Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Sample Weight
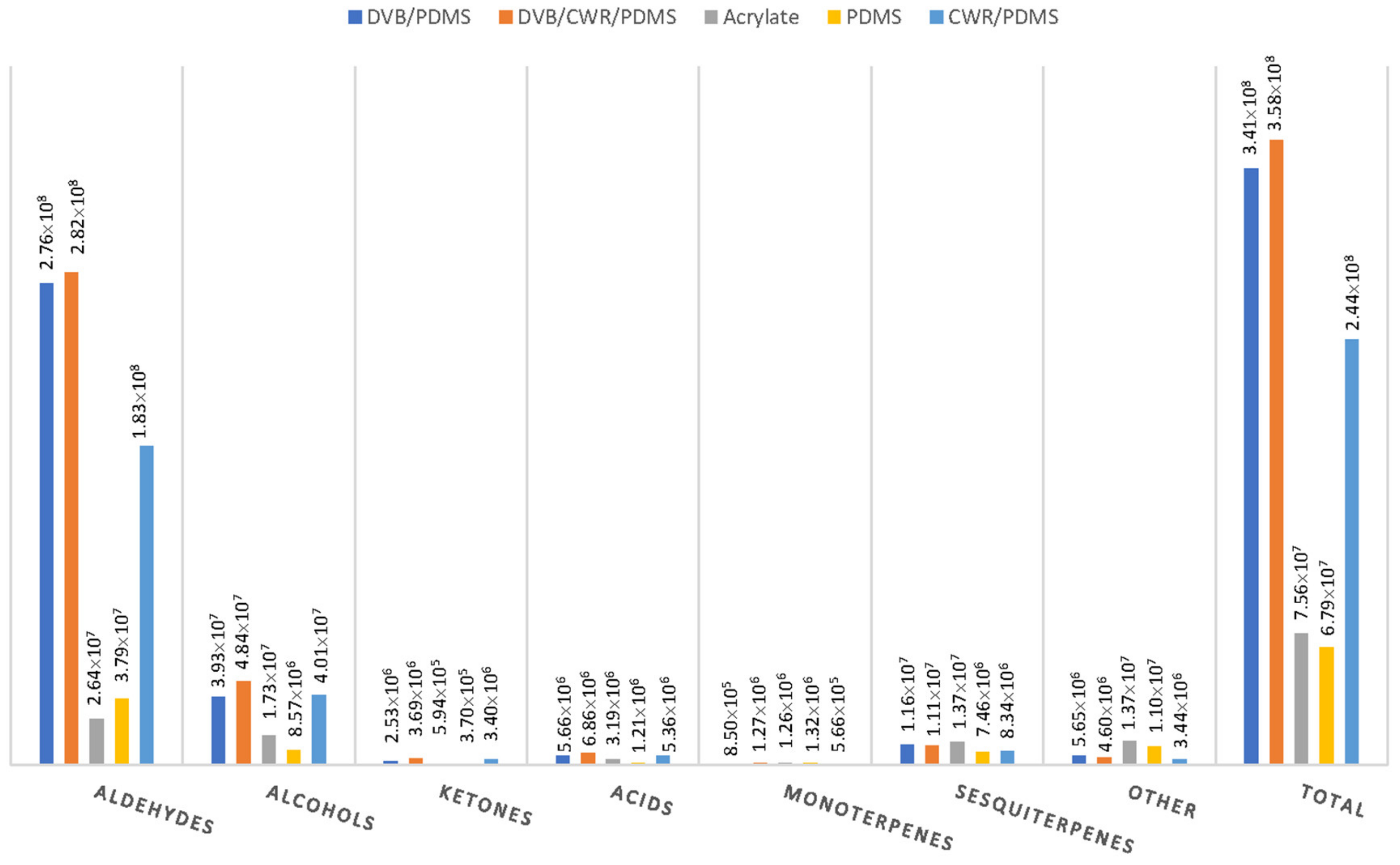
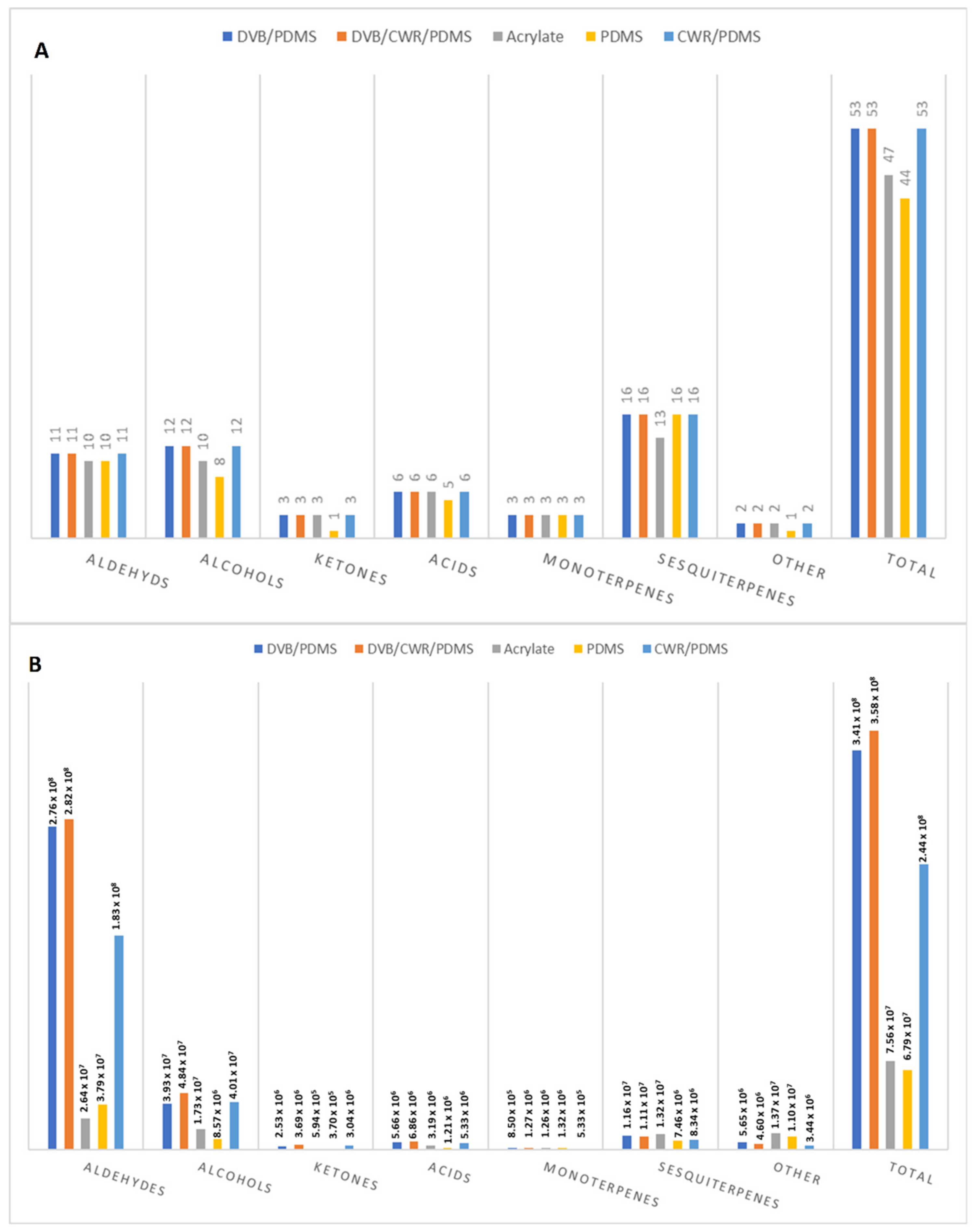
2.2. Selection of the SPME-Arrow Fibre Coating
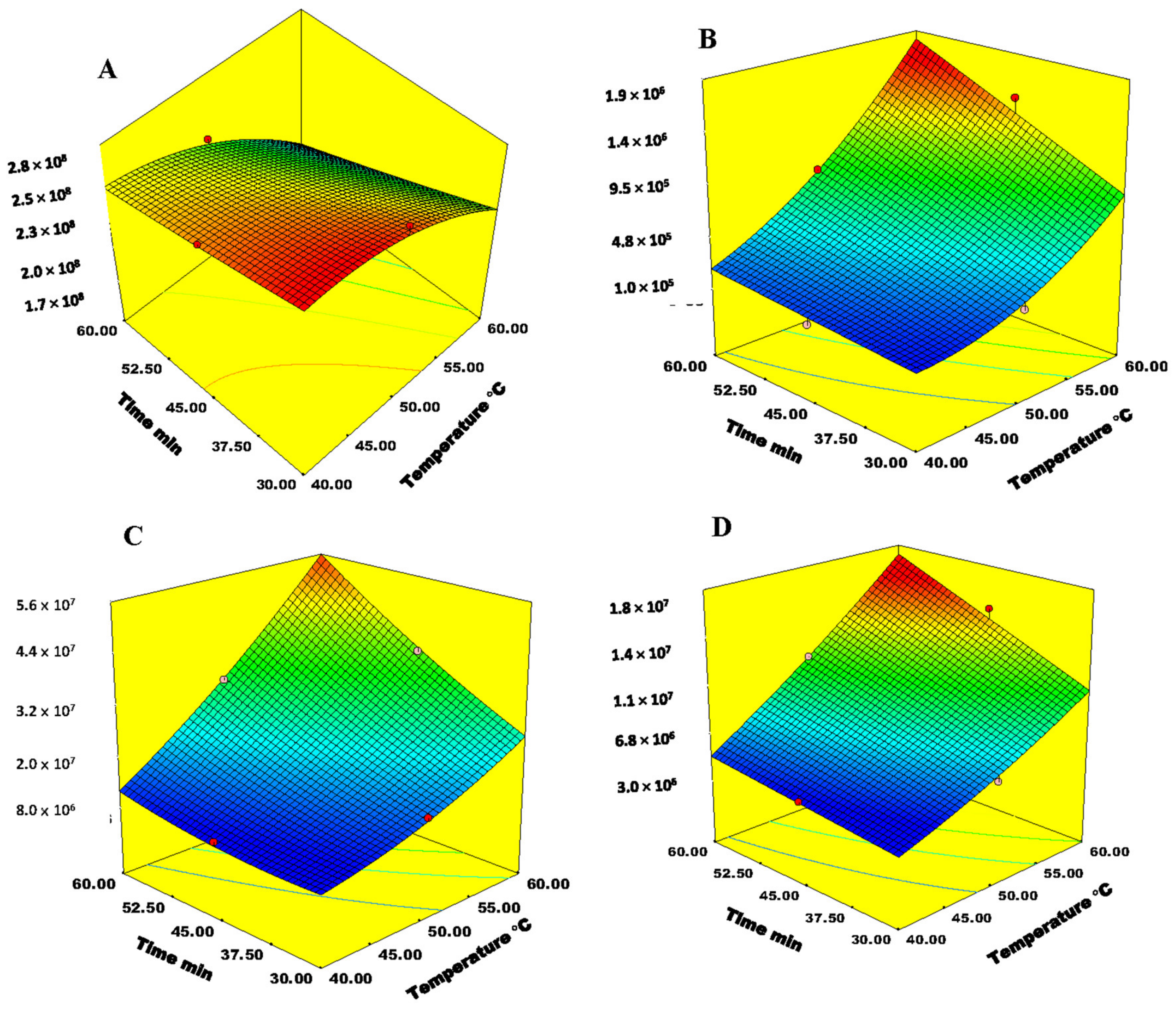
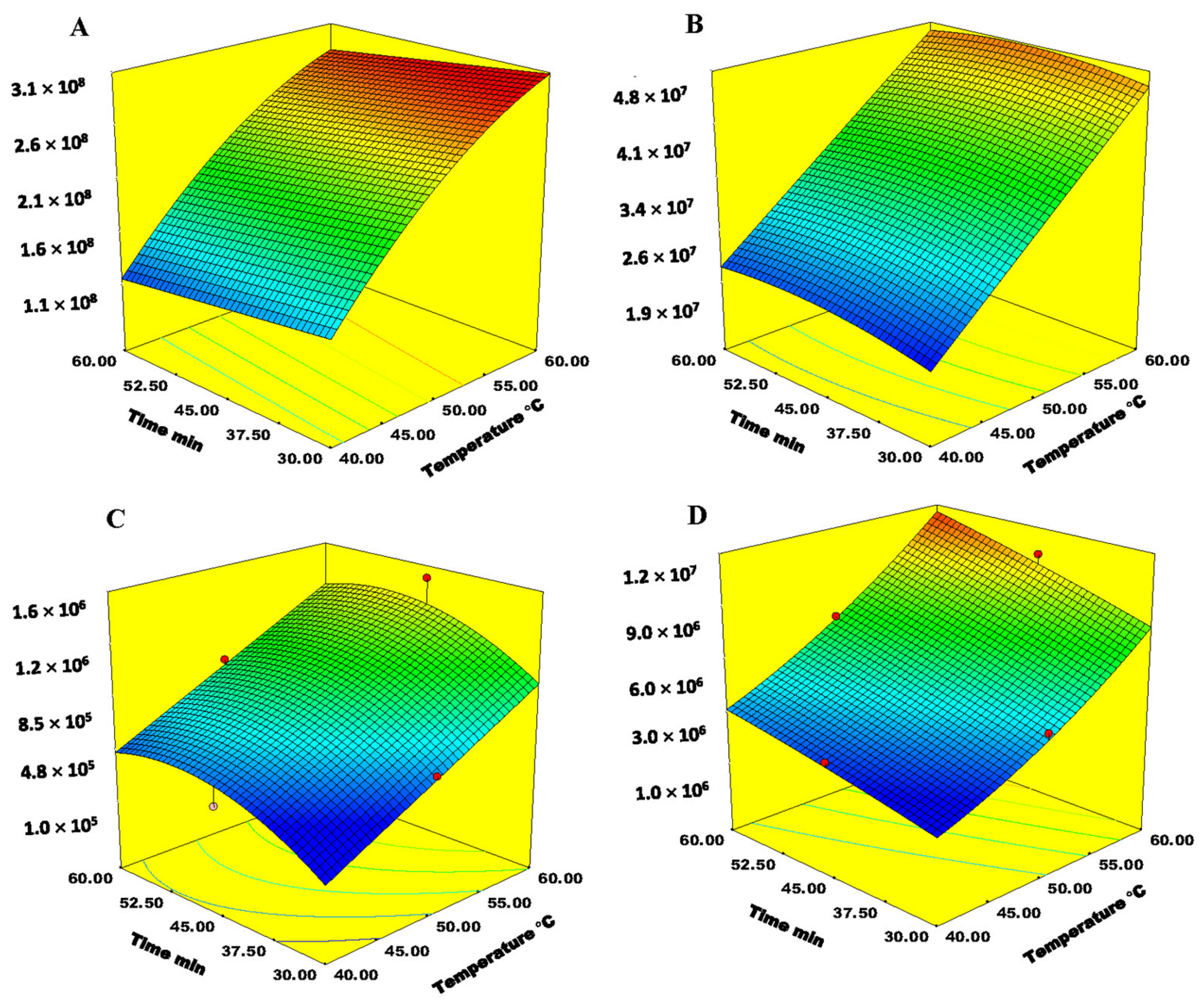
2.3. Optimization of SPME-Arrow Conditions
2.4. Optimization of SPME-Arrow Conditions for Analysis of Bound VOCs
2.5. Method Validation
3. Materials and Methods
3.1. Materials and Reagents
3.2. SPME-Arrow and GC/MS Analysis
3.3. Optimization of SPME-Arrow Method for Determination of Free VOCs
3.3.1. Determination of Sample Weight
3.3.2. Selection of SPME-Arrow Coating
3.4. Experimental Design and Statistical Analysis
3.5. Acid Hydrolysis
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Bisson, L.F.; Waterhouse, A.L.; Ebeler, S.E.; Walker, M.A.; Lapsley, J.T. The present and future of the international wine industry. Nature 2002, 418, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Polaskova, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Zhu, X.L.; Ullah, N.; Tao, Y.S. Aroma Glycosides in Grapes and Wine. J. Food Sci. 2017, 82, 248–259. [Google Scholar] [CrossRef]
- Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Comparison of the Suitability of Different Hydrolytic Strategies to Predict Aroma Potential of Different Grape Varieties. J. Agric. Food Chem. 2009, 57, 2468–2480. [Google Scholar] [CrossRef]
- Roman, S.M.S.; Rubio-Breton, P.; Perez-Alvarez, E.P.; Garde-Cerdan, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef]
- Canuti, V.; Conversano, M.; Calzi, M.L.; Heymann, H.; Matthews, M.A.; Ebeler, S.E. Headspace solid-phase microextraction-gas chromatography-mass spectrometry for profiling free volatile compounds in Cabernet Sauvignon grapes and wines. J. Chromatogr. A 2009, 1216, 3012–3022. [Google Scholar] [CrossRef]
- Panighel, A.; Flamini, R. Applications of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry (SPME-GC/MS) in the Study of Grape and Wine Volatile Compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Palomo, E.; Diaz-Maroto, M.C.; Perez-Coello, M.S. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC-MS. Talanta 2005, 66, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Camara, J.S. Optimisation of solid-phase microextraction combined with gas chromatography-mass spectrometry based methodology to establish the global volatile signature in pulp and skin of Vitis vinifera L. grape varieties. Talanta 2011, 85, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Caldeira, M.; Camara, J.S. Solid phase microextraction as a reliable alternative to conventional extraction techniques to evaluate the pattern of hydrolytically released components in Vitis vinifera L. grapes. Talanta 2012, 95, 1–11. [Google Scholar] [CrossRef]
- Rice, S.; Maurer, D.L.; Fennell, A.; Dharmadhikari, M.; Koziel, J.A. Evaluation of Volatile Metabolites Emitted In-Vivo from Cold-Hardy Grapes during Ripening Using SPME and GC-MS: A Proof-of-Concept. Molecules 2019, 24, 536. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mas, M.C.; Garcia-Riano, L.M.; Alfaro, C.; Rambla, J.L.; Padilla, A.I.; Gutierrez, A. Headspace-based techniques to identify the principal volatile compounds in red grape cultivars. Int. J. Food Sci. Technol. 2009, 44, 510–518. [Google Scholar] [CrossRef]
- Yang, C.X.; Wang, Y.J.; Liang, Z.C.; Fan, P.G.; Wu, B.H.; Yang, L.; Wang, Y.N.; Li, S.H. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC-MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Comparison of major volatile compounds from Riesling and Cabernet Sauvignon grapes (Vitis vinifera L.) from fruitset to harvest. Aust. J. Grape Wine Res. 2010, 16, 337–348. [Google Scholar] [CrossRef]
- Nam, T.G.; Lee, J.Y.; Kim, B.K.; Song, N.E.; Jang, H.W. Analyzing volatiles in brown rice vinegar by headspace solid-phase microextraction (SPME)-Arrow: Optimizing the extraction conditions and comparisons with conventional SPME. Int. J. Food Prop. 2019, 22, 1195–1204. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef]
- Risticevic, S.; Lord, H.; Gorecki, T.; Arthur, C.L.; Pawliszyn, J. Protocol for solid-phase microextraction method development. Nat. Protoc. 2010, 5, 122–139. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyaci, E. Thin film microextraction: Towards faster and more sensitive microextraction. Trac-Trends Anal. Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- Helin, A.; Ronkko, T.; Parshintsev, J.; Hartonen, K.; Schilling, B.; Laubli, T.; Riekkola, M.L. Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J. Chromatogr. A 2015, 1426, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, J.Y.; Choi, Y.S.; Sung, J.M.; Jang, H.W. Comparison of Different Types of SPME Arrow Sorbents to Analyze Volatile Compounds in Cirsium setidens Nakai. Foods 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Chin, Y.W.; Lee, J.Y.; Kim, T.W.; Jang, H.W. Analysis of Volatile Compounds in Soju, a Korean Distilled Spirit, by SPME-Arrow-GC/MS. Foods 2020, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.Z.; Ronkko, T.; Parshintsev, J.; Hartonen, K.; Gan, N.; Sakeye, M.; Sarfraz, J.; Riekkola, M.L. Modified zeolitic imidazolate framework-8 as solid-phase microextraction Arrow coating for sampling of amines in wastewater and food samples followed by gas chromatography-mass spectrometry. J. Chromatogr. A 2017, 1486, 76–85. [Google Scholar] [CrossRef]
- Song, N.E.; Lee, J.Y.; Lee, Y.Y.; Park, J.D.; Jang, H.W. Comparison of headspace-SPME and SPME-Arrow-GC-MS methods for the determination of volatile compounds in Korean salt-fermented fish sauce. Appl. Biol. Chem. 2019, 62, 16. [Google Scholar] [CrossRef]
- Xu, X.B.; Murtada, K.; Pawliszyn, J. Determination of selected volatile terpenes in fish samples via solid phase microextraction arrow coupled with GC-MS. Talanta 2021, 221, 121446. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, W.S.; Lee, Y.Y.; Choi, Y.S.; Choi, H.; Jang, H.W. Solid-phase microextraction Arrow for the volatile organic compounds in soy sauce. J. Sep. Sci. 2019, 42, 2942–2948. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Zachariadis, G.A. Solid-Phase Microextraction Arrow for the Sampling of Volatile Organic Compounds in Milk Samples. Separations 2020, 7, 75. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, C.C.; Wang, L.L.; Chen, S.; Xu, Y. Optimization and validation of a head space solid-phase microextraction-arrow gas chromatography-mass spectrometry method using central composite design for determination of aroma compounds in Chinese liquor (Baijiu). J. Chromatogr. A 2020, 1610, 460584. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.T.; Laboyrie, J.; Marchand-Marion, S.; de Revel, G.; Moio, L.; Riquier, L.; Franc, C. Minty aroma compounds in red wine: Development of a novel automated HS-SPME-arrow and gas chromatography-tandem mass spectrometry quantification method. Food Chem. 2021, 361, 130029. [Google Scholar] [CrossRef]
- Herrington, J.S.; Gomez-Rios, G.A.; Myers, C.; Stidsen, G.; Bell, D.S. Hunting Molecules in Complex Matrices with SPME Arrows: A Review. Separations 2020, 7, 12. [Google Scholar] [CrossRef]
- Tankiewicz, M.; Morrison, C.; Biziuk, M. Application and optimization of headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-flame-ionization detector (GC-FID) to determine products of the petroleum industry in aqueous samples. Microchem. J. 2013, 108, 117–123. [Google Scholar] [CrossRef]
- Carasek, E.; Pawliszyn, J. Screening of tropical fruit volatile compounds using solid-phase microextraction (SPME) fibers and internally cooled SPME fiber. J. Agric. Food Chem. 2006, 54, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Zini, C.A. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, C. Changes in Volatile Compounds. In Sweet, Reinforced and Fortified Wines; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 91–103. [Google Scholar] [CrossRef]
- Baumes, R.; Wirth, J.; Bureau, S.; Gunata, Y.; Razungles, A. Biogeneration of C-13-norisoprenoid compounds: Experiments supportive for an apo-carotenoid pathway in grapevines. Anal. Chim. Acta 2002, 458, 3–14. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Bescansa, L.; Masa, A.; Oliveira, J.M. Changes in free and bound fractions of aroma compounds of four Vitis vinifera cultivars at the last ripening stages. Phytochemistry 2012, 74, 196–205. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Babushok, V.I.; Zenkevich, I.G. Retention Indices for Most Frequently Reported Essential Oil Compounds in GC. Chromatographia 2009, 69, 257–269. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupic, D.; Preiner, D.; Asperger, D.; Kontic, J.K. Solid-liquid Extraction of Phenolics from Red Grape Skins. Acta Chim. Slov. 2016, 63, 287–297. [Google Scholar] [CrossRef][Green Version]

| Terms | Aldehydes | Alcohols | Acids | Ketones | Monoterpenes | Sesquiterpenes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Model | <0.0001 | <0.0001 | <0.0001 | 0.0332 | 0.0235 | <0.0001 | ||||||
| Lack of Fit | 0.5139 | 0.5554 | 0.4554 | 0.9861 | 0.9949 | 0.8611 | ||||||
| Intercept | 1.44 × 1010 | 3.71 × 1010 | 5.84 × 109 | 5.18 × 109 | 1.09 × 109 | 5.80 × 109 | ||||||
| A-Temperature | 4.62 × 109 | <0.0001 | 1.11 × 1010 | <0.0001 | 1.66 × 109 | <0.0001 | −56,416.67 | 0.0403 | 4.10 × 108 | 0.0001 | 3.92 × 109 | <0.0001 |
| B-Incubation | 6.85 × 108 | 0.0761 | 1.29 × 109 | 0.2066 | 2.18 × 108 | 0.2066 | 10,134.22 | 0.9384 | 29810.39 | 0.7139 | 7.56 × 108 | 0.0040 |
| C-Exposure | 1.07 × 109 | 0.0079 | 3.05 × 109 | 0.0063 | 8.62 × 108 | 0.0063 | 2.07 × 108 | 0.0157 | 1.64 × 108 | 0.0412 | 1.05 × 109 | 0.0002 |
| D-Desorption | 5.68 × 108 | 0.1047 | 1.49 × 109 | 0.1200 | 2.33 × 108 | 0.1200 | −1.56 × 108 | 0.2099 | 61,333.33 | 0.4160 | 2.56 × 108 | 0.2151 |
| AB | −1.75 × 108 | 0.7600 | −1.93 × 109 | 0.2342 | −1.16 × 108 | 0.2342 | −2.02 × 108 | 0.3419 | −25,250.00 | 0.8446 | 47807.50 | 0.8900 |
| AC | −2.90 × 108 | 0.0140 | −3.24 × 108 | 0.0370 | 4500.00 | 0.0570 | −4.27 × 108 | 0.0582 | −24000.00 | 0.0422 | 3.15 × 108 | 0.0397 |
| AD | 1.73 × 108 | 0.7633 | −60000.00 | 0.9696 | −1.15 × 108 | 0.9696 | −1.95 × 108 | 0.3583 | 85,750.00 | 0.5094 | 2.35 × 108 | 0.5000 |
| BC | 25880.06 | 0.9712 | 2.59 × 109 | 0.2043 | 1.15 | 0.2043 | −21,152.65 | 0.9354 | −5681.18 | 0.9719 | −4.61 × 108 | 0.2985 |
| BD | 3.05 × 108 | 0.5960 | −1.51 × 109 | 0.3447 | 4.26 × 108 | 0.3447 | 1.98 × 108 | 0.3518 | −1.54 × 108 | 0.2469 | −7.86 × 108 | 0.0387 |
| CD | 15000.00 | 0.9791 | −1.43 × 109 | 0.3709 | −1.20 × 108 | 0.3709 | 100,000.00 | 0.6326 | −76,750.00 | 0.5541 | −3.19 × 108 | 0.3640 |
| A² | 5.78 × 108 | 0.2481 | −22,087.99 | 0.9868 | 4.44 × 108 | 0.9868 | −4.41 × 108 | 0.0257 | −60,420.60 | 0.5834 | 6.93 × 108 | 0.0331 |
| B² | 1.54 × 108 | 0.7642 | −4.91 × 108 | 0.7279 | 3.67 × 108 | 0.7279 | −1149.79 | 0.9951 | −1.10 × 108 | 0.3493 | 2.86 × 108 | 0.3643 |
| C² | −2.17 × 108 | 0.6746 | −1.33 × 109 | 0.3559 | −2.69 × 108 | 0.3559 | −4.24 × 108 | 0.0391 | −2.18 × 108 | 0.0782 | −1.23 × 108 | 0.6934 |
| D² | −4.82 × 108 | 0.3314 | −1.92 × 109 | 0.1683 | 1.28 × 108 | 0.1683 | −89,476.12 | 0.6151 | −2.07 × 108 | 0.0776 | −3.63 × 108 | 0.2310 |
| R2 | 0.9501 | 0.9379 | 0.9408 | 0.9640 | 0.9919 | 0.9752 | ||||||
| Adapted R2 | 0.8918 | 0.8655 | 0.8655 | 0.8719 | 0.8490 | 0.9463 | ||||||
| Precision | 124.7404 | 123.606 | 123.606 | 55.799 | 62.073 | 196.936 | ||||||
| Group | Temperature (°C) | Incubation Time (min) | Exposure Time (min) | Desorption Time (min) | Predicted Value (Peak Area × 106) | Obtained Value (Peak Area × 106, Mean ± SD) |
|---|---|---|---|---|---|---|
| Aldehydes | 60 | 20 | 49 | 7 | 293.00 | 298.00 ± 2.50 |
| Alcohols | 48.00 | 47.50 ± 0.95 | ||||
| Ketones | 4.80 | 4.76 ± 0.05 | ||||
| Acids | 8.34 | 8.39 ± 0.09 | ||||
| Monoterpenes | 1.32 | 1.37 ± 0.02 | ||||
| Sesquiterpenes | 10.30 | 11.00 ± 0.31 |
| Terms | Alcohols | Acids | Carbonyls | Norisoprenoids | Monoterpenes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Model | 0.0064 | <0.0001 | 0.0364 | 0.0008 | 0.0012 | |||||
| Lack of Fit | 0.6491 | 0.6201 | 0.7224 | 0.2524 | 0.6034 | |||||
| Intercept | 2.29 × 1011 | 2.30 × 1010 | 3.10 × 1010 | 4.28 × 108 | 8.37 × 109 | |||||
| A-Temperature | −2.92 × 1010 | 0.0027 | 1.40 × 1010 | <0.0001 | 3.83 × 109 | 0.0436 | 6.14 × 108 | <0.0001 | 4.02 × 109 | <0.0001 |
| B-Incubation | −9.16 × 109 | 0.1450 | −4.94 × 108 | 0.4751 | 3.89 × 109 | 0.0415 | 12,522.50 | 0.7835 | −2.35 × 108 | 0.4811 |
| C-Exposure | −1.60 × 1010 | 0.0296 | 8.24 × 109 | <0.0001 | 6.86 × 109 | 0.0048 | 2.73 × 108 | 0.0015 | 2.15 × 109 | 0.0009 |
| AB | 2.03 × 109 | 0.7981 | −1.13 × 109 | 0.2686 | −4.94 × 108 | 0.8162 | −24,545.00 | 0.7043 | −7.39 × 108 | 0.1516 |
| AC | −6.91 × 109 | 0.0397 | 6.64 × 109 | 0.0007 | −1.98 × 109 | 0.1424 | 1.99 × 108 | 0.0226 | 1.32 × 109 | 0.0293 |
| BC | 5.68 × 109 | 0.4833 | −3.58 × 108 | 0.7089 | 1.19 × 109 | 0.5796 | −20,000.00 | 0.7566 | −32425.00 | 0.9437 |
| A² | −1.62 × 1010 | 0.0936 | 2.25 × 109 | 0.0622 | 4.40 × 108 | 0.8424 | 2.00 × 108 | 0.0255 | 4.44 × 109 | 0.3735 |
| B² | 1.02 × 1010 | 0.2482 | −1.80 × 109 | 0.1141 | 4.81 × 109 | 0.0706 | 1.24 × 108 | 0.1090 | 1.46 × 109 | 0.7614 |
| C² | 9.63 × 108 | 0.9067 | 1.57 × 109 | 0.1557 | −4.57 × 108 | 0.8364 | −7315.00 | 0.9129 | −1.93 × 109 | 0.6885 |
| R2 | 0.9095 | 0.9931 | 0.8991 | 0.9816 | 0.9788 | |||||
| Adapted R2 | 0.8467 | 0.9806 | 0.9176 | 0.9484 | 0.9407 | |||||
| Precision | 89.161 | 317.821 | 87.989 | 177.890 | 172.746 | |||||
| Group | Temperature (°C) | Incubation Time (min) | Exposure Time (min) | Predicted Value (Peak Area × 106) | Obtained Value (Peak Area × 106, Mean ± SD) |
|---|---|---|---|---|---|
| Alcohols | 60 | 20 | 60 | 202.00 | 209.00 ± 9.14 |
| Acids | 49.30 | 49.90 ± 1.35 | |||
| Carbonyls | 40.20 | 39.80 ± 0.49 | |||
| Norisoprenoids | 1.53 | 1.47 ± 0.15 | |||
| Monoterpenes | 15.50 | 16.20 ± 0.89 |
| Factors | Factor Levels | ||
|---|---|---|---|
| Coded levels | −1 | 0 | 1 |
| A: Extraction temperature (°C) | 40 | 50 | 60 |
| B: Incubation time (min) | 10 | 20 | 30 |
| C: Exposure time (min) | 30 | 45 | 60 |
| D: Desorption time (min) | 5 | 7.5 | 10 |
| Factors | Factor Levels | ||
|---|---|---|---|
| Coded levels | −1 | 0 | 1 |
| A: Extraction temperature (°C) | 40 | 50 | 60 |
| B: Incubation time (min) | 10 | 20 | 30 |
| C: Exposure time (min) | 30 | 45 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šikuten, I.; Štambuk, P.; Karoglan Kontić, J.; Maletić, E.; Tomaz, I.; Preiner, D. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules 2021, 26, 7409. https://doi.org/10.3390/molecules26237409
Šikuten I, Štambuk P, Karoglan Kontić J, Maletić E, Tomaz I, Preiner D. Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules. 2021; 26(23):7409. https://doi.org/10.3390/molecules26237409
Chicago/Turabian StyleŠikuten, Iva, Petra Štambuk, Jasminka Karoglan Kontić, Edi Maletić, Ivana Tomaz, and Darko Preiner. 2021. "Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins" Molecules 26, no. 23: 7409. https://doi.org/10.3390/molecules26237409
APA StyleŠikuten, I., Štambuk, P., Karoglan Kontić, J., Maletić, E., Tomaz, I., & Preiner, D. (2021). Optimization of SPME-Arrow-GC/MS Method for Determination of Free and Bound Volatile Organic Compounds from Grape Skins. Molecules, 26(23), 7409. https://doi.org/10.3390/molecules26237409








