Yukmijihwang-Tang Suppresses Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Mediated Bone Loss
Abstract
:1. Introduction
2. Results
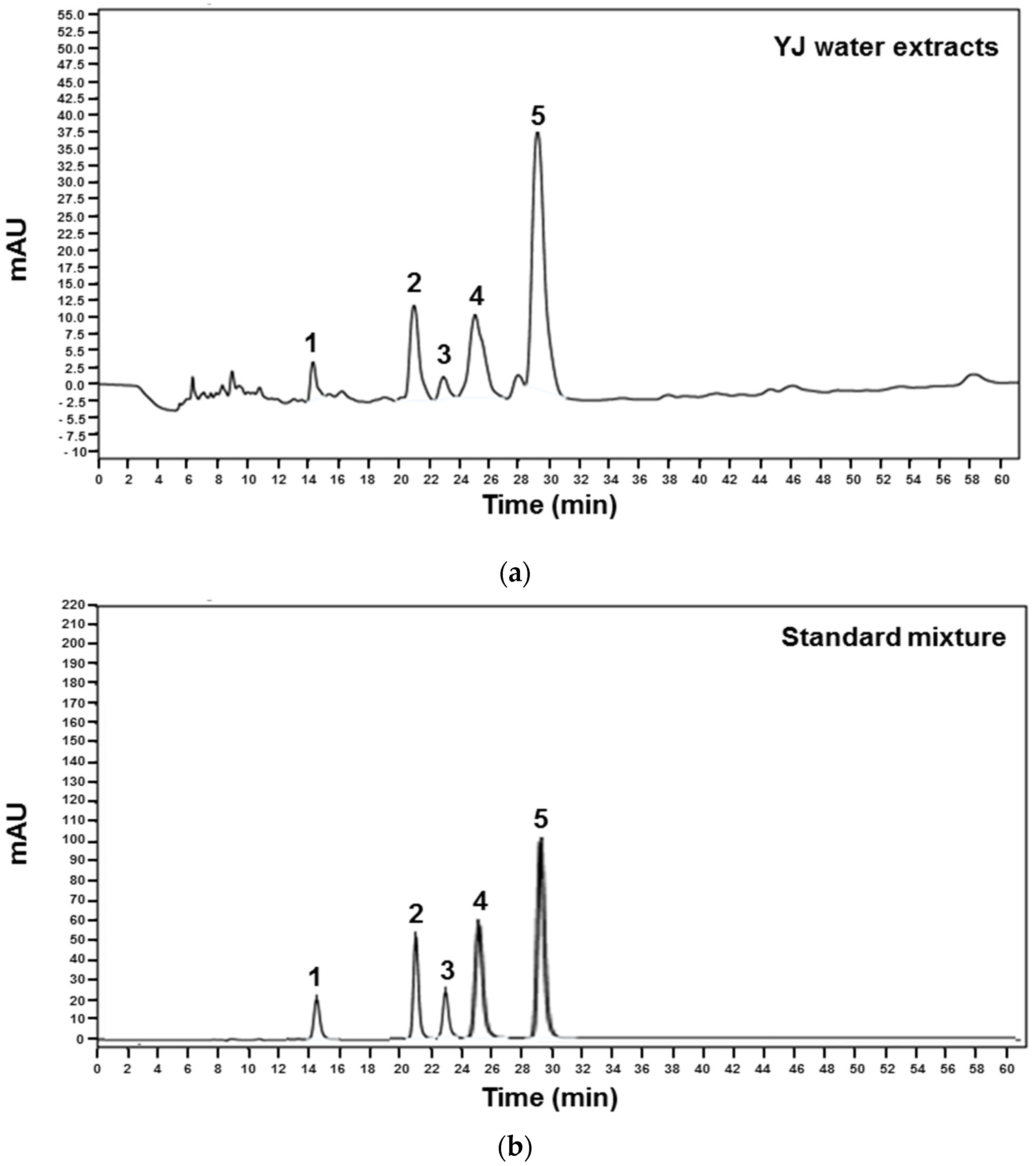
2.1. High-Performance Liquid Chromatography (HPLC) Analysis of YJ Water Extracts
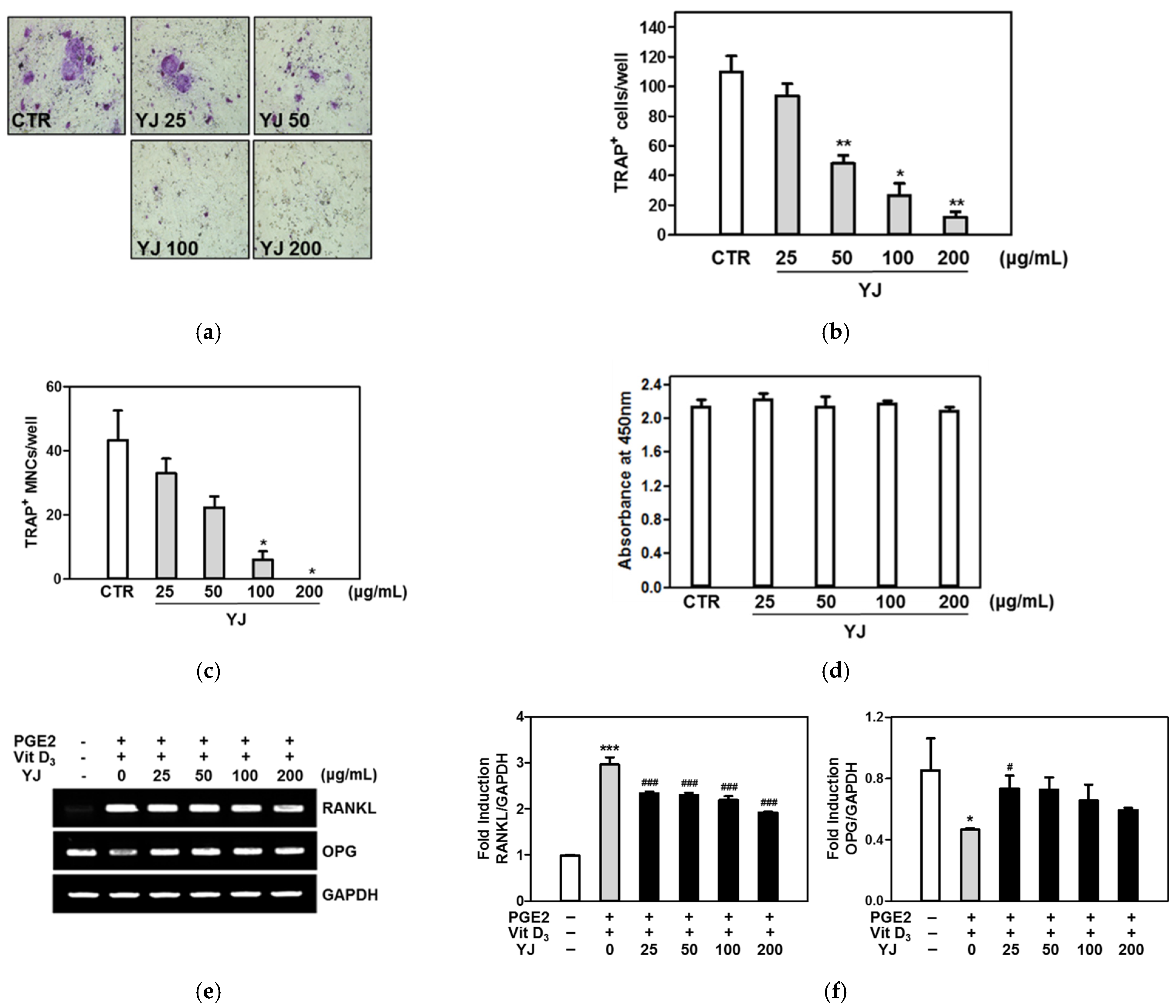
2.2. YJ Inhibited Osteoclast Differentiation in the Co-Culture System of BMCs and Osteoblasts
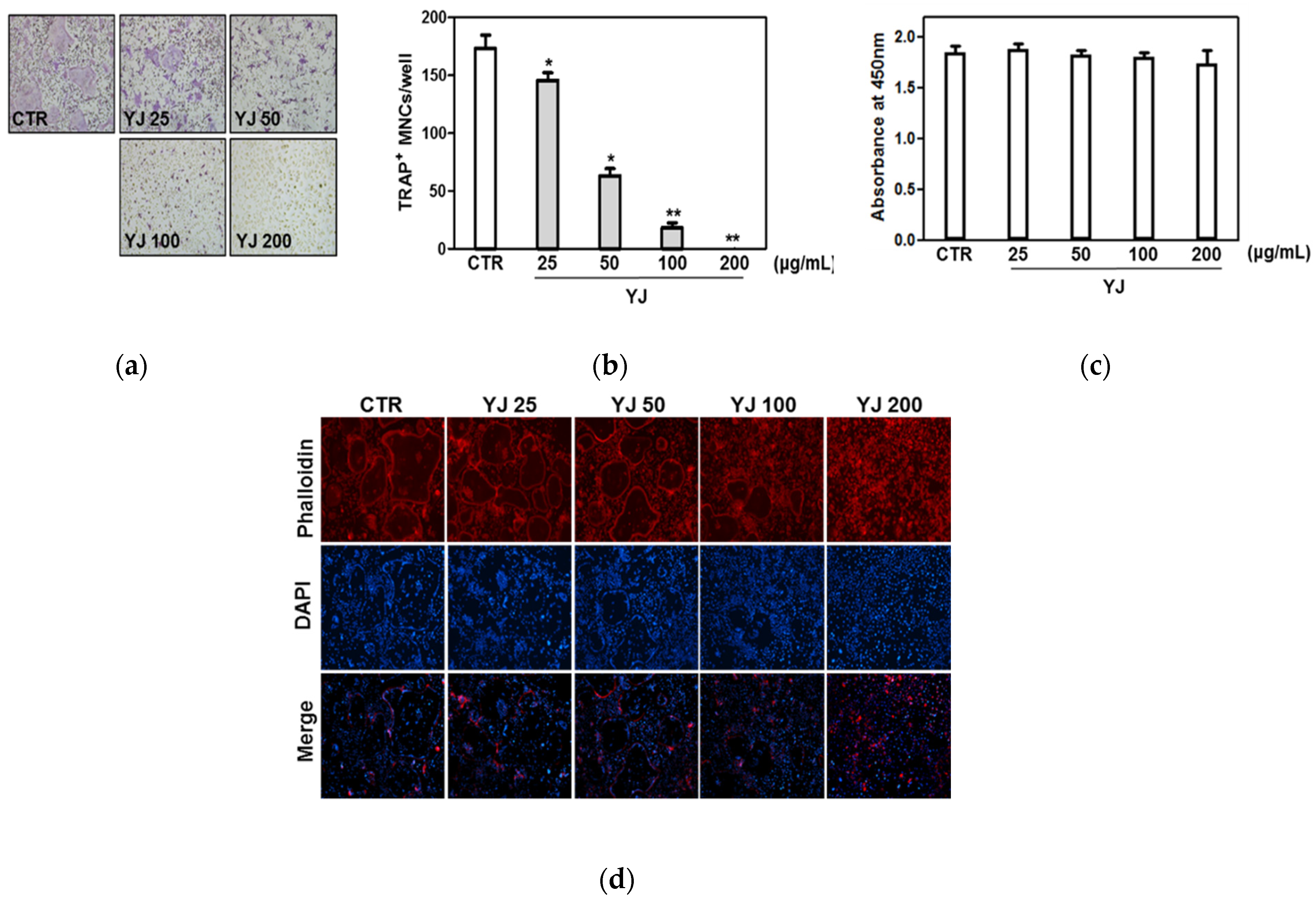
2.3. YJ Inhibited RANKL-Mediated Osteoclast Differentiation in BMMs
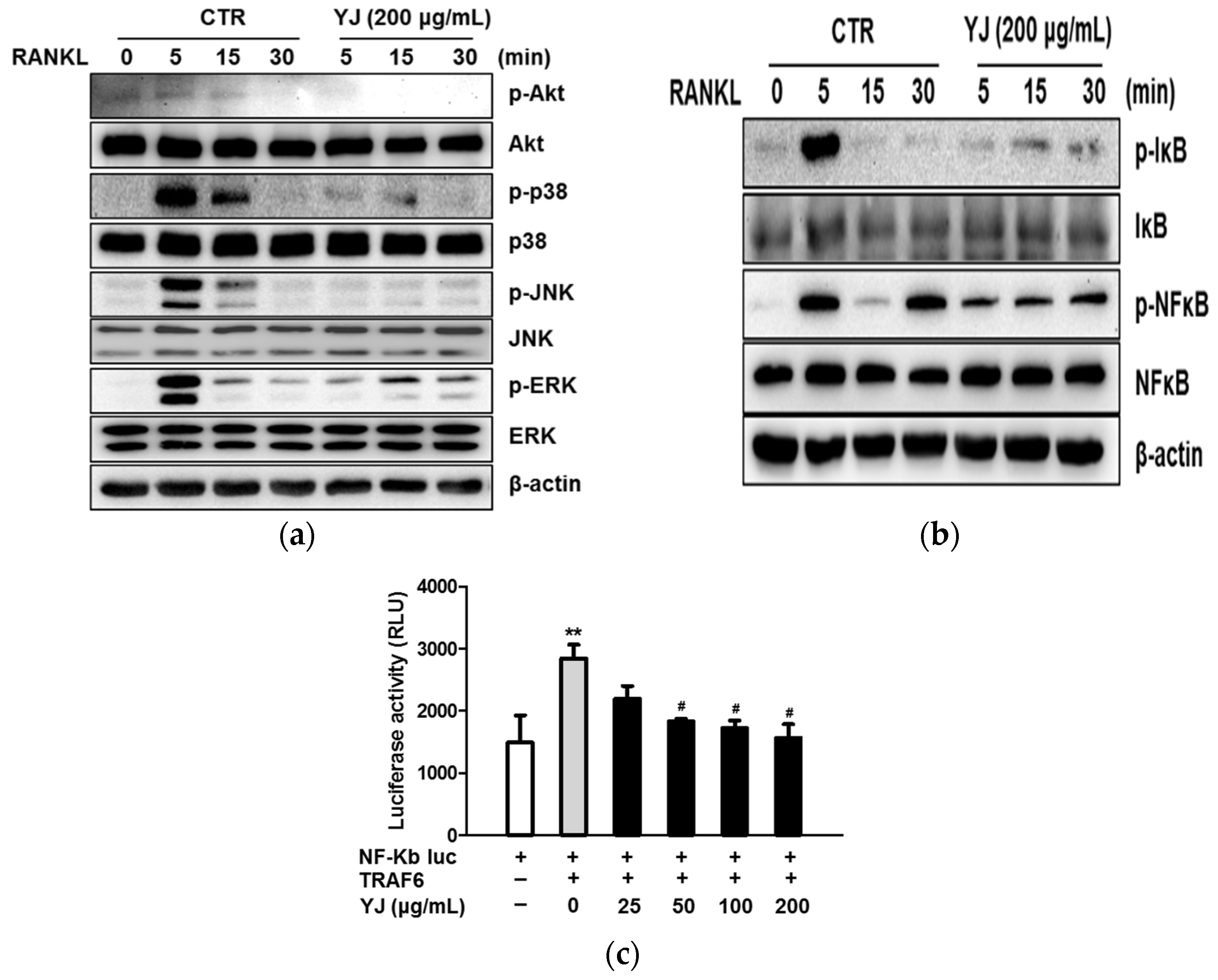
2.4. YJ Inhibited RANKL-Mediated MAPK Signaling Pathway in BMMs
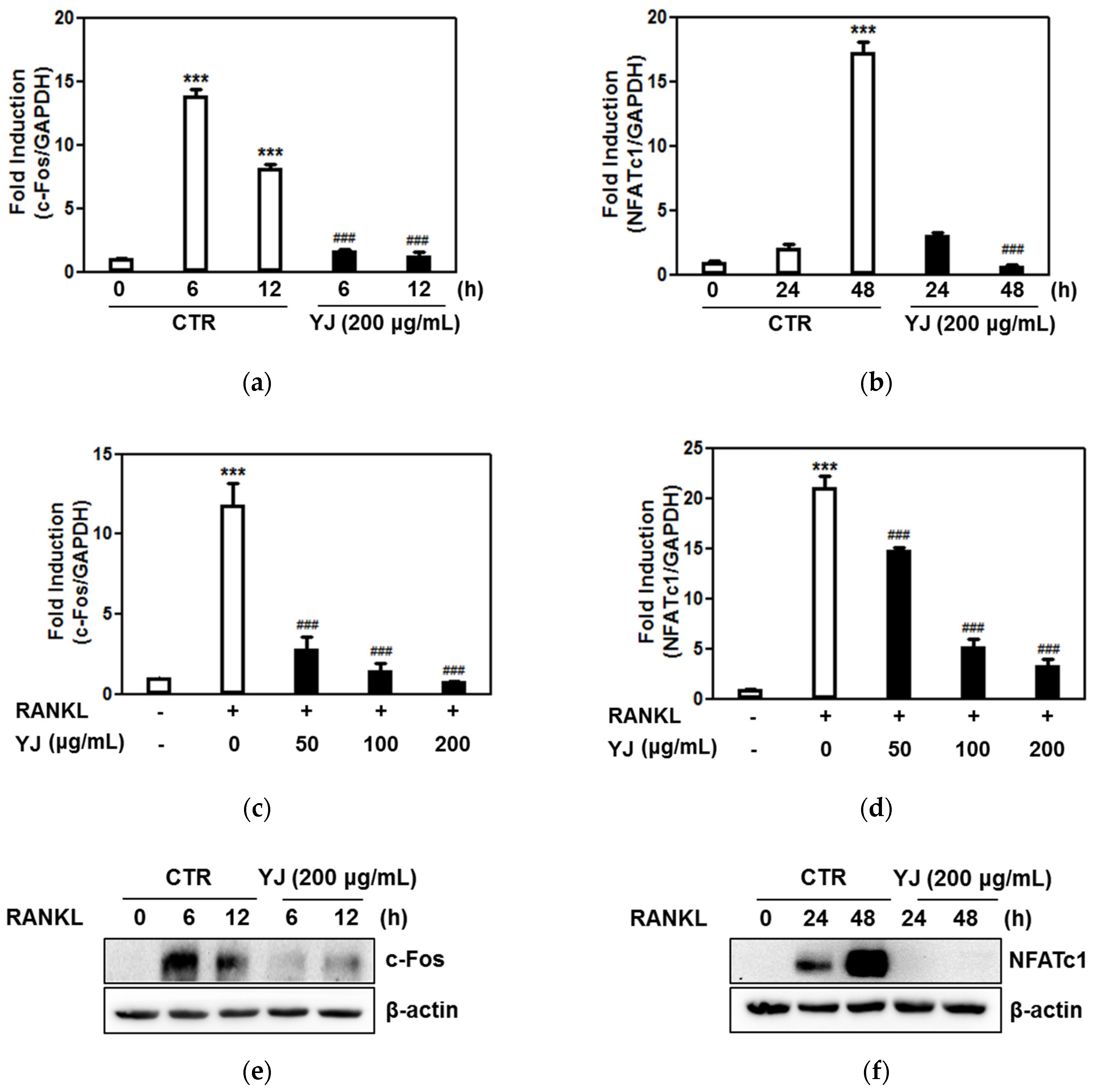
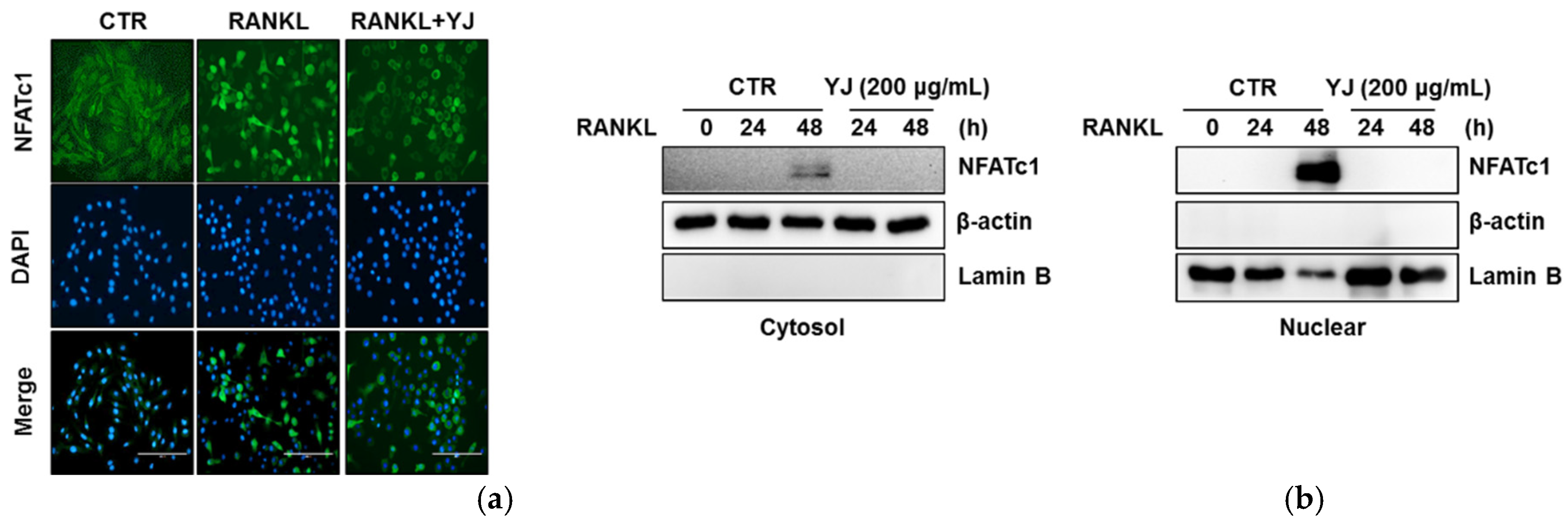
2.5. YJ Inhibited RANKL-Mediated NFATc1 and c-Fos Expression in BMMs
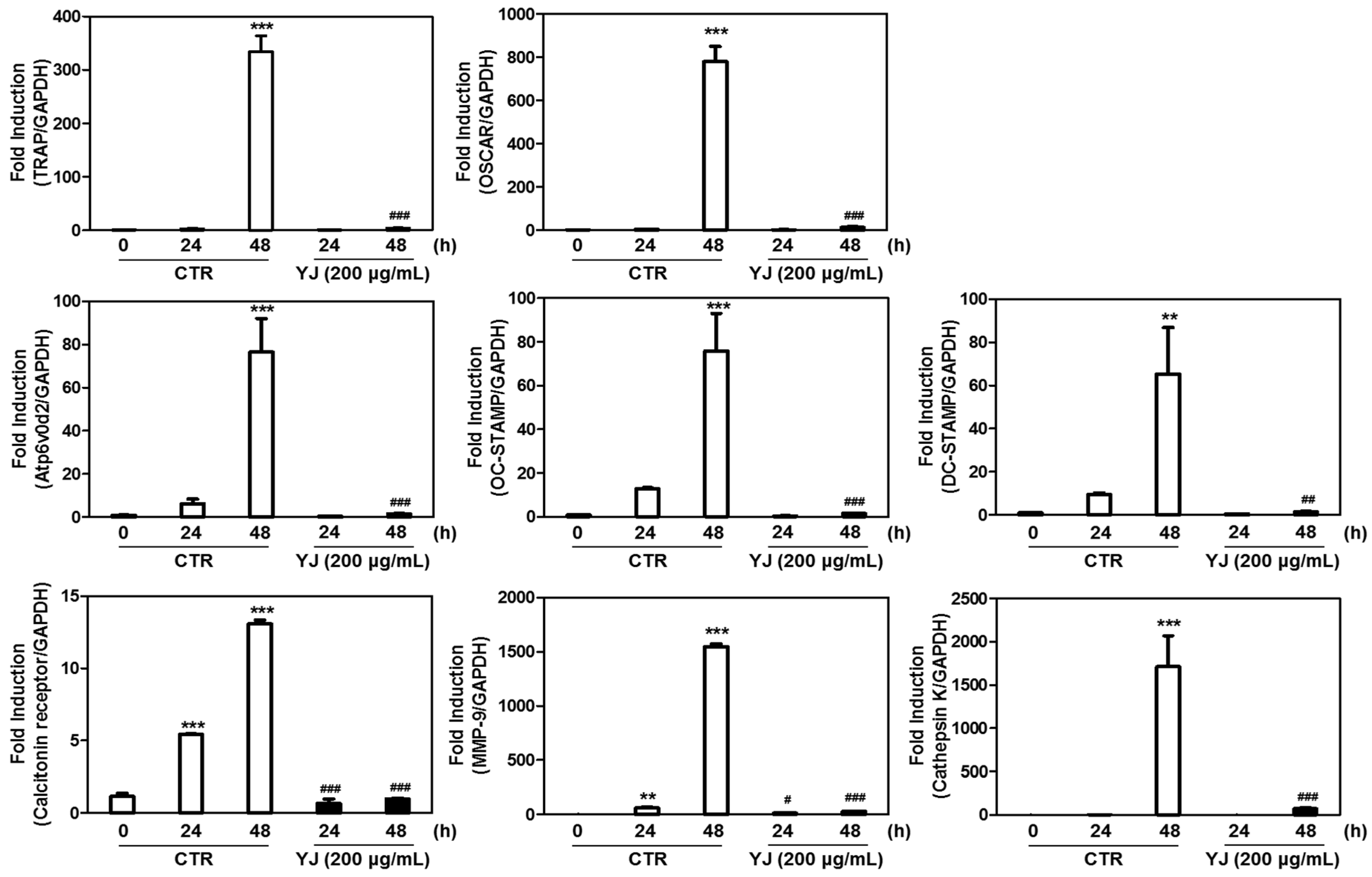
2.6. YJ Reduced RANKL-Mediated Expression of Osteoclast-Related Genes in BMMs
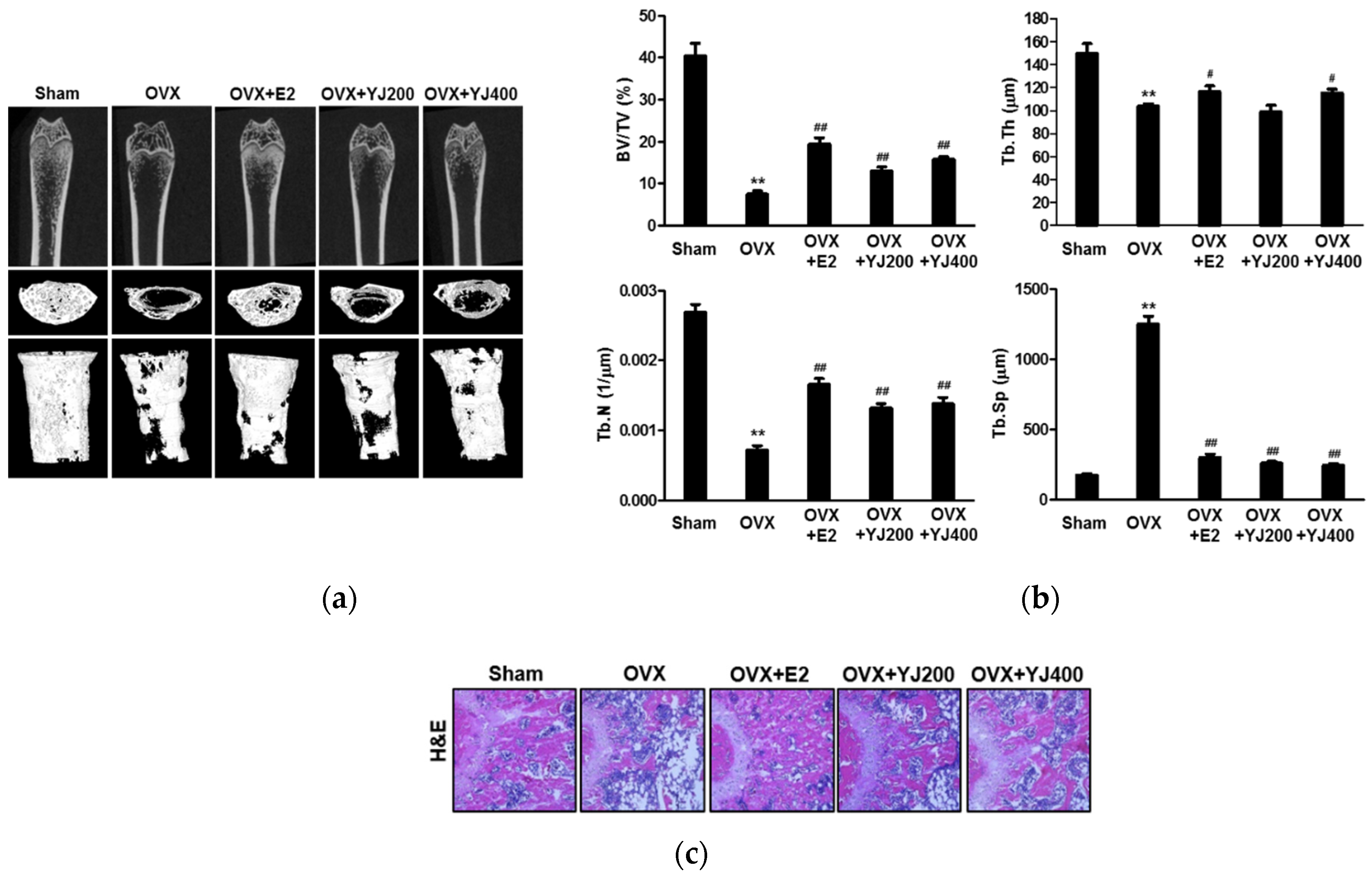
2.7. YJ Led to Increased Bone Density in the OVX Rat Model
3. Discussion
4. Materials and Methods
4.1. Animals and Reagents
4.2. Preparation of YJ
4.3. HPLC Analysis
4.4. Cell Culture and Osteoclast Differentiation
4.5. Co-Culture of Osteoblastic and BMCs
4.6. F-Actin Ring Staining
4.7. Cytotoxicity Assay
4.8. Quantitative Real-Time RT-PCR Analysis and RT-PCR
4.9. Western Blotting
4.10. Luciferase Reporter Assay
4.11. Immunocytochemistry Analysis
4.12. OVX-Mediated Bone Loss Model
4.13. Micro-CT and Histological Analysis
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lerner, U. Bone Remodeling in Post-menopausal Osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, M.T.; Felson, D.T.; Dawson-Hughes, B.; Tucker, K.L.; Cupples, L.A.; Wilson, P.W.F.; Kiel, D.P. Risk Factors for Longitudinal Bone Loss in Elderly Men and Women: The Framingham Osteoporosis Study. J. Bone Miner. Res. 2010, 15, 710–720. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canalis, E. Management of endocrine disease: Novel anabolic treatments for osteoporosis. Eur. J. Endocrinol. 2018, 178, R33–R44. [Google Scholar] [CrossRef]
- Fierro, F.A.; Nolta, J.A.; Adamopoulos, I.E. Concise Review: Stem Cells in Osteoimmunology. Stem Cell 2017, 35, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Takahashi, N.; Udagawa, N.; Tamura, T.; Akatsu, T.; Stanley, E.R.; Kurokawa, T.; Suda, T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J. Clin. Investig. 1993, 91, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Fuller, K.; Wong, B.; Fox, S.; Choi, Y.W.; Chambers, T.J. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J. Exp. Med. 1998, 188, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.P. M-CSF, c-Fms, and Signaling in Osteoclasts and their Precursors. Ann. N. Y. Acad. Sci. 2006, 1068, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.R.; Besser, D.; Kim, N.; Arron, J.; Vologodskaia, M.; Hanafusa, H.; Choi, Y. TRANCE, a TNF Family Member, Activates Akt/PKB through a Signaling Complex Involving TRAF6 and c-Src. Mol. Cell 1999, 4, 1041–1049. [Google Scholar] [CrossRef]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-I.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Ovitt, C.; Grigoriadis, A.E. Bone and haematopoietic defects in mice lacking c-fos. Nature 1992, 360, 741–745. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J.-I. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravallese, E.M.; Harada, Y.; Wang, J.T.; Gorn, A.H.; Thornhill, T.S.; Goldring, S.R. Identification of cell types respon-sible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am. J. Pathol. 1998, 152, 943. [Google Scholar]
- Barrow, A.D.; Raynal, N.; Andersen, T.L.; Slatter, D.A.; Bihan, D.; Pugh, N.; Cella, M.; Kim, T.; Rho, J.; Negishi-Koga, T.; et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Investig. 2011, 121, 3505–3516. [Google Scholar] [CrossRef] [Green Version]
- Okada, Y.; Naka, K.; Kawamura, K.; Matsumoto, T.; Nakanishi, I.; Fujimoto, N.; Sato, H.; Seiki, M. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: Implications for bone resorption. Lab. Investig. 1995, 72, 311–322. [Google Scholar]
- Nakashima, T.; Takayanagi, H. Osteoimmunology: Crosstalk between the Immune and Bone Systems. J. Clin. Immunol. 2009, 29, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.-H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 Induces Osteoclast Fusion via Up-Regulation of Atp6v0d2 and the Dendritic Cell-Specific Transmembrane Protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuhong, H.; Qian, L.; Yu, L.; Yingqiang, Z.; Yanfen, L.; Shujing, Y.; Shufang, Q.; Lanjun, S.; Shuxuan, Z.; Baohe, W. An n-of-1 Trial Service in Clinical Practice: Testing the Effectiveness of Liuwei Dihuang Decoction for Kidney-Yin Deficiency Syndrome. Evid.-Based Complement. Altern. Med. 2013, 2013, 827915. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.G.; Sohn, E.J.; Moon, M.K.; Mun, Y.J.; Woo, W.H.; Kim, M.K.; Lee, H.S. Yukmijihwang-tang ameliorates is-chemia/reperfusion-induced renal injury in rats. J. Ethnopharmacol. 2006, 104, 47–53. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Yang, S.; Qiao, H.; Zhou, W.; Zhang, Y. Liuwei Dihuang decoction facilitates the induction of long-term potentiation (LTP) in senescence accelerated mouse/prone 8 (SAMP8) hippocampal slices by inhibiting volt-age-dependent calcium channels (VDCCs) and promoting N-methyl-d-aspartate receptor (NMDA) receptors. J. Ethnopharmacol. 2012, 140, 384–390. [Google Scholar]
- Perry, B.; Zhang, J.; Sun, C.; Saleh, T.; Wang, Y. Liuwei Dihuang Lowers Body Weight and Improves Insulin and Leptin Sensitivity in Obese Rats. Evid.-Based Complement. Altern. Med. 2011, 2012, 847167. [Google Scholar] [CrossRef]
- Song, X.-Y.; Chen, Q.; Qi, X.-Y. Effect of liuwei dihuang pill on erythrocyte aldose reductase activity in early diabetic nephropathy patients. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi = Chin. J. Integr. Tradit. West. Med. 2004, 24, 1087–1090. [Google Scholar]
- Szeto, Y.-T.; Lei, P.-C.; Ngai, K.-L.; Yiu, A.T.-W.; Chan, C.S.-P.; Kok, E.W.-F.; Leong, C.-W. An in vitro study of the antioxidant activities and effect on human DNA of the Chinese herbal decoction ‘Liu Wei Di Huang’. Int. J. Food Sci. Nutr. 2009, 60, 1–6. [Google Scholar] [CrossRef]
- Shim, K.S.; Ma, C.J.; Kim, D.S.; Ma, J.Y. Yukmijihwang-tang inhibits receptor activator for nuclear Factor-kappaB lig-and-induced osteoclast differentiation. J. Med. Food 2011, 14, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Kim, N.-I.; Lee, T.-K.; Lee, D.-N.; Kim, J.-K.; Lee, I.-S.; Kim, C.-H. Herbal formulation, Yukmi-jihang-tang-Jahage, regulates bone resorption by inhibition of phosphorylation mediated by tyrosine kinase Src and cyclooxygenase expression. J. Ethnopharmacol. 2006, 106, 333–343. [Google Scholar] [CrossRef]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor ne-crosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Dai, J.; Zhu, Y.; Zhong, W.; Lu, S.; Chen, H.; Chai, Y. Paeoniflorin regulates osteoclastogenesis and osteoblastogenesis via manipulating NF-κB signaling pathway both in vitro and in vivo. Oncotarget 2017, 9, 7372–7388. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.Y.; Lin, H.Y.; Fong, Y.C.; Wu, J.B.; Chen, Y.F.; Tsuzuki, M.; Tang, C.H. Paeonol inhibits RANKL-induced osteoclastogenesis by inhibiting ERK, p38 and NF-kappaB pathway. Eur. J. Pharmacol. 2008, 588, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-L.; Zhang, Y.-H.; Cai, J.-P.; Zhu, L.-H.; Ge, W.-J.; Zhang, X. 5-(Hydroxymethyl)-2-furaldehyde Inhibits Adipogenic and Enhances Osteogenic Differentiation of Rat Bone Mesenchymal Stem Cells. Nat. Prod. Commun. 2014, 9, 529–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, H.; Lee, J.K.; Lee, H.Y.; Koh, W.S.; Seo, C.S.; Lee, M.Y.; Huang, D.S.; Shin, H. Safety Evaluation of Yukmiji-hwang-tang: Assessment of Acute and Subchronic Toxicity in Rats. Evid. Based Complement. Alternat. Med. 2011, 2011, 672136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.-M.; Lee, S.H.; Lee, D.; Lee, J.H.; Chang, G.T.; Kim, H.; Lee, J.Y. The Korean herbal formulation Yukmiji-hwangtang stimulates longitudinal bone growth in animal models. BMC Complement. Altern. Med. 2017, 17, 239. [Google Scholar] [CrossRef]
| Time (min) | A | B | Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 15 | 85 | 0.4 |
| 30 | 40 | 60 | 0.4 |
| 40 | 60 | 40 | 0.4 |
| 60 | 100 | 0 | 0.4 |
| Target Gene | Primer Sequence (5′–3′) | |
|---|---|---|
| c-Fos | Forward | CTGGTGCAGCCCACTCTGGTC |
| Reverse | CTTTCAGCAGATTGGCAATCTC | |
| NFATc1 | Forward | CAACGCCCTGACCACCGATAG |
| Reverse | GGCTGCCTTCCGTCTCATAGT | |
| TRAP | Forward | ACTTCCCCAGCCCTTACTAC |
| Reverse | TCAGCACATAGCCCACACCG | |
| OSCAR | Forward | GAACACCAGAGGCTATGACT |
| Reverse | CCGTGGAGCTGAGGAAAAGG | |
| Atp6v0d2 | Forward | TCAGATCTCTTCAAGGCTGTGCTG |
| Reverse | GTGCCAAATGAGTTCAGAGTGATG | |
| OC-STAMP | Forward | TCACTGACCTGCGTTTCGACAA |
| Reverse | GCGTAGGCCTGTAGCCACCAA | |
| DC-STAMP | Forward | GCAAGGACCCCAAGGAGTCG |
| Reverse | CAGTTGGCCCAGAAAGAGGG | |
| Cathepsin K | Forward | ACGGAGGCATTGACTCTGAAGATG |
| Reverse | GTTGTTCTTATTCCGAGCCAAGAG | |
| Calcitonin receptor | Forward | TGGTTGAGGTTGTGCCCA |
| Reverse | CTCGTGGGTTTGCCTCATC | |
| MMP-9 | Forward | TCCAACCTCACGGACACCC |
| Reverse | AGCAAAGCCGGCCGTAGA | |
| GAPDH | Forward | ACCACAGTCCATGCCATCAC |
| Reverse | TCCACCACCCTGTTGCTGTA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-Y.; Kim, Y.-K. Yukmijihwang-Tang Suppresses Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Mediated Bone Loss. Molecules 2021, 26, 7579. https://doi.org/10.3390/molecules26247579
Han S-Y, Kim Y-K. Yukmijihwang-Tang Suppresses Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Mediated Bone Loss. Molecules. 2021; 26(24):7579. https://doi.org/10.3390/molecules26247579
Chicago/Turabian StyleHan, Sang-Yong, and Yun-Kyung Kim. 2021. "Yukmijihwang-Tang Suppresses Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Mediated Bone Loss" Molecules 26, no. 24: 7579. https://doi.org/10.3390/molecules26247579
APA StyleHan, S.-Y., & Kim, Y.-K. (2021). Yukmijihwang-Tang Suppresses Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)-Induced Osteoclast Differentiation and Prevents Ovariectomy (OVX)-Mediated Bone Loss. Molecules, 26(24), 7579. https://doi.org/10.3390/molecules26247579






