Metal Emulsion-Based Synthesis, Characterization, and Properties of Sn-Based Microsphere Phase Change Materials
Abstract
1. Introduction
2. Results and Discussion
2.1. In LiCl–KCl–CsCl Eutectic System
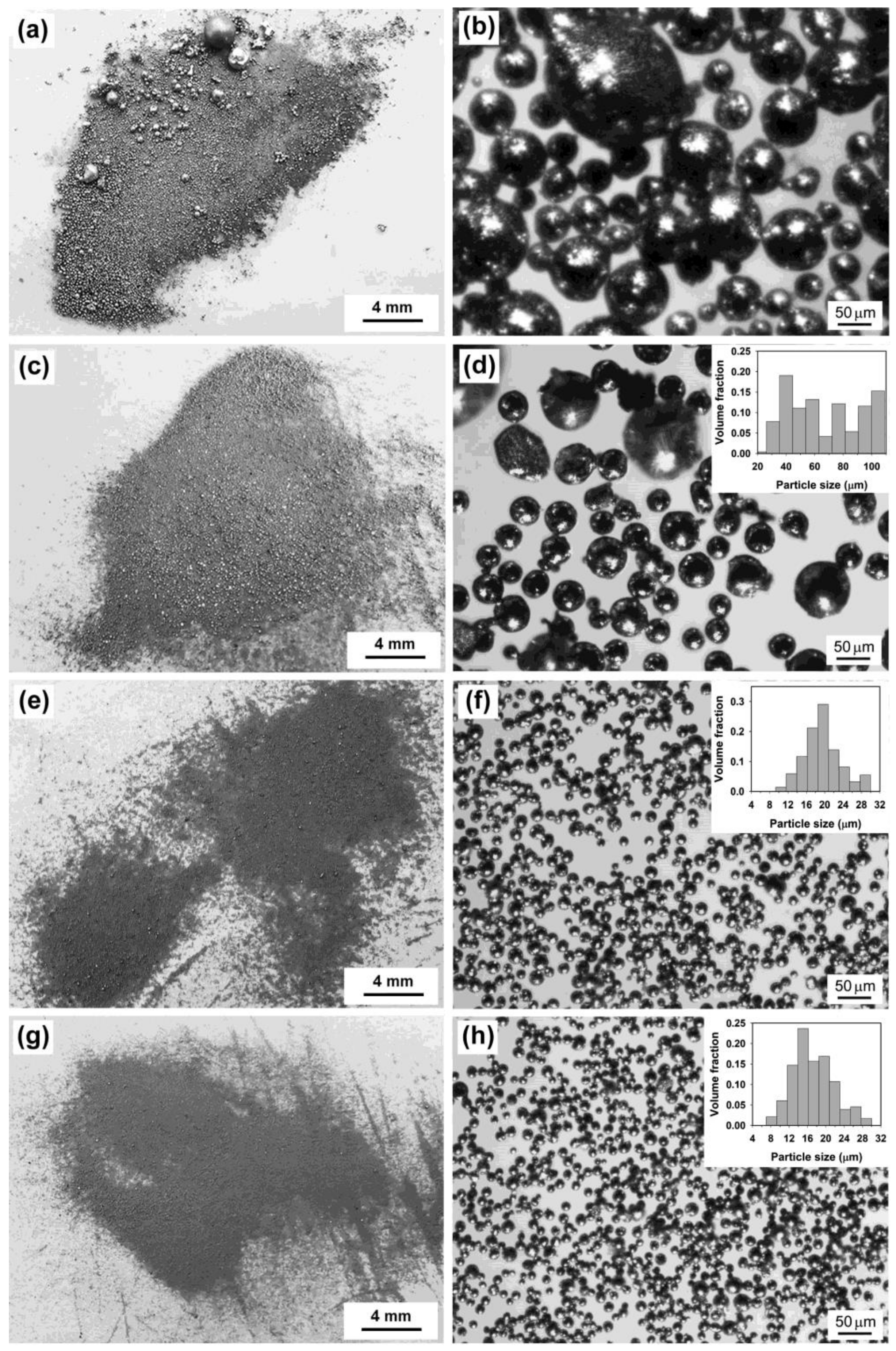
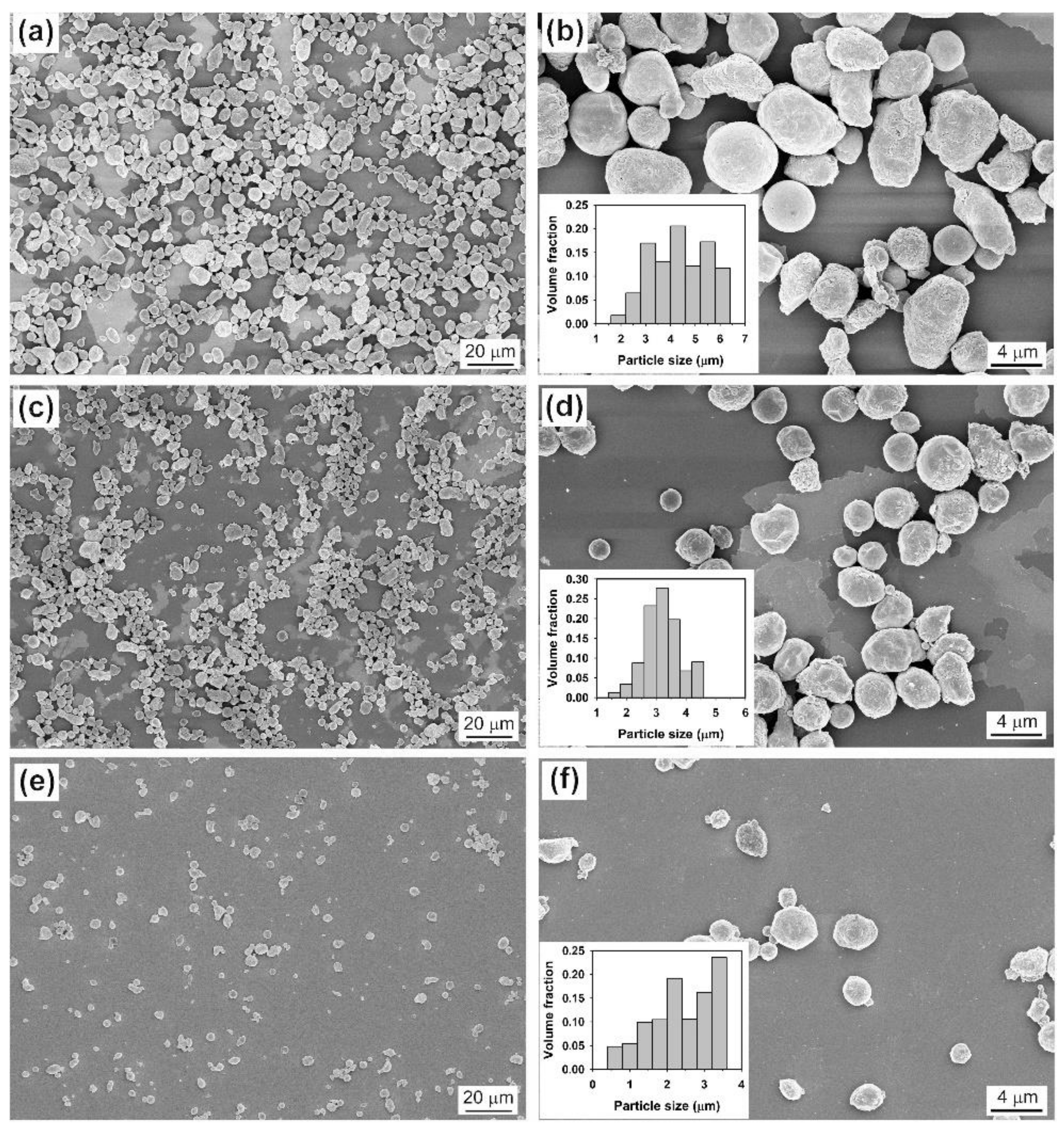
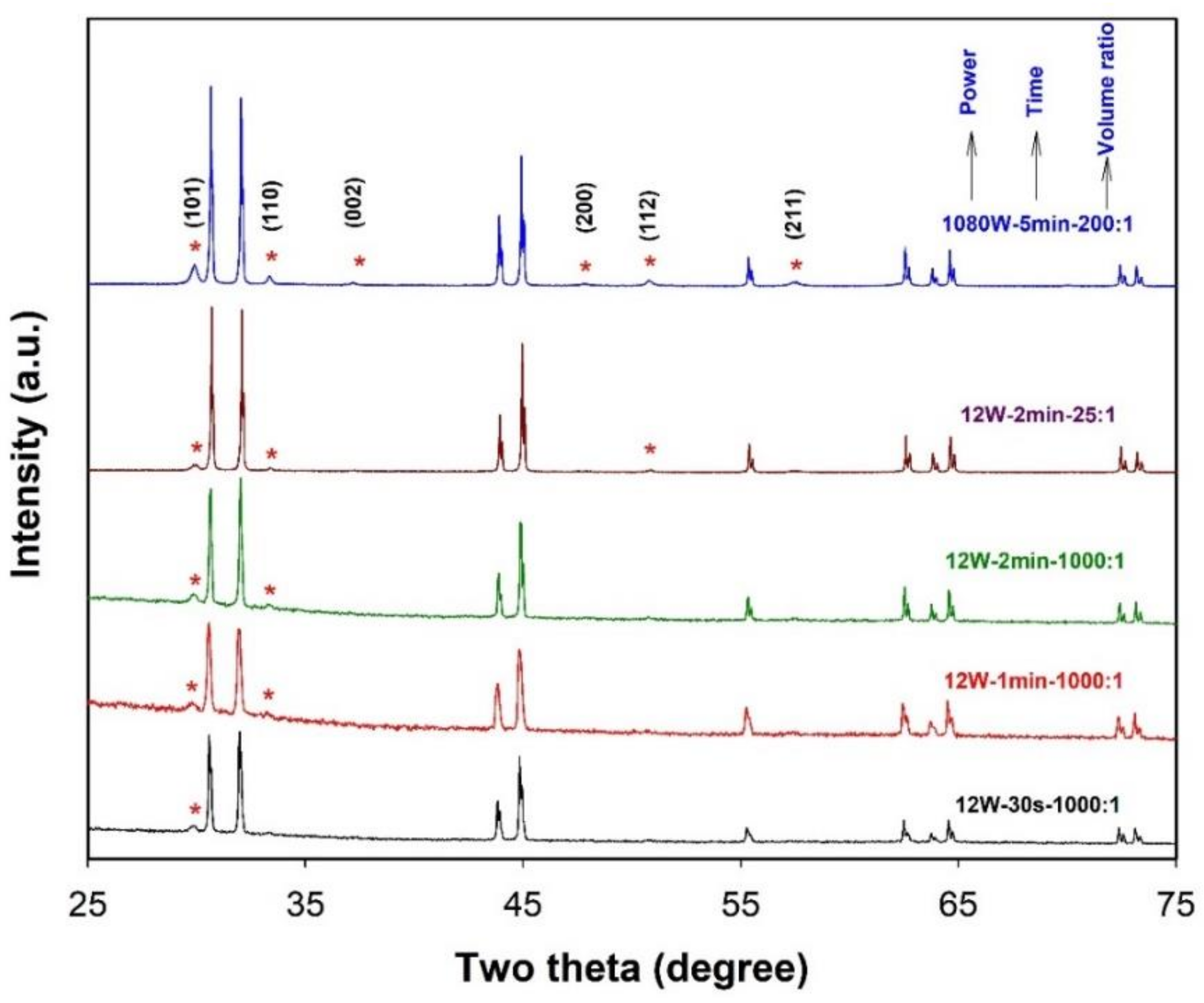
2.1.1. The Effect of Ultrasonic Power, Sonication Time, and the Volume Ratio of Salts to Metal
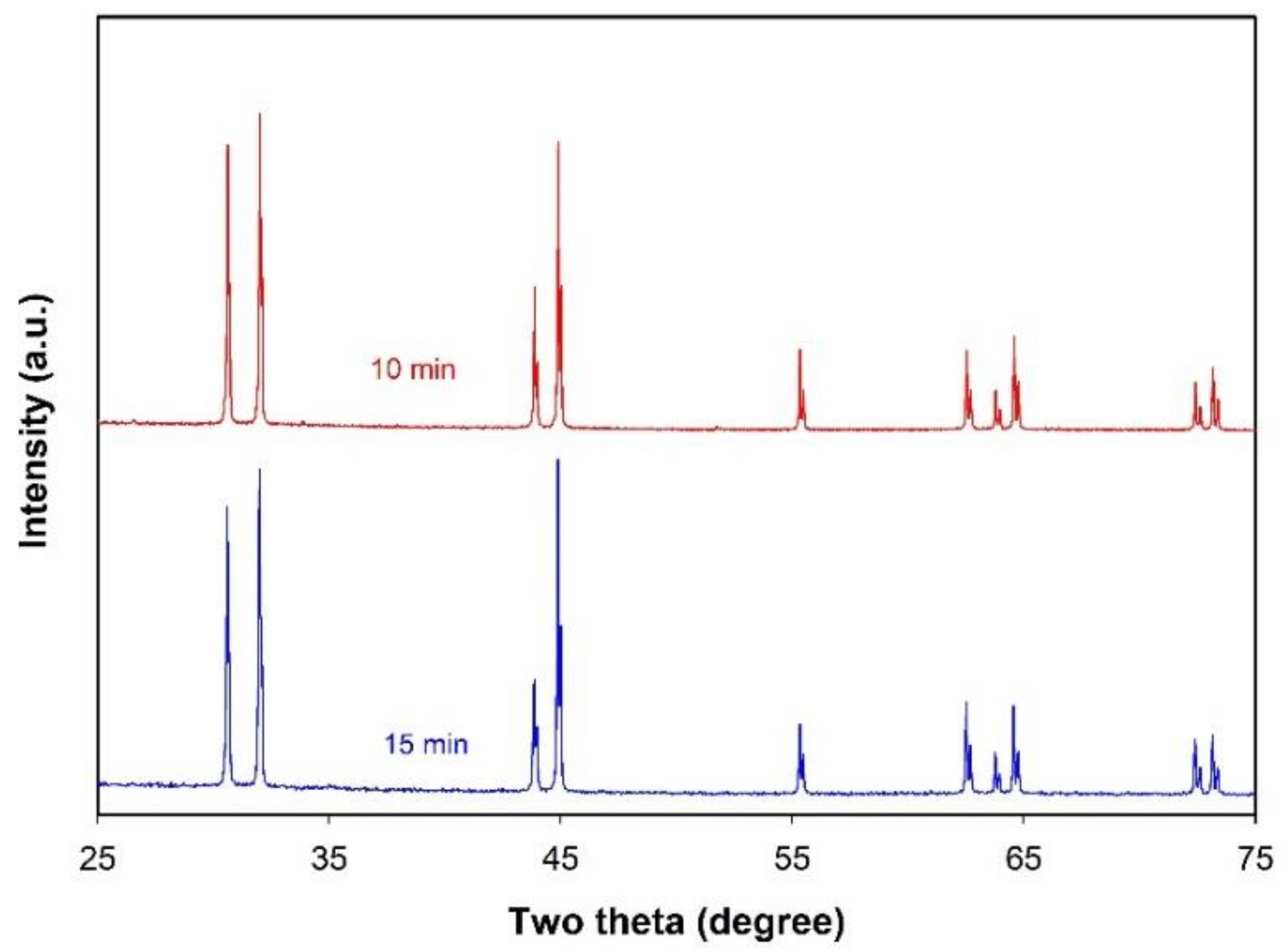
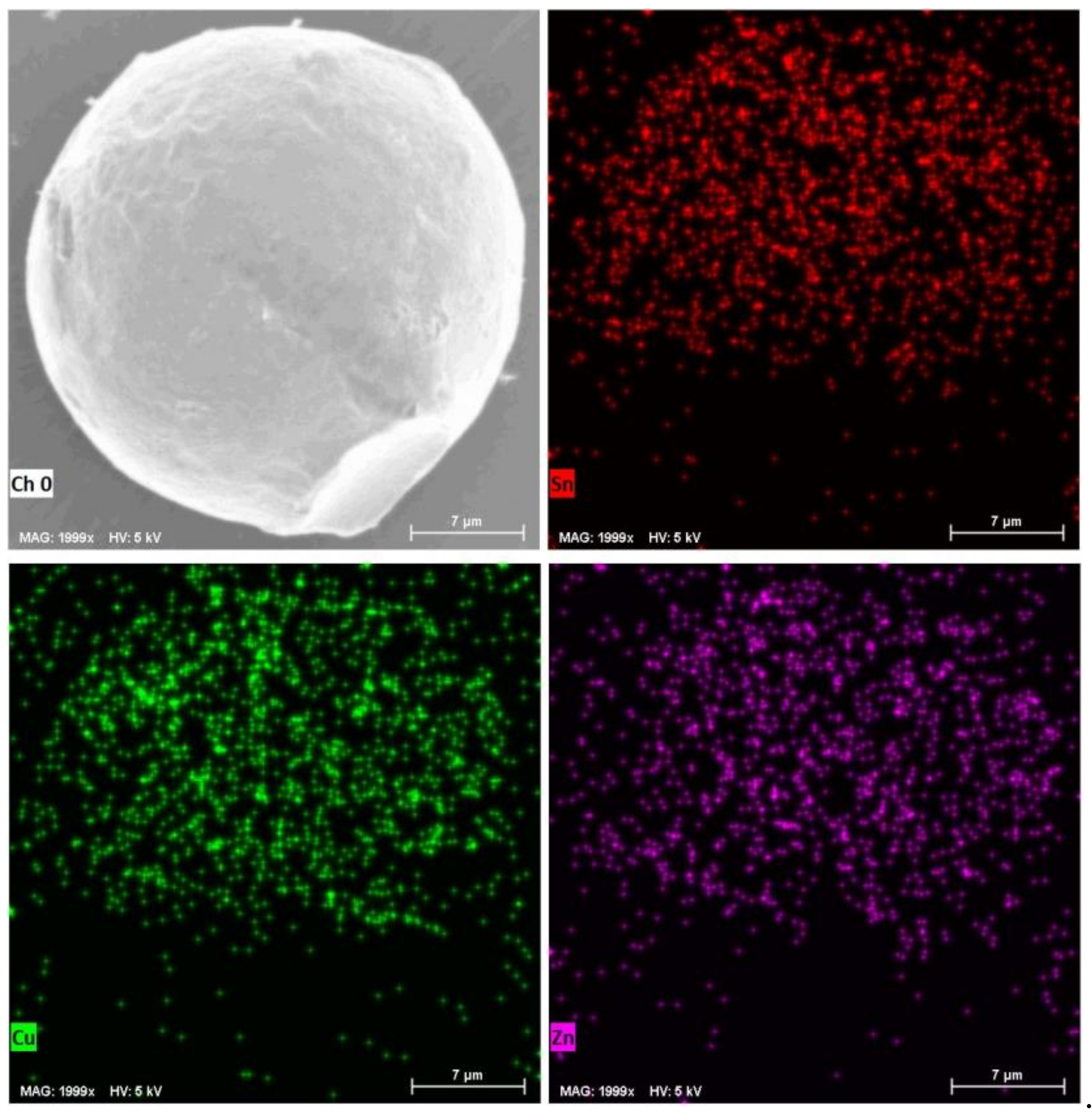
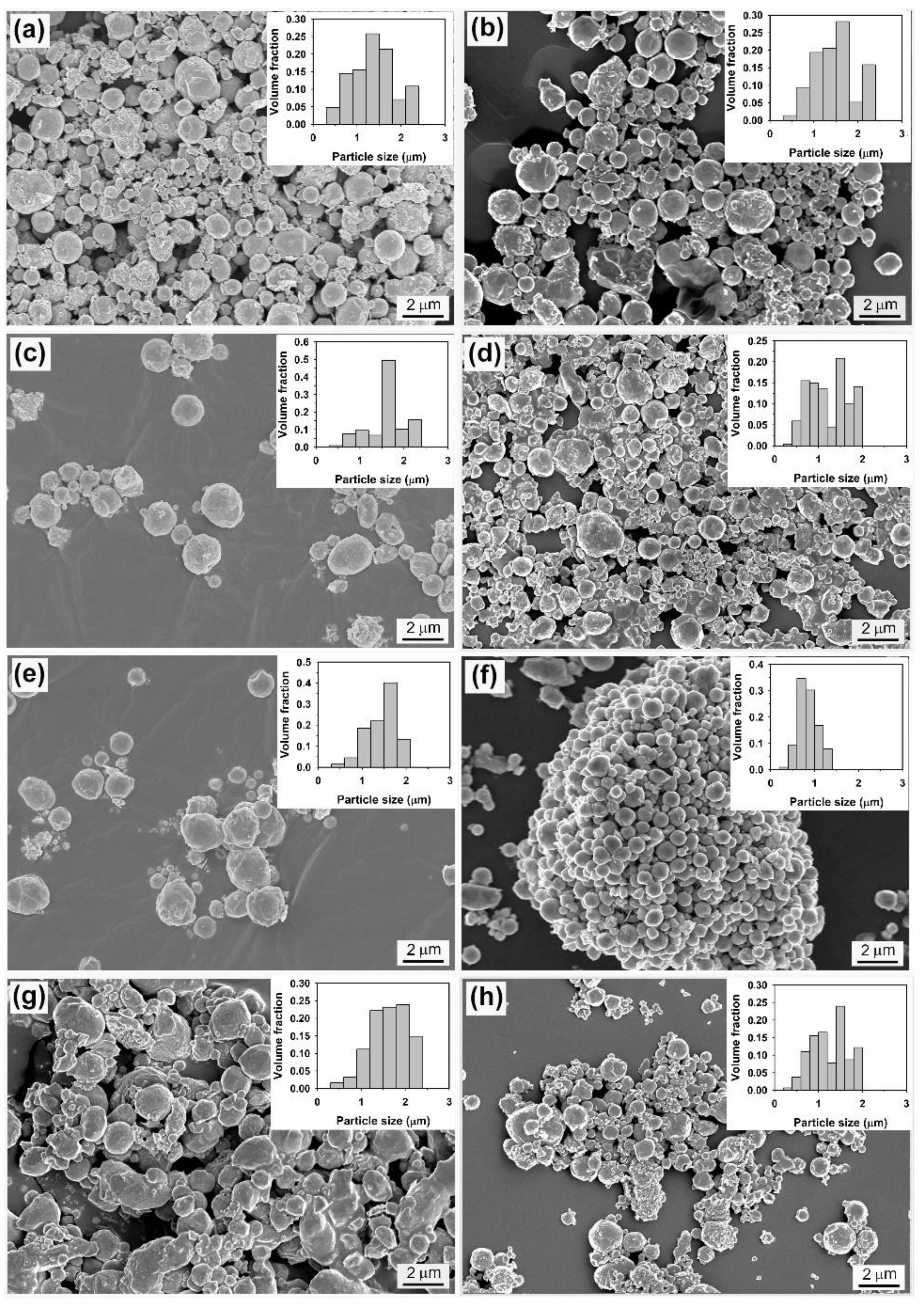
2.1.2. Preparation of Sn-Based Alloy Microspheres
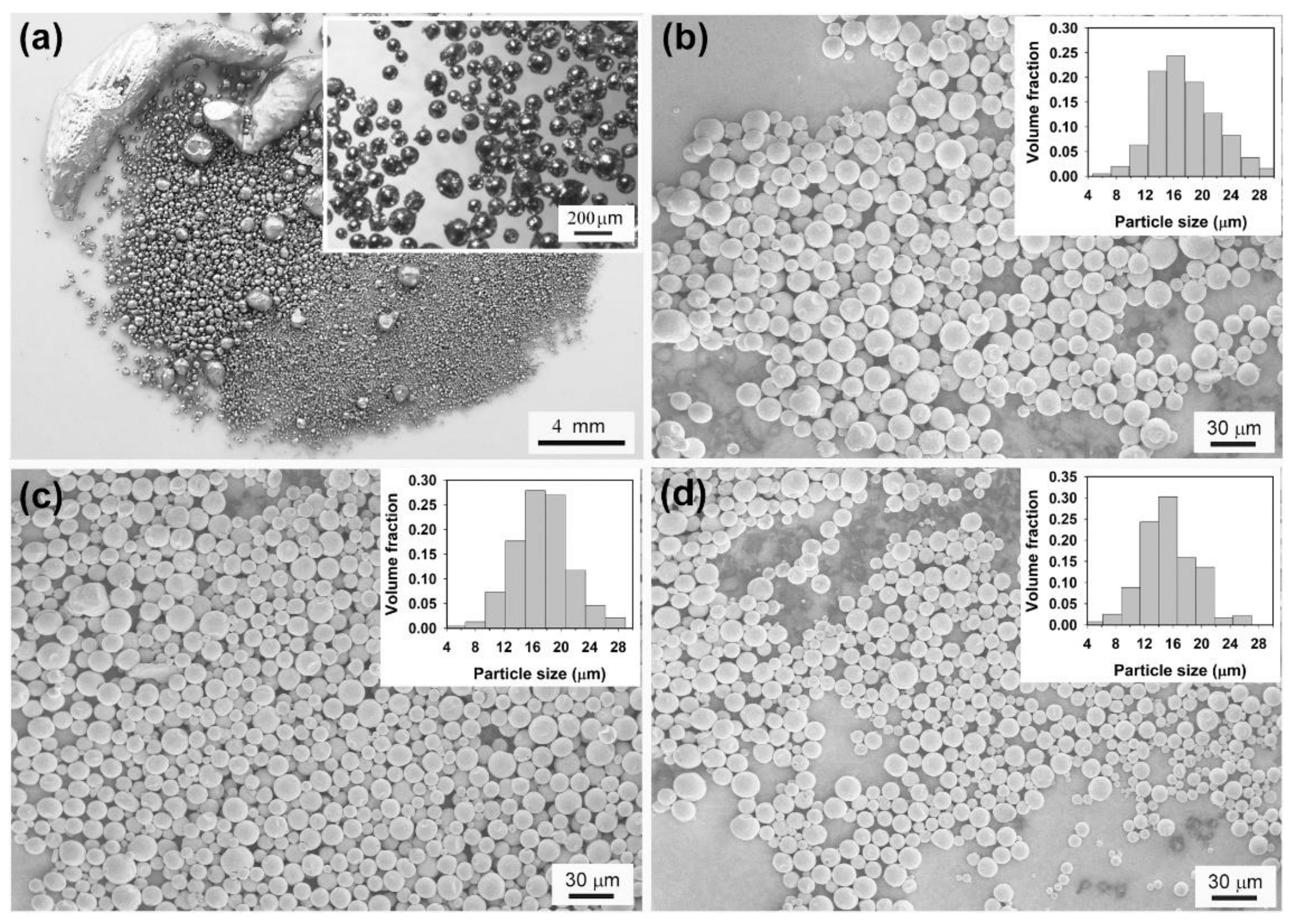
2.2. In LiNO3-NaNO3-KNO3 System
2.3. Phase Change Properties of Sn-Based Microspheres
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Sn-Based Materials via a Molten Salt-Based Metal Emulsion Method and Encapsulation of Sn-Based Microspheres
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.H.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Tran. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhang, X.L.; Hua, W.S. Review of preparation technologies of organic composite phase change materials in energy storage. J. Mol. Liq. 2021, 336, 115923. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Du, Z.; Jiang, F.; Ding, Y.L. A review of performance investigation and enhancement of shell and tube thermal energy storage device containing molten salt based phase change materials for medium and high temperature applications. Appl. Energy 2019, 255, 113806. [Google Scholar] [CrossRef]
- Fernández, A.I.; Barreneche, C.; Belusko, M.; Segarra, M.; Bruno, F.; Cabeza, L.F. Considerations for the use of metal alloys as phase change materials for high temperature applications. Sol. Energy Mater. Sol. Cells 2017, 171, 275–281. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.W.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Fang, D.; Cheng, X.M.; Li, Y.Y.; Sun, Z. Microstructure and thermal characteristics of Mg–Sn alloys as phase change materials for thermal energy storage. RSC Adv. 2016, 6, 96327–96333. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sheng, N.; Saito, G.; Akiyama, T. Microencapsulation of metal-based phase change material for high–temperature thermal energy storage. Sci. Rep. 2015, 5, 9117. [Google Scholar] [CrossRef]
- Zhu, S.L.; Nguyen, M.T.; Tokunaga, T.; Yonezawa, T. Size–tunable alumina–encapsulated Sn-based phase change materials for thermal energy storage. ACS Appl. Nano Mater. 2019, 2, 3752–3760. [Google Scholar] [CrossRef]
- Li, Q.L.; Ma, X.D.; Zhang, X.Y.; Zhang, J.B.; Ma, J.Q.; Hu, X.W.; Lan, Y.F. Preparation of a new capsule phase change material for high temperature thermal energy storage. J. Alloys Compd. 2021, 868, 159179. [Google Scholar] [CrossRef]
- Bao, J.M.; Zou, D.Q.; Zhu, S.X.; Ma, Q.; Wang, Y.S.; Hu, Y.P. A medium–temperature, metal–based, microencapsulated phase change material with a void for thermal expansion. Chem. Eng. J. 2021, 415, 128965. [Google Scholar] [CrossRef]
- Moser, Z.; Dutkiewicz, J.; Gasior, W.; Salawa, J. The Sn–Zn (tin–zinc) system. Bull. Alloy Phase Diagr. 1985, 6, 330–334. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, J.; Makov, G. Thermodynamic calculation of Bi–Sn alloy phase diagram under pressure with advanced density measurements. Metals Mater. Int. 2020, 26, 586–590. [Google Scholar] [CrossRef]
- Friedman, H.; Reich, S.; Popovitz–Biro, R.; Huth, V.P.; Halevy, I.; Koltypin, Y.; Gedanken, A.; Porat, Z. Micro–and nano–spheres of low melting point metals and alloys formed by ultrasonic cavitation. Ultrason. Sonochem. 2013, 20, 432–444. [Google Scholar] [CrossRef]
- Song, D.Y.; Maruoka, N.; Maeyama, T.; Shibata, H.; Kitamura, S.Y. Influence of bottom bubbling condition on metal emulsion formation in lead–salt system. ISIJ Int. 2010, 50, 1539–1545. [Google Scholar] [CrossRef][Green Version]
- Song, D.Y.; Maruoka, N.; Shibata, H.; Kitamura, S.Y.; Sasaki, N. Influence of bottom bubbling rate on formation of metal emulsion in Sn–Sb–Cu alloy and molten salt system. ISIJ Int. 2017, 57, 236–244. [Google Scholar] [CrossRef]
- Song, D.Y.; Maruoka, N.; Gupta, G.S.; Shibata, H.; Kitamura, S.; Sasaki, N.; Ogawa, Y.; Matsuo, M. Influence of bottom bubbling rate on formation of metal emulsion in Al–Cu alloy and molten salt system. ISIJ Int. 2012, 52, 1018–1025. [Google Scholar] [CrossRef]
- Roy, R.R.; Utlgard, T.A. Interfacial tension between aluminum and NaCl–KCl–based salt systems. Metall. Mater. Trans. B 1998, 29, 821–827. [Google Scholar] [CrossRef]
- Tanaka, T.; Goto, H.; Nakamoto, M.; Suzuki, M.; Hanao, M.; Zeze, M.; Yamamura, H.; Yoshikawa, T. Dynamic changes in interfacial tension between liquid Fe alloy and molten slag induced by chemical reactions. ISIJ Int. 2016, 56, 944–952. [Google Scholar] [CrossRef]
- Reding, J.N. Interfacial tensions between molten magnesium and salts of the MgCl2–KCl–BaCl2 system. J. Chem. Eng. Data 1971, 16, 190–195. [Google Scholar] [CrossRef]
- Silny, A.; Utigard, T.A. Interfacial tension between aluminum and chloride–fluoride melts. J. Chem. Eng. Data 1996, 41, 1340–1345. [Google Scholar] [CrossRef]
- Ye, J.; Yogeshwar, S. Interaction and interfacial tension between aluminum alloys and molten salts. Mater. Trans. JIM 1996, 37, 1479–1485. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal stability of the eutectic composition in LiNO3–NaNO3–KNO3 ternary system used for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
- Wu, Y.T.; Li, Y.; Ren, N.; Zhi, R.P.; Ma, C.F. Experimental study on the thermal stability of a new molten salt with low melting point for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2018, 176, 181–189. [Google Scholar] [CrossRef]
- Zeppieri, S.; Rodrıguez, J.; Lopez de Ramos, A.L. Interfacial tension of alkane + water systems. J. Chem. Eng. Data 2001, 46, 1086–1088. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Good, R.J. A theory for the estimation of surface and interfacial energies. I. derivation and application to interfacial tension. J. Phys. Chem. 1957, 61, 904–909. [Google Scholar] [CrossRef]
- Pickering, S.U. Emulsions. J. Chem. Soc. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.A.; Xu, Y.; Dong, J. A review on of phase change materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Zahir, M.H.; Mohamed, S.A.; Saidur, R.; Al–Sulaiman, F.A. Supercooling of phase–change materials and the techniques used to mitigate the phenomenon. Appl. Energy 2019, 240, 793–817. [Google Scholar] [CrossRef]
- Cho, M.G.; Kang, S.K.; Seo, S.K.; Shih, D.Y.; Lee, H.M. Effects of under bump metallization and nickel alloying element on the undercooling behavior of Sn–based, Pb–free solders. J. Mater. Res. 2009, 24, 534–543. [Google Scholar] [CrossRef]
- Kang, S.K.; Cho, M.G.; Lauro, P.; Shih, D.Y. Critical factors affecting the undercooling of Pb–free, flip–chip solder bumps and in–situ observation of solidification process. In Proceedings of the IEEE 57th Electronic Components and Technology Conference, Sparks, NV, USA, 29 May–1 June 2007; pp. 1597–1603. [Google Scholar]
- Bin, Y.; Gao, Y.L.; Zou, C.D.; Zhai, Q.J.; Zhuravlev, E.; Schick, C. Size-dependent undercooling of pure Sn by single particle DSC measurements. Chin. Sci. Bull. 2010, 55, 2063–2065. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Luo, W.; Yu, Y.; Xue, Z.; Zheng, Y.; Liu, Z. Metal Emulsion-Based Synthesis, Characterization, and Properties of Sn-Based Microsphere Phase Change Materials. Molecules 2021, 26, 7449. https://doi.org/10.3390/molecules26247449
Zheng X, Luo W, Yu Y, Xue Z, Zheng Y, Liu Z. Metal Emulsion-Based Synthesis, Characterization, and Properties of Sn-Based Microsphere Phase Change Materials. Molecules. 2021; 26(24):7449. https://doi.org/10.3390/molecules26247449
Chicago/Turabian StyleZheng, Xiali, Wei Luo, Yun Yu, Zebin Xue, Yifan Zheng, and Zongjian Liu. 2021. "Metal Emulsion-Based Synthesis, Characterization, and Properties of Sn-Based Microsphere Phase Change Materials" Molecules 26, no. 24: 7449. https://doi.org/10.3390/molecules26247449
APA StyleZheng, X., Luo, W., Yu, Y., Xue, Z., Zheng, Y., & Liu, Z. (2021). Metal Emulsion-Based Synthesis, Characterization, and Properties of Sn-Based Microsphere Phase Change Materials. Molecules, 26(24), 7449. https://doi.org/10.3390/molecules26247449





