing, A.M. (Alexander Majouga) and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 21-13-00023(synthesis), Russian Foundation for Basic Research, grant numbers 19-03-00201 and 18-29-08060, and Applied Genetic Resource Faculty of MITP (Support Grant 075-15-2021-684) (biological evaluation).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in file mail.ru repository at https://cloud.mail.ru/public/svf7/4u5R7wxqq, accessed on 20 November 2021.
Acknowledgments
The-NMR and X-ray studies of this work were supported by the M.V. Lomonosov Moscow State University Program of Development.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Tsukano, C.; Takemoto, Y. Synthetic Approaches to Spiro-oxindoles and Iminoindolines Based on Formation of C2AC3 Bond. Heterocycles 2014, 89, 2271–2302. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Li, Z.; Gu, C.; Fishlock, D. Synthesis of a SpiroindolinonePyrrolidinecarboxamide MDM2 Antagonist. Org. Process. Res. Dev. 2013, 17, 247–256. [Google Scholar] [CrossRef]
- Gollner, A.; Rudolph, D.; Arnhof, H.; Bauer, M.; Blake, S.M.; Boehmelt, G.; Cockroft, X.-L.; Dahmann, G.; Ettmayer, P.; Gerstberger, T.; et al. Discovery of Novel Spiro[3 H-Indole-3,2-pyrrolidin]-2(1H)-one Compounds as Chemically Stable and Orally Active Inhibitors of the MDM2–p53 Interaction. J. Med. Chem. 2016, 59, 10147–10162. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, Q.; Liu, J.-J.; Zhang, J.; Jiang, N.; Chu, X.-J.; Bartkovitz, D.; Luk, K.-C.; Janson, C.; Tovar, C.; et al. Discovery of Potent and Selective Spiroindolinone MDM2 Inhibitor, RO8994, for Cancer Therapy. Bioorg. Med. Chem. 2014, 22, 4001–4009. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Wang, G.; Qiu, S.; Shangary, S.; Gao, W.; Qin, D.; Stuckey, J.; Krajewski, K.; et al. Structure-Based Design of Spiro-Oxindoles as Potent, SpecificSmall-Molecule Inhibitors of the MDM2p53 Interaction. J. Med. Chem. 2006, 49, 3432–3435. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-Spirooxindole Natural Products as Inspirations for the Development of Potential Therapeutic Agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef] [PubMed]
- Poulos, Z. Spirooxindole Alkaloids; Lambert Academic Publishing: Weinheim, Germany, 2014; pp. 1–280. [Google Scholar]
- Pavlovska, T.L.; Redkin, R.; Lipson, V.V.; Atamanuk, D.V. Molecular Diversity of Spirooxindoles. Synthesis and Biological Activity. Mol. Divers. 2016, 20, 299–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising Scaffolds for Anticancer Agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef]
- Santos, M.M.M. Recent Advances in the Synthesis of Biologically Active Spirooxindoles. Tetrahedronn 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Wu, X.; Bayle, J.H.; Olson, D.; Levine, A.J. Thep53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993, 7, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Lane, D.P.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Levine, A.J. p53, the Cellular Gatekeeper for Growth and Division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Monterrey, I.; Bertamino, A.; Portaб, A.; Carotenuto, A.; Musella, S.; Aquino, C.; Granata, I.; Sala, M.; Brancaccio, D.; Picone, D.; et al. Identification of the Spiro(Oxindole-3,30-thiazo-lidine)-Based Derivatives as Potential p53 Activity Modulators. J. Med. Chem. 2010, 53, 8319–8329. [Google Scholar] [CrossRef]
- Nakamaru, K.; Seki, T.; Tazaki, K.; Tse, A. Preclinical Characterization of a Novel Orally-Available MDM2 Inhibitor DS-3032b: Anti-Tumor Profile and Predictive Biomarkers for Sensitivity. Mol. Cancer Ther. 2015, 14, B5. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.; Zhao, Y.; McEachern, D.; Meaux, I.; Barrierem, C.; Stuckey, J.A.; Meagher, J.L.; Bai, L.; Liu, L.; et al. SAR405838: An Optimized Inhibitor of MDM2-p53 Interaction That Induces Complete and Durable Tumor Regression. Cancer Res. 2014, 74, 5855–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Cai, L.; Chen, C.; Xie, X.; Zhao, Q.; Zhao, X.; Zhou, H.; Han, B.; Peng, C. Computational Analysis of Spiro-Oxindole Inhibitors of the MDM2-p53 Interaction: Insights and Selection of Novel Inhibitors. J. Biomol. Struct. Dyn. 2016, 34, 341–351. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Liu, J.-J.; Jiang, N.; Zhang, J.; Ross, T.M.; Chu, X.-J.; Bartkovitz, D.; Podlaski, F.; Janson, C.; et al. Discovery of RG7388, a Potent and Selective p53–MDM2 Inhibitor in Clinical Development. J. Med. Chem. 2013, 56, 5979–5983. [Google Scholar] [CrossRef]
- Shangary, S.; Qin, D.; McEachern, D.; Liu, M.; Miller, R.S.; Qiu, S.; Nikolovska-Coleska, Z.; Ding, K.; Wang, G.; Chen, J.; et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc. Natl. Acad. Sci. USA 2008, 105, 3933–3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.; Liao, W.; Zeng, S.X.; Lu, H. Reviving the guardian of the genome: Small molecule activators of p53. Pharmacol. Ther. 2017, 178, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Anil, B.; Riedinger, C.; Endicott, J.A.; Noble, M.E. The structure of an MDM2-Nutlin-3a complex solved by the use of a validated MDM2 surface-entropy reduction mutant. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 1358–1366. [Google Scholar] [CrossRef]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-based design of potent non-peptide MDM2 inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Vasilevski, S.V.; Beloglazkina, E.K.; Kukushkin, M.E.; Machulkin, A.E.; Veselov, M.S.; Chufarova, N.V.; Vanzcool, A.; Zyk, N.V.; Skvortsov, D.A.; et al. Design, Synthesis and Biological Evaluation of Novel Potent MDM2/p53 Small-Molecule Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 404–409. [Google Scholar] [CrossRef]
- Kukushkin, M.E.; Skvortsov, D.A.; Kalinina, M.A.; Tafeenko, V.A.; Burmistrov, V.V.; Butov, G.M.; Zyk, N.V.; Majouga, A.G.; Beloglazkina, E.K. Synthesis and cytotoxicity of oxindoles dispiro derivatives with thiohydantoin and adamantane fragments. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 544–555. [Google Scholar] [CrossRef]
- Beloglazkina, A.A.; Karpov, N.A.; Mefedova, S.R.; Polyakov, V.S.; Skvortsov, D.A.; Kalinina, M.A.; Tafeenko, V.A.; Majouga, A.G.; Zyk, N.V.; Beloglazkina, E.K. Synthesis of dispirooxindoles containing n-unsubstituted heterocyclic moieties and study of their anticancer activity. Russ. Chem. Bull. 2019, 68, 1006–1013. [Google Scholar] [CrossRef]
- Novotortsev, V.K.; Kukushkin, M.E.; Tafeenko, V.A.; Skvortsov, D.A.; Kalinina, M.A.; Timoshenko, R.V.; Chmelyuk, N.S.; Vasilyeva, L.A.; Tarasevich, B.N.; Gorelkin, P.V.; et al. Dispirooxindoles based on 2-selenoxo-imidazolidin-4-ones: Synthesis, cytotoxicity and ROS generation ability. IJMS 2021, 22, 2613. [Google Scholar] [CrossRef] [PubMed]
- Beloglazkina, A.; Barashkin, A.; Polyakov, V.; Kotovsky, G.; Karpov, N.; Mefedova, S.; Zagribelny, B.; Ivanenkov, Y.; Kalinina, M.; Skvortsov, D.; et al. Synthesis and biological evaluation of novel dispiro compounds based on 5-arylidenehydantoins and isatins as inhibitors of p53–mdm2 protein–protein interaction. Chem. Heterocycl. Compd. 2020, 56, 5613. [Google Scholar] [CrossRef]
- Majouga, A.G.; Beloglazkina, E.K.; Beloglazkina, A.A.; Kukushkin, M.E.; Ivanenkov, Y.A.; Veselov, M.S. New Dispiro-Indolinones, Inhibitors of MDM2/p53 Interaction, Method of Synthesis and Application. Patent #RU2629750C2, 9 April 2015. [Google Scholar]
- Shangary, S.; Ding, K.; Qiu, S.; Nikolovska-Coleska, Z.; Bauer, J.A.; Liu, M.; Wang, G.; Lu, Y.; McEachern, N.; Bernard, D.; et al. Reactivation of p53 by a specific MDM2 antagonist (MI-43) leads to p21-mediated cell cycle arrest and selective cell death in colon cancer. Mol. Cancer Ther. 2008, 7, 1533–1542. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, O.Y.; Antipin, R.L.; Udina, A.U.; Krasnovskaya, O.O.; Beloglazkina, E.K.; Terenin, V.I.; Oteliansky, V.E.; Zyk, N.V.; Majouga, A.G. An improved protocol for synthesis of 3-substituted 5-arylidene-2-thiohydantoins: Two-step procedure alternative to classical methods. J. Heterocyclic Chem. 2016, 53, 1570–1577. [Google Scholar] [CrossRef]
- Wirth, T. Small Organoselenium Compounds: More than just Glutathione Peroxidase Mimics. Angew. Chem. Int. Ed. 2015, 54, 10074–10076. [Google Scholar] [CrossRef]
- Müller, A.; Cadenas, E.; Graf, P.; Sies, H. A novel biologically active seleno-organic compound-1. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem. Pharmacol. 1984, 33, 3235–3239. [Google Scholar] [CrossRef]
- Schewe, T. Molecular actions of ebselen-an antiinflammatory antioxidant. Gen. Pharmacol. 1995, 26, 1153–1169. [Google Scholar] [CrossRef]
- Novotortsev, V.K.; Kukushkin, M.E.; Tafeenko, V.A.; Zyk, N.V.; Beloglazkina, E.K. New spiro-linked indolinonepyrrolidineselenoxoimidazolones. Mendeleev Commun. 2020, 30, 320–321. [Google Scholar] [CrossRef]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Hietanen, S.; Lain, S.; Krausz, E.; Blattner, C.; Lane, D.P. Activation of p53 in cervical carcinoma cells by small molecules. Proc. Natl. Acad. Sci. USA 2000, 97, 8501–8506. [Google Scholar] [CrossRef] [Green Version]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Arriola, E.L.; Lopez, A.R.; Chresta, C.M. Differential regulation of p21waf-1/cip-1 and Mdm2 by etoposide: Etoposide inhibits the p53-Mdm2 autoregulatory feedback loop. Oncogene 1999, 18, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Rosenthal, J.; Andreeff, M.; Zernovak, O.; Kumar, P.; Gajee, R.; Chen, S.; Rosen, M.; Song, S.; Kochan, J.; et al. Phase 1 Dose Escalation Study of MDM2 Inhibitor DS-3032b in Patients with Hematological Malignancies-Preliminary Results. Blood 2016, 128, 593. [Google Scholar] [CrossRef]
- Aguilar, A.; Lu, J.; Liu, L.; Du, D.; Bernard, D.; McEachern, D.; Przybranowski, S.; Li, X.; Luo, R.; Wen, B.; et al. Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017, 60, 2819–2839. [Google Scholar] [CrossRef] [PubMed]
- Bertamino, A.; Soprano, M.; Musella, S.; Rusciano, M.R.; Sala, M.; Vernieri, E.; Di Sarno, V.; Limatola, A.; Carotenuto, A.; Cosconati, S.; et al. Synthesis, in Vitro, and in Cell Studies of a New Series of [Indoline-3,2′-thiazolidine]-Based p53 Modulators. J. Med. Chem. 2013, 56, 5407–5421. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, C.; Bressac-de Pailleret, B.; Lefrere, I.; Ronsi, M.; Feunteun, J.; Tursz, T.; Wiels, J. Overexpression of MDM2, due to enhanced translation, results in inactivation of wild-type p53 in Burkitt’s lymphoma cells. Oncogene 1998, 16, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Kussie, P.H.; Gorina, S.; Marechal, V.; Elenbaas, B.; Moreau, J.; Levine, A.J.; Pavletich, N.P. Structure of the MDM2 Oncoprotein Bound to the p53 Tumor Suppressor Transactivation Domain. Science 1996, 274, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Molsoft L.L.C. Available online: https://www.molsoft.com (accessed on 31 January 2018).
- Kravchenko, J.E.; Ilyinskaya, G.V.; Komarov, P.G.; Agapova, L.S.; Kochetkov, D.V.; Strom, E.; Frolova, E.I.; Kovriga, I.; Gudkov, A.V.; Feinstein, E.; et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 6302–6307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, B.; Thompson, T.; Xia, M.; Janson, C.; Lukacs, C.; Deo, D.; Di Lello, P.; Fry, D.; Garvie, C.; Huang, K.S.; et al. Activation of the p53 pathway by small-molecule-induced MDM2 and MDMX dimerization. Proc. Natl. Acad. Sci. USA 2012, 109, 11788–11793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majouga, A.G.; Beloglazkina, E.K.; Vatsadze, S.Z.; Moiseeva, A.A.; Moiseev, F.S.; Butin, K.P.; Zyk, N.V. The first example of a reversibly reducible Co-II complex with an anionic 2-thiohydantoin-type ligand. Mend. Commun. 2005, 15, 48–50. [Google Scholar] [CrossRef]
- Beloglazkina, E.K.; Majouga, A.G.; Moiseeva, A.A.; Zyk, N.V.; Zefirov, N.S. Oxidation of triphenylphosphine and norbornene by nitrous oxide in the presence of CoIILCl2 [L = 3-phenyl-5-(2-pyridylmethylidene)-2-thiohydantoin]: The first example of CoII-catalyzed alkene oxidation by N2O. Mend. Commun. 2009, 19, 69–71. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).