Energetic Co-Crystal of a Primary Metal-Free Explosive with BTF. Ideal Pair for Co-Crystallization
Abstract
1. Introduction
2. Experimental and Computational Part
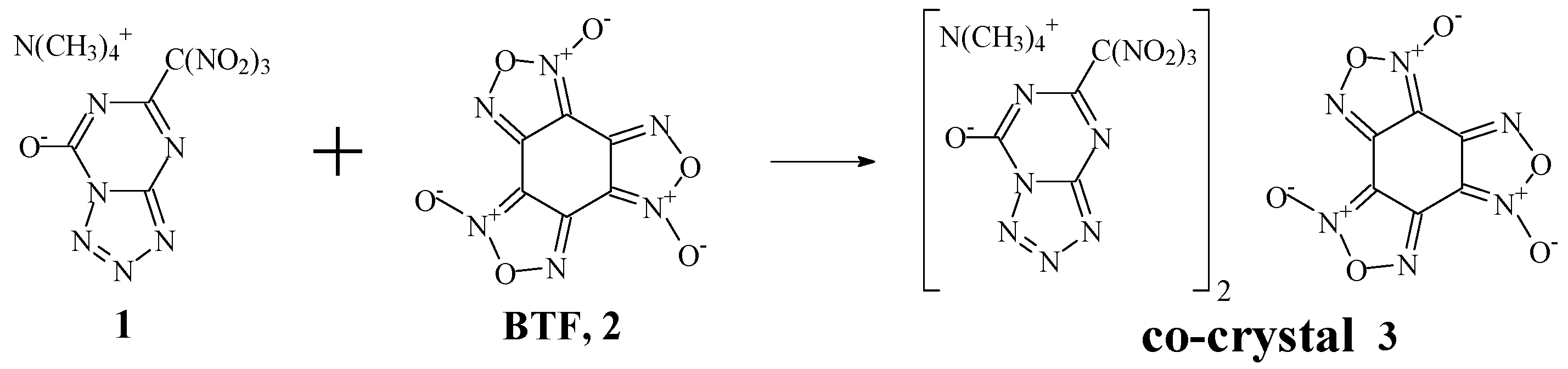
2.1. Preparation of the Co-Crystal 3
2.2. Thermal Analysis
2.3. Sensitivity Test
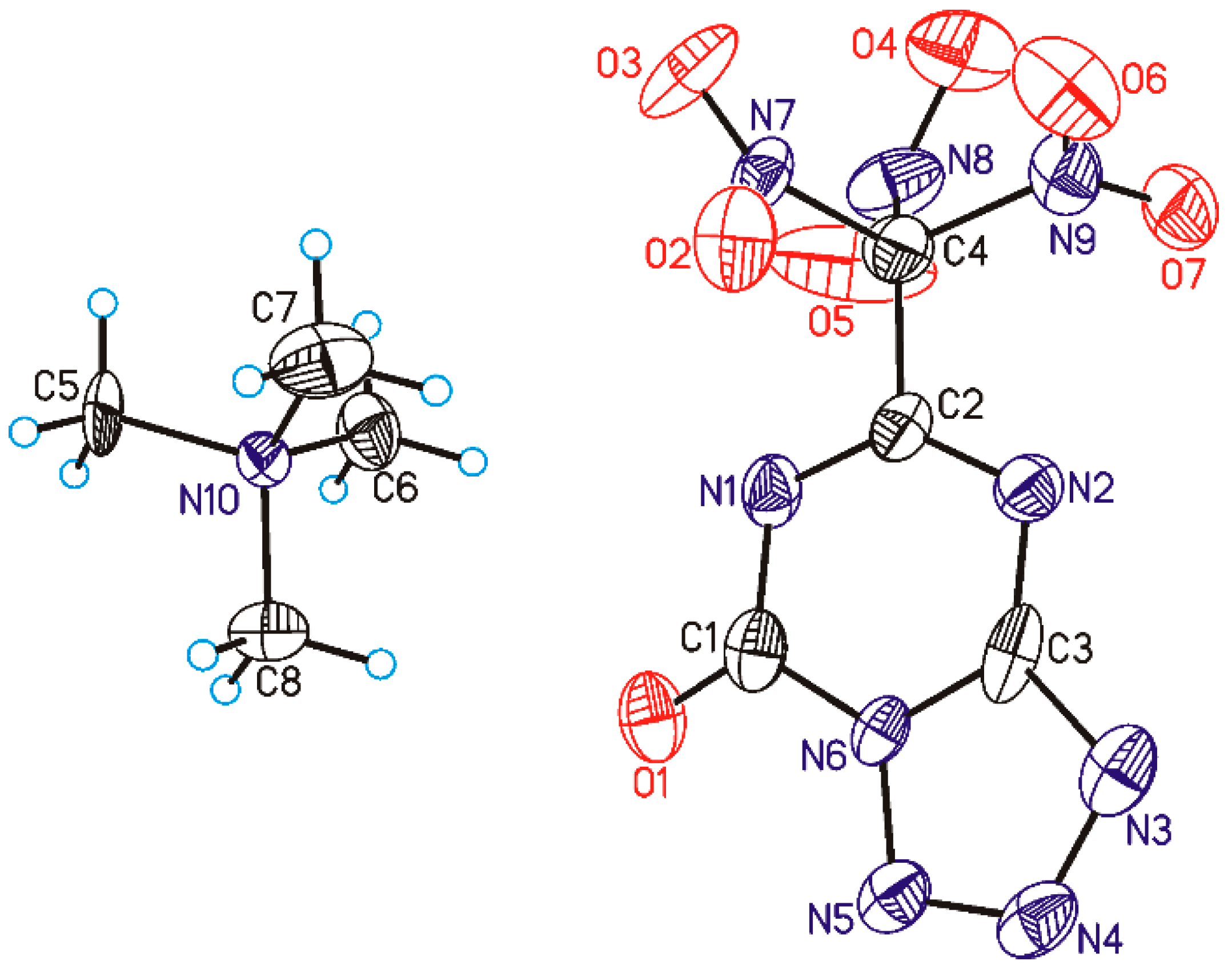
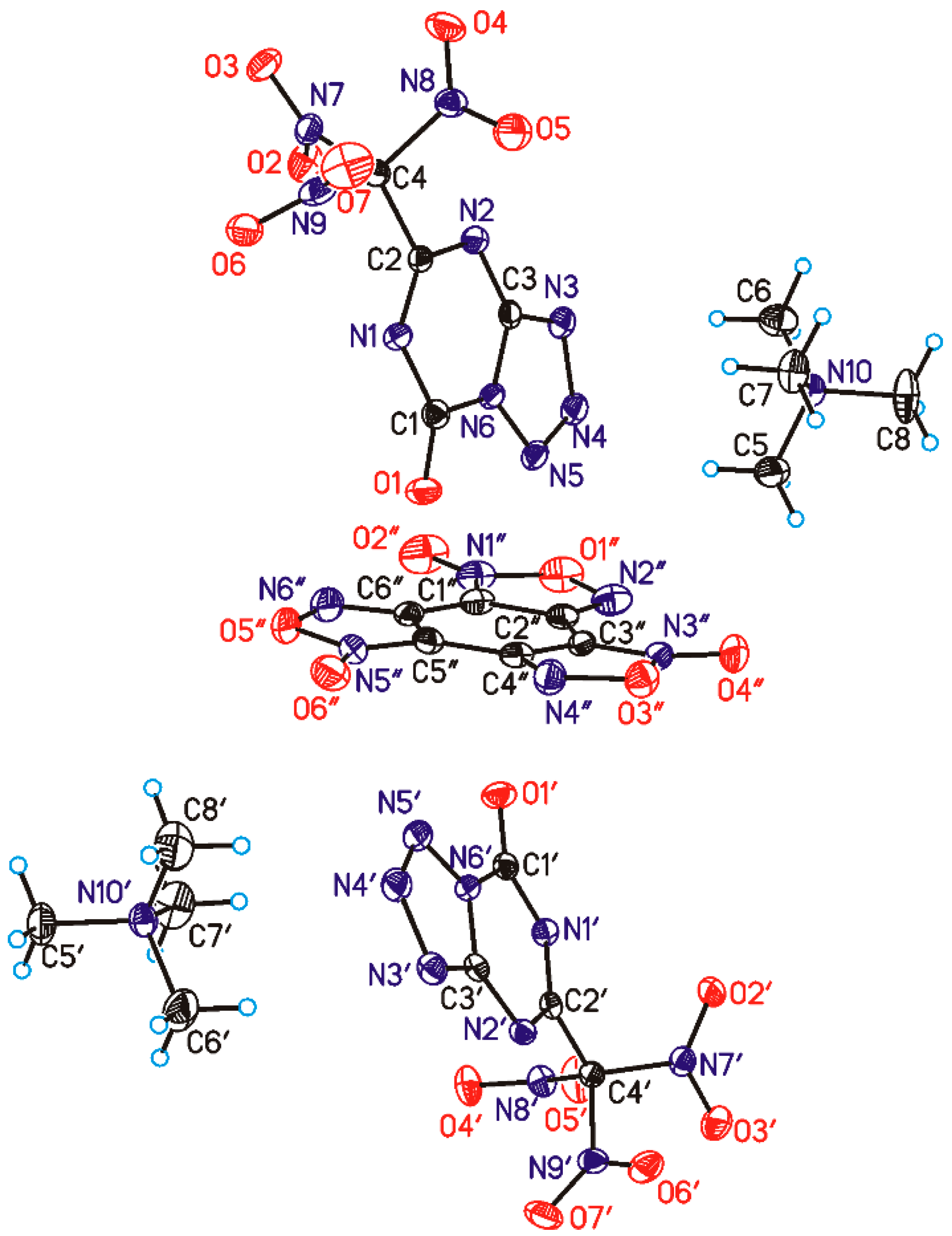
2.4. Single Crystal X-ray Diffraction
2.5. Methodology of Crystal Structure Investigation
3. Results and Discussion
3.1. Choice of Co-Former
3.2. The Structure and Physical Properties of Co-Crystal 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kitaygorodsky, A.I. Mixed Crystals; Nauka: Moscow, Russia, 1983. [Google Scholar]
- Aakeröy, C.B.; Salmon, D.J. Building Co-Crystals with Molecular Sense and Supramolecular Sensibility. CrystEngComm 2005, 7, 439. [Google Scholar] [CrossRef]
- Wood, P.A.; Feeder, N.; Furlow, M.; Galek, P.T.A.; Groom, C.R.; Pidcock, E. Knowledge-Based Approaches to Co-Crystal Design. CrystEngComm 2014, 16, 5839. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Kaur, R.; Guru Row, T.N. Co-Crystallization and Small Molecule Crystal Form Diversity: From Pharmaceutical to Materials Applications. CrystEngComm 2016, 18, 8528–8555. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Sinha, A.S. (Eds.) Co-Crystals: Preparation, Characterization and Applications; Monographs in Supramolecular Chemistry; Royal Society of Chemistry: Cambridge, UK, 2018; ISBN 978-1-78801-115-0. [Google Scholar]
- Mir, N.A.; Dubey, R.; Desiraju, G.R. Strategy and Methodology in the Synthesis of Multicomponent Molecular Solids: The Quest for Higher Cocrystals. Acc. Chem. Res. 2019, 52, 2210–2220. [Google Scholar] [CrossRef]
- Zhang, J.; Shreeve, J.M. Time for Pairing: Cocrystals as Advanced Energetic Materials. CrystEngComm 2016, 18, 6124–6133. [Google Scholar] [CrossRef]
- Sarkar, N.; Gonnella, N.C.; Krawiec, M.; Xin, D.; Aakeröy, C.B. Evaluating the Predictive Abilities of Protocols Based on Hydrogen-Bond Propensity, Molecular Complementarity, and Hydrogen-Bond Energy for Cocrystal Screening. Cryst. Growth Des. 2020, 20, 7320–7327. [Google Scholar] [CrossRef]
- Shan, N.; Zaworotko, M.J. The Role of Cocrystals in Pharmaceutical Science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef]
- Blagden, N.; Coles, S.J.; Berry, D.J. Pharmaceutical Co-Crystals—Are We There Yet? CrystEngComm 2014, 16, 5753–5761. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Cao, H.-L.; Zhou, J.-R.; Cai, F.-Y.; Lü, J.; Cao, R. Two-Component Pharmaceutical Cocrystals Regulated by Supramolecular Synthons Comprising Primary N···H···O Interactions. Cryst. Growth Des. 2019, 19, 3–16. [Google Scholar] [CrossRef]
- Yousef, M.A.E.; Vangala, V.R. Pharmaceutical Cocrystals: Molecules, Crystals, Formulations, Medicines. Cryst. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, S.; Huang, S.; Tian, Y.; Liu, Y.; Zhang, H.; Sun, J. Host–Guest Energetic Materials Constructed by Incorporating Oxidizing Gas Molecules into an Organic Lattice Cavity toward Achieving Highly-Energetic and Low-Sensitivity Performance. Chem. Commun. 2019, 55, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, B.; Peng, R.; Niu, C.; Xiao, L.; Guo, Z.; Zhang, Q. Novel Strategies for Synthesizing Energetic Materials Based on BTO with Improved Performances. Dalton Trans. 2019, 48, 11848–11854. [Google Scholar] [CrossRef] [PubMed]
- Landenberger, K.B.; Matzger, A.J. Cocrystal Engineering of a Prototype Energetic Material: Supramolecular Chemistry of 2,4,6-Trinitrotoluene. Cryst. Growth Des. 2010, 10, 5341–5347. [Google Scholar] [CrossRef]
- Gamekkanda, J.C.; Sinha, A.S.; Aakeröy, C.B. Cocrystals and Salts of Tetrazole-Based Energetic Materials. Cryst. Growth Des. 2020, 20, 2432–2439. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Crystal Engineering of Energetic Materials: Co-Crystals of Ethylenedinitramine (EDNA) with Modified Performance and Improved Chemical Stability. Chem. Eur. J. 2015, 21, 11029–11037. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Xu, J.; Zhang, H.; Guan, Y.; Jiang, H.; Huang, S.; Huang, H.; Wang, Z. Three Energetic 2,2′,4,4′,6,6′-Hexanitrostilbene Cocrystals Regularly Constructed by H-Bonding, π-Stacking, and van Der Waals Interactions. Cryst. Growth Des. 2018, 18, 1940–1943. [Google Scholar] [CrossRef]
- Fedyanin, I.V.; Lyssenko, K.A.; Fershtat, L.L.; Muravyev, N.V.; Makhova, N.N. Crystal Solvates of Energetic 2,4,6,8,10,12-Hexanitro-2,4,6,8,10,12-Hexaazaisowurtzitane Molecule with [Bmim]-Based Ionic Liquids. Cryst. Growth Des. 2019, 19, 3660–3669. [Google Scholar] [CrossRef]
- Orlenko, L.P. (Ed.) Physics of Explosion, 3rd ed.; Fismatlit: Moscow, Russia, 2002; Volume 1. [Google Scholar]
- Bogdanova, Y.U.A.; Gubin, S.A.; Korsunskii, B.L.; Pepekin, V.I. Detonation Characteristics of Powerful Insensitive Explosives. Combust. Explos. Shock Waves 2009, 45, 738–743. [Google Scholar] [CrossRef]
- Kubota, N. Propellants and Explosives: Thermochemical Aspects of Combustion; Wiley-VCH: Weinheim, Germany, 2002; ISBN 978-3-527-30210-9. [Google Scholar]
- Pavlovets, G.; Tsutsuran, V. Physicochemical Properties of Powders and Propellants; Russian Ministry of Defense Publishing House: Moscow, Russia, 2009. [Google Scholar]
- Landenberger, K.B.; Matzger, A.J. Cocrystals of 1,3,5,7-Tetranitro-1,3,5,7-Tetrazacyclooctane (HMX). Cryst. Growth Des. 2012, 12, 3603–3609. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, Y.; Li, H.; Zhou, Y.; Zhou, J.; Gao, T.; Zhang, H.; Yang, Z.; Jiang, G. Toward Low-Sensitive and High-Energetic Cocrystal I: Evaluation of the Power and the Safety of Observed Energetic Cocrystals. CrystEngComm 2013, 15, 4003. [Google Scholar] [CrossRef]
- Bennion, J.C.; Siddiqi, Z.R.; Matzger, A.J. A Melt Castable Energetic Cocrystal. Chem. Commun. 2017, 53, 6065–6068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiao, F.; Li, H. Crystal Engineering for Creating Low Sensitivity and Highly Energetic Materials. Cryst. Growth Des. 2018, 18, 5713–5726. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Zhou, X.; Zhang, C.; Huang, H.; Li, J.; Nie, F. Characterization and Properties of a Novel Energetic–Energetic Cocrystal Explosive Composed of HNIW and BTF. Cryst. Growth Des. 2012, 12, 5155–5158. [Google Scholar] [CrossRef]
- Herrmannsdörfer, D.; Klapötke, T.M. Semibatch Reaction Crystallization for Scaled-Up Production of High-Quality CL-20/HMX Cocrystal: Efficient Because of Solid Dosing. Cryst. Growth Des. 2021, 21, 1708–1717. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Wu, Y.; Jin, S.; Wang, X.; Wang, Y.; Shang, F.; Chen, K.; Du, J.; Shu, Q. A Novel Cocrystal Composed of CL-20 and an Energetic Ionic Salt. Chem. Commun. 2018, 54, 13268–13270. [Google Scholar] [CrossRef]
- Landenberger, K.B.; Bolton, O.; Matzger, A.J. Energetic–Energetic Cocrystals of Diacetone Diperoxide (DADP): Dramatic and Divergent Sensitivity Modifications via Cocrystallization. J. Am. Chem. Soc. 2015, 137, 5074–5079. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, B.S.; Fadeev, M.A.; Gidaspov, A.A.; Kosareva, E.A.; Bakharev, V.V. Condensed Tetrazolo-1,3,5-Triazines. 1. Synthesis of 5-Polynitromethyltetrazolo[1,5-a]-1,3,5-Triazin-7-One Salts. Chem. Heterocycl. Compd. 2005, 41, 228–234. [Google Scholar] [CrossRef]
- Trache, D.; Klapötke, T.M.; Maiz, L.; Abd-Elghany, M.; DeLuca, L.T. Recent Advances in New Oxidizers for Solid Rocket Propulsion. Green Chem. 2017, 19, 4711–4736. [Google Scholar] [CrossRef]
- Tang, J.; Chen, D.; Zhang, G.; Yang, H.; Cheng, G. A “Green” Primary Explosive: Design, Synthesis, and Testing. Synlett 2019, 30, 885–892. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Ignat’ev, N.V.; Schulte, M. Straightforward One-Pot Synthesis of Benzofuroxans from o-Halonitrobenzenes in Ionic Liquids. Mendeleev Commun. 2012, 22, 95–97. [Google Scholar] [CrossRef]
- Moschinskii, Y.V. Differential scanning calorimeter DSK-500. Instrum. Exp. Tech. 2003, 46, 143–144. [Google Scholar]
- Rekshinskii, V.A.; Pyzhov, A.M.; Gidaspov, A.A. Sensitivity of Energetic Materials to Thermal and Mechanical Stimules: A Textbook; Samara State Technical University: Samara, Russia, 2010. [Google Scholar]
- Golovina, N.I.; Titkov, A.N.; Raevskii, A.V.; Atovmyan, L.O. Kinetics and Mechanism of Phase Transitions in the Crystals of 2,4,6-Trinitrotoluene and Benzotrifuroxane. J. Solid State Chem. 1994, 113, 229–238. [Google Scholar] [CrossRef]
- APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kitaigorodskii, A.I. Organic Chemical Crystallography; Consultant’s Bureau: New York, NY, USA, 1961; ISBN 978-0-592-01213-1. [Google Scholar]
- Gavezzotti, A. Calculation of Intermolecular Interaction Energies by Direct Numerical Integration over Electron Densities. I. Electrostatic and Polarization Energies in Molecular Crystals. J. Phys. Chem. B 2002, 106, 4145–4154. [Google Scholar] [CrossRef]
- Gavezzotti, A. Calculation of Intermolecular Interaction Energies by Direct Numerical Integration over Electron Densities. 2. An Improved Polarization Model and the Evaluation of Dispersion and Repulsion Energies. J. Phys. Chem. B 2003, 107, 2344–2353. [Google Scholar] [CrossRef]
- Turner, M.J.; Grabowsky, S.; Jayatilaka, D.; Spackman, M.A. Accurate and Efficient Model Energies for Exploring Intermolecular Interactions in Molecular Crystals. J. Phys. Chem. Lett. 2014, 5, 4249–4255. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules—A Quantum Theory; Oxford University Press: Oxford, UK, 1990; ISBN 0-19-855168-1. [Google Scholar]
- Suponitsky, K.Y.; Tsirelson, V.G.; Feil, D. Electron-Density-Based Calculations of Intermolecular Energy: Case of Urea. Acta Crystallogr. A Found Crystallogr. 1999, 55, 821–827. [Google Scholar] [CrossRef]
- Gidaspov, A.A.; Bakharev, V.V.; Suponitsky, K.Y.; Nikitin, V.G.; Sheremetev, A.B. High-Density Insensitive Energetic Materials: 2,4,6-Tris(2-Fluoro-2,2-Dinitroethoxy)-1,3,5-Triazine. RSC Adv. 2016, 6, 104325–104329. [Google Scholar] [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-Mechanical Condensed Matter Simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Peintinger, M.F.; Oliveira, D.V.; Bredow, T. Consistent Gaussian Basis Sets of Triple-Zeta Valence with Polarization Quality for Solid-State Calculations. J. Comput. Chem. 2013, 34, 451–459. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Gatti, C.; Saunders, V.R.; Roetti, C. Crystal Field Effects on the Topological Properties of the Electron Density in Molecular Crystals: The Case of Urea. J. Chem. Phys. 1994, 101, 10686. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Ananyev, I.V.; Karnoukhova, V.A.; Dmitrienko, A.O.; Lyssenko, K.A. Toward a Rigorous Definition of a Strength of Any Interaction Between Bader’s Atomic Basins. J. Phys. Chem. A 2017, 121, 4517–4522. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, WA, Australia, 2017. [Google Scholar]
- Jayatilaka, D.; Grimwood, D.J. Tonto: A Fortran Based Object-Oriented System for Quantum Chemistry and Crystallography. In Computational Science—ICCS 2003; Sloot, P.M.A., Abramson, D., Bogdanov, A.V., Gorbachev, Y.E., Dongarra, J.J., Zomaya, A.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 2660, pp. 142–151. ISBN 978-3-540-40197-1. [Google Scholar]
- Dalinger, I.L.; Kormanov, A.V.; Suponitsky, K.Y.; Muravyev, N.V.; Sheremetev, A.B. Pyrazole-Tetrazole Hybrid with Trinitromethyl, Fluorodinitromethyl, or (Difluoroamino)Dinitromethyl Groups: High-Performance Energetic Materials. Chem. Asian J. 2018, 13, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Palysaeva, N.V.; Gladyshkin, A.G.; Vatsadze, I.A.; Suponitsky, K.Y.; Dmitriev, D.E.; Sheremetev, A.B. N-(2-Fluoro-2,2-Dinitroethyl)Azoles: A Novel Assembly of Diverse Explosophoric Building Blocks for Energetic Compound Design. Org. Chem. Front. 2019, 6, 249–255. [Google Scholar] [CrossRef]
- Suponitsky, K.Y.; Smol’yakov, A.F.; Ananyev, I.V.; Khakhalev, A.V.; Gidaspov, A.A.; Sheremetev, A.B. 3,4-Dinitrofurazan: Structural Nonequivalence of Ortho-Nitro Groups as a Key Feature of the Crystal Structure and Density. ChemistrySelect 2020, 5, 14543–14548. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Mel’nikova, S.F.; Kokareva, E.S.; Nekrutenko, R.E.; Strizhenko, K.V.; Suponitsky, K.Y.; Pham, T.D.; Pivkina, A.N.; Sinditskii, V.P. Nitroxy- and Azidomethyl Azofurazans as Advanced Energetic Materials. Def. Technol. 2021, in press. [Google Scholar] [CrossRef]
- Larin, A.A.; Muravyev, N.V.; Pivkina, A.N.; Suponitsky, K.Y.; Ananyev, I.V.; Khakimov, D.V.; Fershtat, L.L.; Makhova, N.N. Assembly of Tetrazolylfuroxan Organic Salts: Multipurpose Green Energetic Materials with High Enthalpies of Formation and Excellent Detonation Performance. Chem. Eur. J. 2019, 25, 4225–4233. [Google Scholar] [CrossRef]
- Gunawardana, C.A.; Aakeröy, C.B. Co-Crystal Synthesis: Fact, Fancy, and Great Expectations. Chem. Commun. 2018, 54, 14047–14060. [Google Scholar] [CrossRef]
- Aakeröy, C. Is There Any Point in Making Co-Crystals? Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Desper, J.; Fasulo, M.; Hussain, I.; Levin, B.; Schultheiss, N. Ten Years of Co-Crystal Synthesis; the Good, the Bad, and the Ugly. CrystEngComm 2008, 10, 1816. [Google Scholar] [CrossRef]
- Abeysekera, A.M.; Sinha, A.S.; Aakeroy, C.B. The Impact of Halogen Substituents on the Synthesis and Structure of Co-Crystals of Pyridine Amides. Molecules 2021, 26, 1147. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Görbitz, C.H.; Hersleth, H.-P. On the Inclusion of Solvent Molecules in the Crystal Structures of Organic Compounds. Acta Crystallogr. B Struct. Sci. 2000, 56, 526–534. [Google Scholar] [CrossRef]
- Bu, R.; Xiong, Y.; Wei, X.; Li, H.; Zhang, C. Hydrogen Bonding in CHON-Containing Energetic Crystals: A Review. Cryst. Growth Des. 2019, 19, 5981–5997. [Google Scholar] [CrossRef]
- Sarkar, N.; Aakeröy, C.B. Evaluating Hydrogen-Bond Propensity, Hydrogen-Bond Coordination and Hydrogen-Bond Energy as Tools for Predicting the Outcome of Attempted Co-Crystallisations. Supramol. Chem. 2020, 32, 81–90. [Google Scholar] [CrossRef]
- Fedyanin, I.V.; Samigullina, A.I.; Krutov, I.A.; Gavrilova, E.L.; Zakharychev, D.V. Structures of a Phosphoryl Derivative of 4-Allyl-2,4-Dihydro-3H-1,2,4-Triazole-3-Thione: An Illustrative Example of Conformational Polymorphism. Crystals 2021, 11, 1126. [Google Scholar] [CrossRef]
- Gidaspov, A.A.; Zalomlenkov, V.A.; Bakharev, V.V.; Parfenov, V.E.; Yurtaev, E.V.; Struchkova, M.I.; Palysaeva, N.V.; Suponitsky, K.Y.; Lempert, D.B.; Sheremetev, A.B. Novel Trinitroethanol Derivatives: High Energetic 2-(2,2,2-Trinitroethoxy)-1,3,5-Triazines. RSC Adv. 2016, 6, 34921–34934. [Google Scholar] [CrossRef][Green Version]
- Gilmanov, R.Z.; Nikitin, V.G.; Khayrutdinov, F.G.; Strizhenko, K.V.; Suponitsky, K.Y.; Sheremetev, A.B. 8-Oxo-4H,8H-Bis(Furazano)[3,4-b:3′,4′-e]Pyridine 1,7-Dioxide: A New High-Density Insensitive Explosive. Mendeleev Commun. Mendeleev Commun. 2022. [Google Scholar] [CrossRef]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-π Interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, C.; Wang, X.; Xu, J.; He, X.; Liu, Y.; Liu, X.; Huang, H.; Sun, J. Five Energetic Cocrystals of BTF by Intermolecular Hydrogen Bond and π-Stacking Interactions. Cryst. Growth Des. 2013, 13, 679–687. [Google Scholar] [CrossRef]
- Wei, X.; Ma, Y.; Long, X.; Zhang, C. A Strategy Developed from the Observed Energetic–Energetic Cocrystals of BTF: Cocrystallizing and Stabilizing Energetic Hydrogen-Free Molecules with Hydrogenous Energetic Coformer Molecules. CrystEngComm 2015, 17, 7150–7159. [Google Scholar] [CrossRef]
- Zelenov, V.P.; Baraboshkin, N.M.; Khakimov, D.V.; Muravyev, N.V.; Meerov, D.B.; Troyan, I.A.; Pivina, T.S.; Dzyabchenko, A.V.; Fedyanin, I.V. Time for Quartet: The Stable 3: 1 Cocrystal Formulation of FTDO and BTF—A High-Energy-Density Material. CrystEngComm 2020, 22, 4823–4832. [Google Scholar] [CrossRef]
- Göbel, M.; Klapötke, T.M. Development and Testing of Energetic Materials: The Concept of High Densities Based on the Trinitroethyl Functionality. Adv. Funct. Mater. 2009, 19, 347–365. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Aleksandrova, N.S.; Palysaeva, N.V.; Struchkova, M.I.; Tartakovsky, V.A.; Suponitsky, K.Y. Ionic Liquids as Unique Solvents in One-Pot Synthesis of 4-(N,2,2,2-Tetranitroethylamino)-3-R-Furazans. Chem. Eur. J. 2013, 19, 12446–12457. [Google Scholar] [CrossRef]
- Matyushin, Y.N.; Lebedev, V.P.; Chironov, V.V.; Pepekin, V.I. Energy of the N→O Bond in Benzofuroxanes. Khim. Fiz. 2002, 21, 58–61. [Google Scholar]
- Kustova, L.V.; Kirpichev, E.P.; Rubtsov, Y.U.I.; Avdonin, V.V.; Korepin, A.G.; Eremenko, L.T. Standard Enthalpies of Formation of Certain Nitro Compounds. Russ. Chem. Bull. 1981, 30, 1830–1836. [Google Scholar] [CrossRef]
- Kizin, A.N.; Dvorkin, P.L.; Ryzhova, G.L.; Lebedev, Y.U.A. Parameters for Calculation of Standard Enthalpies of Formation of Organic Compounds in the Liquid State. Russ. Chem. Bull. 1986, 35, 343–346. [Google Scholar] [CrossRef]
- Holmes, J.L.; Aubry, C. Group Additivity Values for Estimating the Enthalpy of Formation of Organic Compounds: An Update and Reappraisal. 2. C, H, N, O, S, and Halogens. J. Phys. Chem. A 2012, 116, 7196–7209. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Wang, B.; Qiu, L.; Xu, R.; Sheremetev, A.B. Recent Synthetic Efforts towards High Energy Density Materials: How to Design High-Performance Energetic Structures? FirePhysChem 2021, in press. [Google Scholar] [CrossRef]
- Smirnov, A.S.; Smirnov, S.P.; Pivina, T.S.; Lempert, D.B.; Maslova, L.K. Comprehensive Assessment of Physicochemical Properties of New Energetic Materials. Russ. Chem. Bull. 2016, 65, 2315–2332. [Google Scholar] [CrossRef]
- Tsyshevsky, R.; Pagoria, P.; Smirnov, A.S.; Kuklja, M.M. Comprehensive End-to-End Design of Novel High Energy Density Materials: II. Computational Modeling and Predictions. J. Phys. Chem. C 2017, 121, 23865–23874. [Google Scholar] [CrossRef]
- Matyáš, R.; Pachman, J. Tetrazoles. In Primary Explosives; Springer: Berlin/Heidelberg, Germany, 2013; pp. 187–226. ISBN 978-3-642-28435-9. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Kudin, K.A., Jr.; Burant, J.C.; Millam, J.M.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, UK, 2004. [Google Scholar]
- Keith, T.A. AIMAll, Version 14.11.23; TK Gristmill Software: Overland Park, KS, USA, 2014; Available online: http://aim.tkgristmill.com (accessed on 1 December 2021).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2021, 33, 580–592. [Google Scholar]
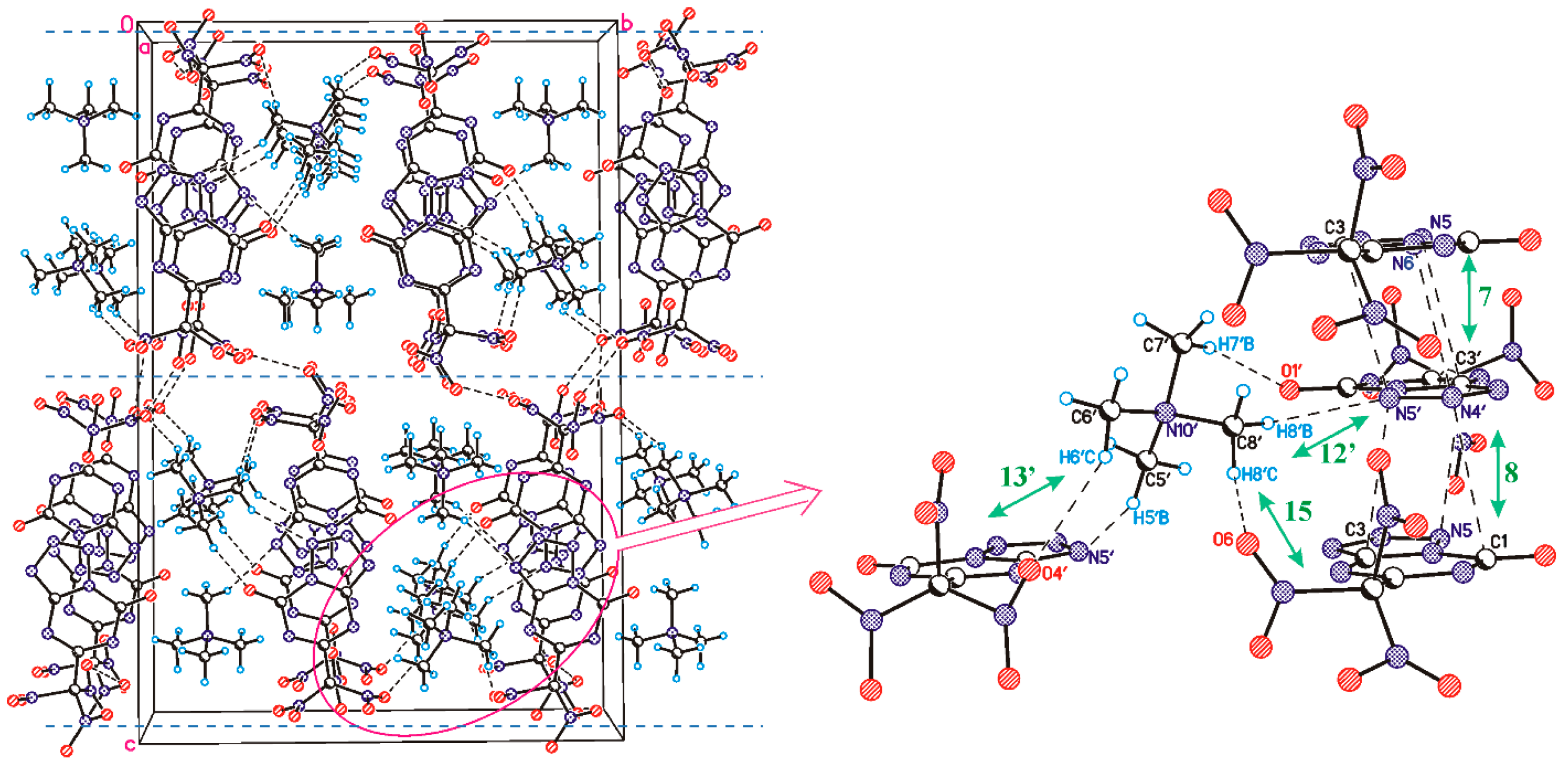
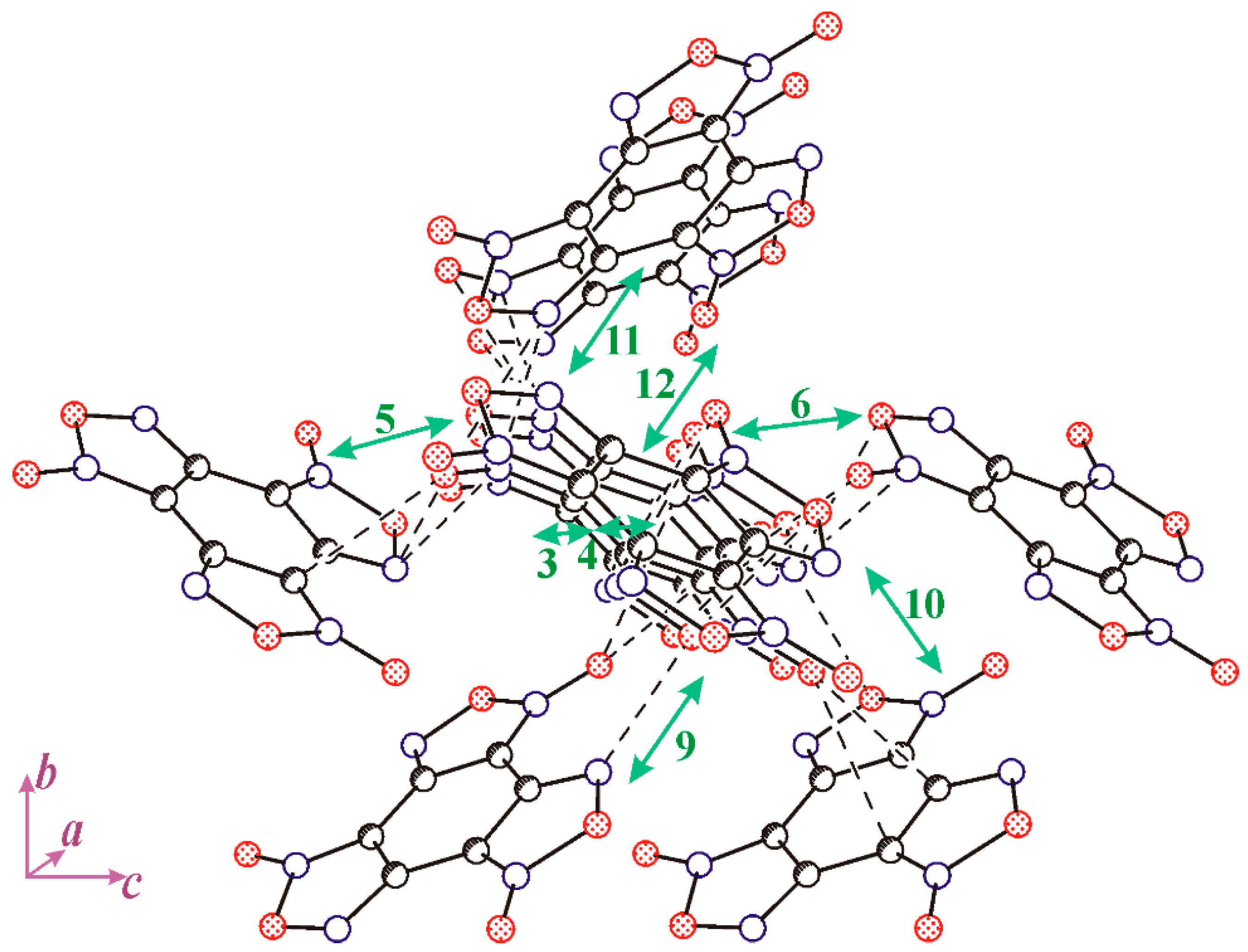
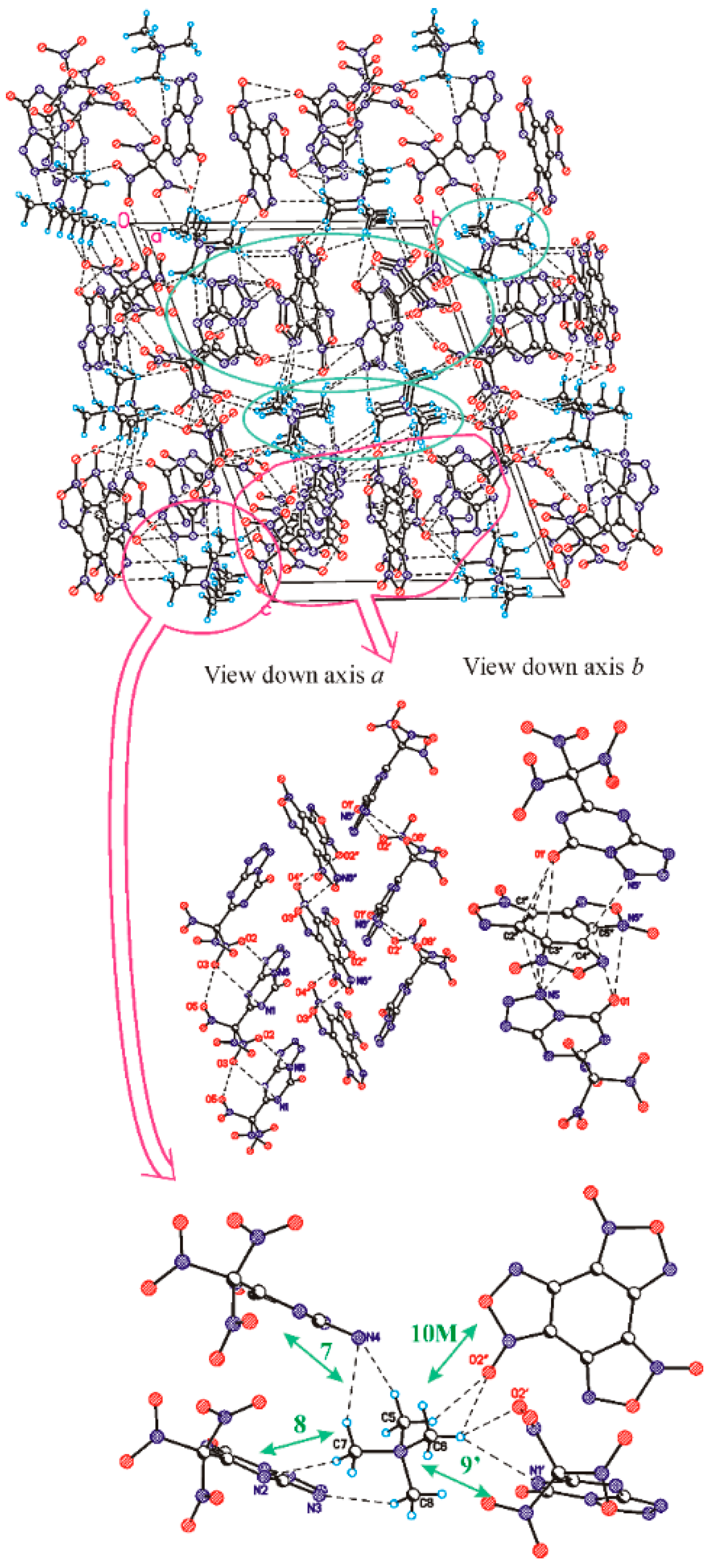
| Neighbour | Symmetry Code | Type of Interaction | Energy | Molecular Pair |
|---|---|---|---|---|
| Anion A | ||||
| 1 | −1 + x, y, z | NO2…NO2 | −1.66 | A…A |
| 2 | 1 + x, y, z | NO2…NO2 | −1.66 | A…A |
| 3 | 1 − x, 1 − y, 1 − z | NO2…NO2 | −1.40 | A…A |
| 4 | 2−x, 1−y, 1−z | NO2…NO2 | −0.54 | A…A |
| 5 | x, y, z | NO2…NO2 | −4.09 | A…A′ |
| 6 | 1 + x, y, z | NO2…NO2 | −0.68 | A…A′ |
| 7 | −1/2 + x, 1.5 − y, z − 1/2 | π…π stack | −2.71 | A…A′ |
| 8 | 1/2 + x, 1.5 − y, −1/2 + z | π…π stack | −4.09 | A…A′ |
| 9 | −1 + x, y, z | C–H…O | −3.02 | A…C |
| 10 | x, y, z | C–H…O(N) | −4.46 | A…C |
| 11 | 1.5 − x, ½ + y, 1/2 − z | C–H…N | −1.83 | A…C |
| 12 | 1 − x, 1 − y, 1 − z | C–H…O | −1.01 | A…C |
| 13 | 2 − x, 1 − y, 1 − z | C–H…O | −3.52 | A…C |
| 14 | x, y, z | C–H…O(N) | −2.49 | A…C′ |
| 15 | 1.5 − x, 1/2 + y, 1/2 − z | C–H…O(N) | −3.51 | A…C′ |
| 16 | 1/2 − x, 1/2 + y, 1/2 − z | C–H…O(N) | −4.10 | A…C′ |
| Anion A′ | ||||
| 1′ | −1 + x, y, z | NO2…NO2 | −2.66 | A′…A′ |
| 2′ | 1 + x, y, z | NO2…NO2 | −2.66 | A′…A′ |
| 3′ | −x, 2 − y, 1 − z | NO2…NO2 | −1.03 | A′…A′ |
| 4′ | 1 − x, 2 − y, 1 − z | NO2…NO2 | −2.04 | A′…A′ |
| 9′ | 1 − x, 1 − y, 1 − z | C–H…O(N) | −4.17 | A′…C |
| 10′ | 2 − x, 1 − y, 1 − z | C–H…O(N) | −2.32 | A′…C |
| 11′ | −1/2 + x, 1.5 − y, ½ + z | C–H…N | −2.60 | A′…C |
| 12′ | 1 − x, 1 − y, 1 − z | C–H…O(N) | −4.84 | A′…C′ |
| 13′ | −1/2 + x, 1.5 − y, ½ + z | C–H…O(N) | −4.03 | A′…C′ |
| 14′ | 1/2 + x, 1.5 − y, ½ + z | C–H…O(N) | −3.91 | A′…C′ |
| 15′ | 1/2 − x, 1/2 + y, 1/2 − z | C–H…O | −0.35 | A′…C′ |
| Cation…Cation | ||||
| 1CC′ | 1 + x, y, z | vdW | −1.19 | C…C′ |
| 2CC′ | x, y, z | vdW | −0.42 | C…C′ |
| Neighbour | Symmetry Code | Type of Interaction | Energy a | Energy b |
|---|---|---|---|---|
| Anion A | ||||
| 1 | −1 + x, y, −1 + z | vdW | −1.30 | −0.32 |
| 2 | 1 + x, y, 1 + z | vdW | −1.30 | −0.32 |
| 3 | −1 + x, y, z | π…π stack | −1.62 | −3.67 |
| 4 | 1 + x, y, z | π…π stack | −1.62 | −3.67 |
| 5 | x, y, −1 + z | π…π stack | −3.77 | −5.73 |
| 6 | x, y, 1 + z | π…π stack | −3.77 | −5.73 |
| 7 | −x, −y, −1/2 + z | vdW | −1.98 | −0.24 |
| 8 | −x, −y, 1/2 + z | vdW | −1.98 | −0.24 |
| 9 | 1 − x, −y, −1/2 + z | O(N)…π | −3.97 | −5.62 |
| 10 | 1 − x, −y, 1/2 + z | O(N)…π | −3.97 | −5.62 |
| 11 | −1/2 + x, 1/2 − y, z | O(N)…π | −3.93 | −3.71 |
| 12 | 1/2 + x, 1/2 − y, z | O(N)…π | 3.93 | −3.71 |
| 13 | −1/2 + x, 1/2 − y, −1 + z | vdW | −0.4 | −0.49 |
| 14 | 1/2 + x, 1/2 − y, 1 + z | vdW | −0.4 | −0.49 |
| Neighbour | Symmetry Code | Type of Interaction | Energy | Molecular Pair |
|---|---|---|---|---|
| Anion A | ||||
| 1 | −1 + x, y, z | weak O…π | −1.86 | A…A |
| 2 | 1 + x, y, z | weak O…π | −1.86 | A…A |
| 3 | 1 − x, 2 − y, −z | NO2…NO2 | −2.48 | A…A |
| 4 | −1 + x, 1 + y, z | NO2…NO2 | −2.48 | A…A |
| 5 | 2 + x, −1 + y, z | NO2…NO2/O…π | −2.68 | A…A′ |
| 6 | 2 + x, −1 + y, z | NO2…NO2 | −2.27 | A…A′ |
| 7 | x, y, z | C–H…N | −4.51 | A…C |
| 8 | 1 + x, y, z | C–H…O(N) | −4.66 | A…C |
| 9 | x, −1 + y, z | C–H…O | −2.07 | A…C′ |
| 10 | 1 + x, −1 + y, z | C–H…O | −3.45 | A…C′ |
| 11 | −x, 1 − y, 1 − z | C–H…O | −0.82 | A…C′ |
| 12 | 1 − x, 1 − y, 1 − z | C–H…O(N) | −5.13 | A…C′ |
| 13 | x, y, z | O(N)…π | −3.95 | A…M |
| 14 | 1 + x, y, z | weak O…π | −2.10 | A…M |
| Anion A′ a | ||||
| 1′ | −1 + x, y, z | weak O…π | −1.83 | A′…A′ |
| 2′ | 1 + x, y, z | weak O…π | −1.83 | A′…A′ |
| 3′ | 1 − x, 2 − y, −z | NO2…NO2 | −2.21 | A′…A′ |
| 6′ | −1 + x, 1 + y, z | C–H…O | −1.82 | A′…C |
| 7′ | x, 1 + y, z | C–H…O | −2.40 | A′…C |
| 8′ | −1 − x, 1 − y, −z | C–H…O | −2.21 | A′…C |
| 9′ | −x, 1 − y, −z | C–H…O(N) | −4.06 | A′…C |
| 10′ | −1 + x, y, z | C–H…O(N) | −4.78 | A′…C′ |
| 11′ | x, y, z | C–H…N | −4.14 | A′…C′ |
| 12′ | −x, 1 − y, 1 − z | C–H…N | −1.29 | A′…C′ |
| 13′ | x, y, z | O(N)…π | −3.82 | A′…M |
| 14′ | −1 + x, y, z | O(N)…π | −2.69 | A′…M |
| BTF molecule (M) b | ||||
| 1 M | −1 + x, y, z | π…π stacking | −2.67 | M…M |
| 2 M | 1 + x, y, z | π…π stacking | −2.67 | M…M |
| 3 M | 1 − x, 1 − y, −z | vdW | −0.46 | M…M |
| 8 M | x, y, z | C–H…O(N) | −2.41 | M…C |
| 9 M | −x, 1 − y, −z | C–H…O(N) | −1.77 | M…C |
| 10 M | 1 − x, 1 − y, −z | C–H…O | −3.14 | M…C |
| 11 M | x, y, z | C–H…O | −0.50 | M…C′ |
| 12 M | −x, 1 − y, 1 − z | C–H…O(N) | −4.52 | M…C′ |
| 13 M | 1 − x, 1 − y, 1 − z | C–H…O | −0.50 | M…C′ |
| Structural Unit | Energy a | Volume | ΔOED Criterion | |||
|---|---|---|---|---|---|---|
| Co-Former | Co-Crystal | Co-Former | Co-Crystal | Co-Former | Co-Crystal | |
| Anion_1 | −40.8 | −38.1 | 251.27 | 254.27 | 0.351 | 0.329 |
| Anion_2 | −42.2 | −40.3 | 250.72 | 253.29 | 0.355 | 0.336 |
| Cation_1 | −24.6 | −26.9 | 124.85 | 124.58 | 0.167 | 0.169 |
| Cation_2 | −24.8 | −27.2 | 123.42 | 122.92 | 0.178 | 0.182 |
| BTF | −34.0 | −31.2 | 214.59 | 220.82 | 0.373 | 0.318 |
| Salt 1 | BTF 2 | Co-Cystal 3 | |
|---|---|---|---|
| Formula | C8H12N10O7 | C6N6O6 | C22H24N26O20 |
| MW | 360.25 | 252.1 | 972.6 |
| α [a] | 0.318 | 0.5 | 0.357 |
| ρ [g⋅cm−3] [b] | 1.543 | 1.898 | 1.594 |
| ΔHfo (s) [kJ mol−1] (kJ g−1) [c] | −157.4 (−0.44) | +580.7 [83] (+2.30) | +245.2 (+0.25) |
| D [m s−1] [d] | 6740 | 8740 | 7200 |
| PC-J [GPa] [e] | 17.0 | 34.0 | 20.0 |
| Qexpl [kJ kg−1] [f] | 3071.1 | 6444.6 | 3861.8 |
| Energetic Material | Tm, [a] °C | Tonset/peak, [b] °C | h100%, [c] cm | Eim, [d] J |
|---|---|---|---|---|
| BTF 2 | 202 | 230.8/254.8 | ND | - |
| Salt 1 | 166 | 177.5/195.2 | 13 | 0.39 |
| Co-crystal | 153 | 167.2/189.8 | 24 | 0.69 |
| Mixture of 1 with 2 (2:1) | 157 | 167.6/190.3 | 20 | 0.6 |
| tetrazene | 135/153 | 10 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suponitsky, K.Y.; Fedyanin, I.V.; Karnoukhova, V.A.; Zalomlenkov, V.A.; Gidaspov, A.A.; Bakharev, V.V.; Sheremetev, A.B. Energetic Co-Crystal of a Primary Metal-Free Explosive with BTF. Ideal Pair for Co-Crystallization. Molecules 2021, 26, 7452. https://doi.org/10.3390/molecules26247452
Suponitsky KY, Fedyanin IV, Karnoukhova VA, Zalomlenkov VA, Gidaspov AA, Bakharev VV, Sheremetev AB. Energetic Co-Crystal of a Primary Metal-Free Explosive with BTF. Ideal Pair for Co-Crystallization. Molecules. 2021; 26(24):7452. https://doi.org/10.3390/molecules26247452
Chicago/Turabian StyleSuponitsky, Kyrill Yu., Ivan V. Fedyanin, Valentina A. Karnoukhova, Vladimir A. Zalomlenkov, Alexander A. Gidaspov, Vladimir V. Bakharev, and Aleksei B. Sheremetev. 2021. "Energetic Co-Crystal of a Primary Metal-Free Explosive with BTF. Ideal Pair for Co-Crystallization" Molecules 26, no. 24: 7452. https://doi.org/10.3390/molecules26247452
APA StyleSuponitsky, K. Y., Fedyanin, I. V., Karnoukhova, V. A., Zalomlenkov, V. A., Gidaspov, A. A., Bakharev, V. V., & Sheremetev, A. B. (2021). Energetic Co-Crystal of a Primary Metal-Free Explosive with BTF. Ideal Pair for Co-Crystallization. Molecules, 26(24), 7452. https://doi.org/10.3390/molecules26247452







