The Polyphenols α-Mangostin and Nordihydroguaiaretic Acid Induce Oxidative Stress, Cell Cycle Arrest, and Apoptosis in a Cellular Model of Medulloblastoma
Abstract
1. Introduction
2. Results
2.1. Effects of α-Mangostin and NDGA on Cell Viability in Daoy Cells
2.2. The α-Mangostin and NDGA Promote Oxidative Stress in Daoy Cells
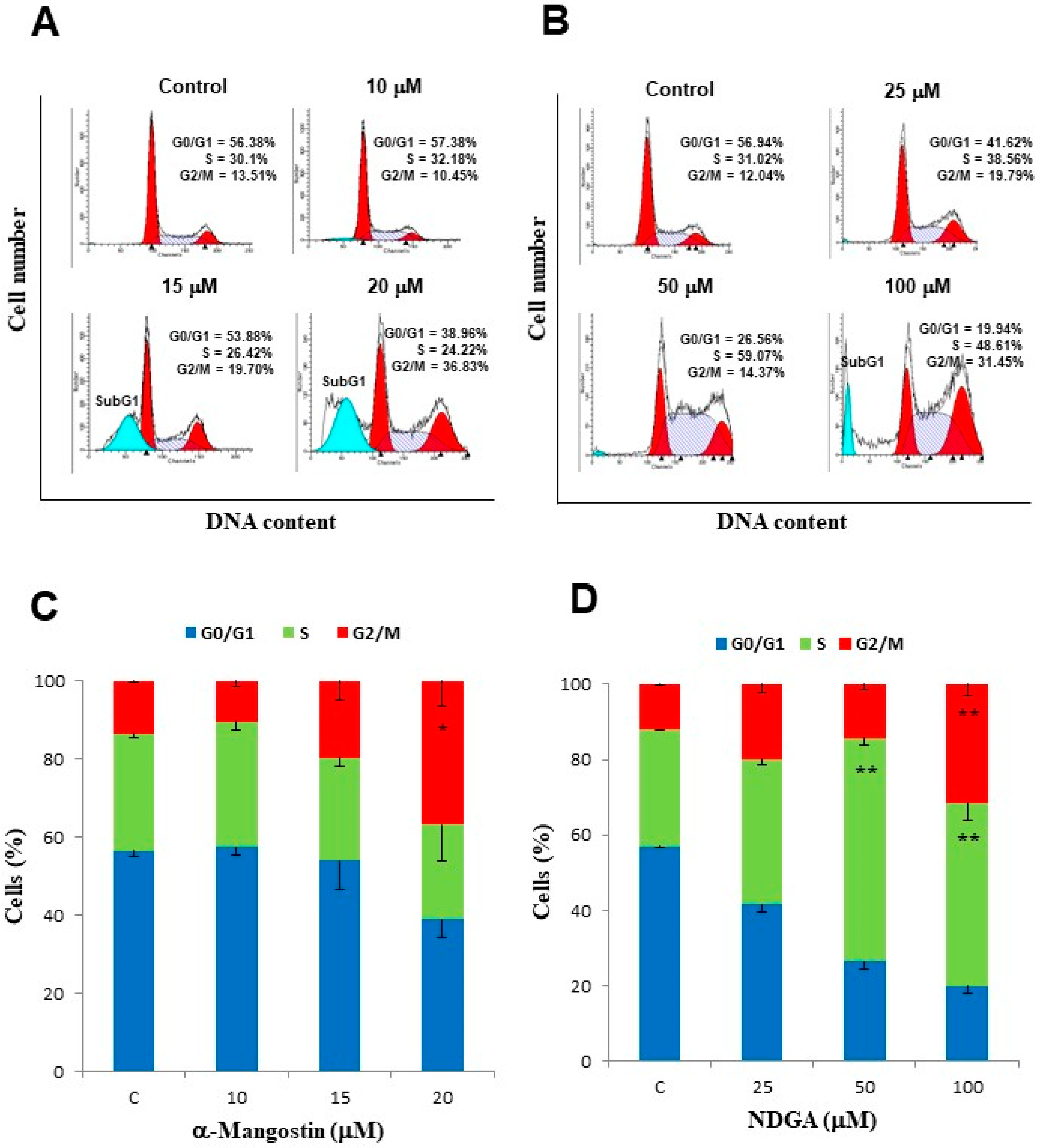
2.3. α-Mangostin and NDGA Induced Arrest in Different Stages of the Cell Cycle in Daoy Cells
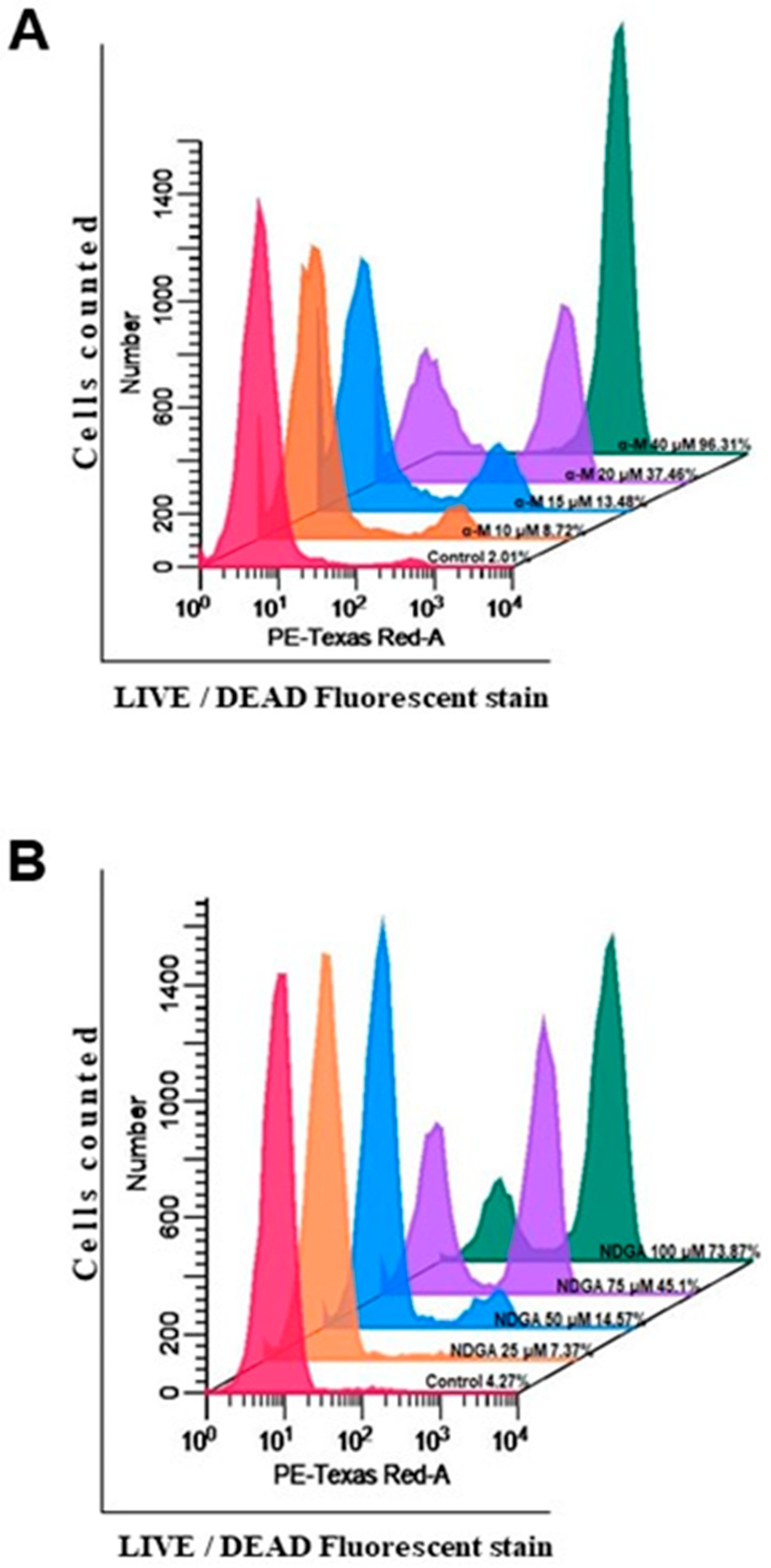
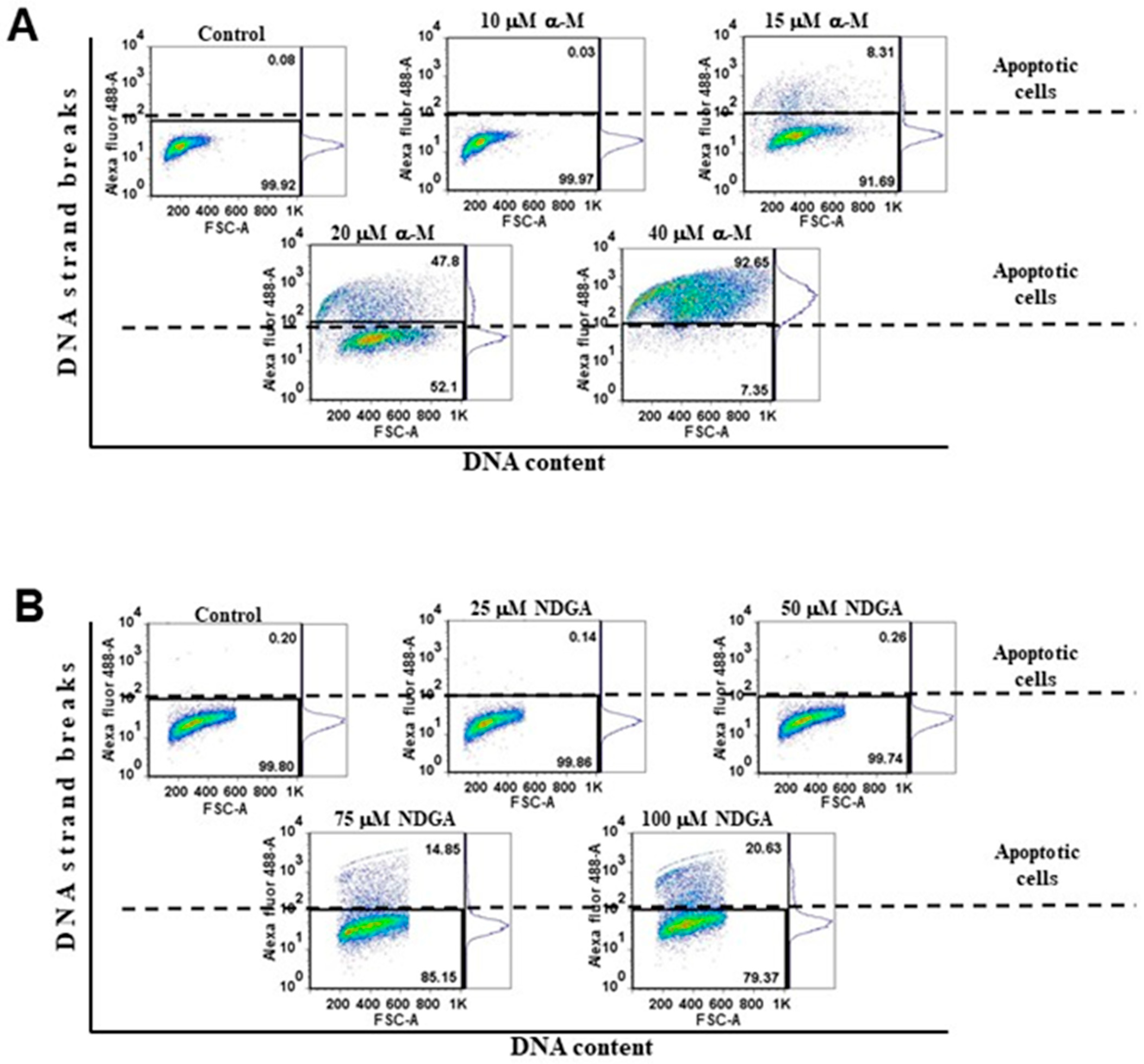
2.4. α-Mangostin and NDGA Promoted Cell Death in Daoy Cells by Triggering Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cell Culture
4.2. Cellular Treatments
4.3. Cell Viability Assays
4.4. Carbonyl Proteins and GSH and GSSG Quantifications
4.5. Cell Cycle Analysis
4.6. Cell Death Assay
4.7. TUNEL Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Packer, R.J.; Cogen, P.; Vezina, G.; Rorke, L.B. Medulloblastoma: Clinical and biologic aspects. Neuro Oncol. 1999, 1, 232–250. [Google Scholar] [CrossRef]
- Packer, R.J.; MacDonald, T.; Vezina, G. Central nervous system tumors. Pediatr. Clin. N. Am. 2008, 55, 121–145. [Google Scholar] [CrossRef]
- Roussel, M.F.; Robinson, G. Medulloblastoma. advances and challenges. F1000 Biol. Rep. 2011, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Dillinger, T.L.; Barriga, P.; Escarcega, S.; Jimenez, M.; Salazar, L.D.; Grivetti, L.E. Food of the gods: Cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000, 130 (Suppl. 8S), 2057S–2072S. [Google Scholar] [CrossRef] [PubMed]
- Ramiro-Puig, E.; Castell, M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr. 2009, 101, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- D’Archivio, M.; Santangelo, C.; Scazzocchio, B.; Vari, R.; Filesi, C.; Masella, R.; Giovannini, C. Modulatory effects of polyphenols on apoptosis induction: Relevance for cáncer prevention. Int. J. Mol. Sci. 2008, 9, 213–228. [Google Scholar] [CrossRef]
- Labbé, D.; Provencal, M.; Lamy, S.; Boivin, D.; Gingras, D.; Béliveau, R. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J. Nutr. 2009, 139, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, S.; Andrade, C.A.; Cárdenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Etnhopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef]
- Chan, J.K.W.; Bittner, S.; Bittner, A.; Atwal, S.; Shen, W.J.; Inayathullah, M.; Rajada, J.; Nicolls, M.R.; Kraemer, F.B.; Azhar, S. Nordihydroguaiaretic acid, a lignan from Larrea tridentata (Creosote Bush), protects against american lifestyle-induced obesity syndrome dietinduced metabolic dysfunction in mice. J. Pharmacol. Exp. Ther. 2018, 365, 281–290. [Google Scholar] [CrossRef]
- Huang, H.J.; Chen, W.L.; Hsieh, R.H.; Hsieh-Li, H.M. Multifunctional effects of mangosteen pericarp on cognition in C57BL/6J and triple transgenic Alzheimer’s mice. Evidence-based complement. Altern. Med. 2014, 2014, 813672. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa, Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Failla, M.L. Biological activities and bioavailability of mangosteen xanthones: A critical review of the current evidence. Nutrients 2013, 5, 3163–3183. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef]
- Li, G.; Petiwala, S.M.; Nonn, L.; Johnson, J.J. Inhibition of CHOP accentuates the apoptotic effect of α-mangostin from the mangosteen fruit (Garcinia mangostana) in 22Rv1 prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 453, 75–80. [Google Scholar] [CrossRef]
- Won, Y.S.; Lee, J.H.; Kwon, S.J.; Kim, J.Y.; Park, K.H.; Lee, M.K.; Seo, K.-I. α-Mangostin-induced apoptosis is mediated by estrogen receptor α in human breast cancer cells. Food Chem. Toxicol. 2014, 66, 158–165. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 2003, 66, 1124–1127. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Yi, H.; Ohguchi, K.; Ito, T.; Tanaka, T.; Kobayashi, E.; Iinuma, M.; Nozawa, Y. Preferential target is mitochondria in α-mangostin-induced apoptosis in human leukemia HL60 cells. Bioorg. Med. Chem. 2004, 12, 5799–5806. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Iinuma, M.; Naoe, T.; Nozawa, Y.; Akao, Y. Characterized mechanism of α-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem. 2007, 15, 5620–5628. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, J.; Lei, J.; Duan, W.; Sheng, L.; Chen, X.; Hu, A.; Wang, Z.; Wu, Z.; Wu, E.; et al. α-Mangostin suppresses the viability and epithelial-mesenchymal transition of pancreatic cancer cells by downregulating the PI3K/Akt pathway. BioMed Res. Int. 2014, 2014, 546353. [Google Scholar] [CrossRef]
- Beninati, S.; Oliverio, S.; Cordella, M.; Rossi, S.; Senatore, C.; Liguori, I.; Lentini, A.; Piredda, L.; Tabolacci, C. Inhibition of cell proliferation, migration and invasion of B16-F10 melanoma cells by α-mangostin. Biochem. Biophys. Res. Commun. 2014, 450, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, S.A.; Yahayu, M.; Ali, L.Z.; ElhagIshag, O. α-Mangostin from Cratoxylum Arborescens: An in Vitro and in Vivo Toxicological Evaluation. Arab. J. Chem. 2015, 8, 129–137. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, F.; Dong, Y.; Ren, F. α-Mangostin Induces Apoptosis in Human Osteosarcoma Cells Through ROS-Mediated Endoplasmic Reticulum Stress via the WNT Pathway. Cell Transplant. 2021, 30, 9636897211035080. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.L.; Ozbay, T.; Bender, L.M.; Nahta, R. Nordihydroguaiaretic acid, a cytotoxic insulin-like growth factor-I receptor/HER2 inhibitor in trastuzumab-resistant breast cancer. Mol. Cancer Ther. 2008, 7, 1900–1908. [Google Scholar] [CrossRef][Green Version]
- Gao, P.; Zhai, F.; Guan, L.; Zheng, J. Nordihydroguaiaretic acid inhibits growth of cervical cancer SiHa cells by up-regulating p21. Oncol. Lett. 2011, 2, 123–128. [Google Scholar] [CrossRef]
- Li, X.; Fan, S.; Pan, X.; Xiaokaiti, Y.; Duan, J.; Shi, Y.; Pan, Y.; Tie, L.; Wang, X.; Li, Y.; et al. Nordihydroguaiaretic acid impairs prostate cancer cell migration and tumor metastasis by suppressing neuropilin 1. Oncotarget 2016, 7, 86225–86238. [Google Scholar] [CrossRef]
- Sahu, S.C.; Ruggles, D.I.; O’Donnell, M.W. Prooxidant Activity and Toxicity of Nordihydroguaiaretic Acid in Clone-9 Rat Hepatocyte Cultures. Food Chem. Toxicol. 2006, 44, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Leon, D.; Parada, D.; Vargas-Uribe, M.; Perez, A.A.; Ojeda, L.; Zambrano, A.; Reyes, A.M.; Salas, M. Effect of Nordihydroguaiaretic Acid on Cell Viability and Glucose Transport in Human Leukemic Cell Lines. FEBS Open Bio 2016, 6, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Zhang, L.; Ji, H.; Kou, Y.; Ou, B. Bioavailability and antioxidant effects of a xanthone-rich mangosteen (Garcinia mangostana) product in humans. J. Agric. Food Chem. 2009, 57, 8788–8792. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef]
- Chang, C.W.; Huang, T.Z.; Chang, W.H.; Tseng, Y.C.; Wu, Y.T.; Hsu, M.C. Acute Garcinia mangostana (mangosteen) supplementation does not alleviate physical fatigue during exercise: A randomized, double-blind, placebocontrolled, crossover trial. J. Int. Soc. Sports Nutr. 2016, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Rojo, A.I.; Martínez-Klimova, E.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic Acid: From Herbal Medicine to Clinical Development for Cancer and Chronic Diseases. Front. Pharmacol. 2020, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, T.W.; Weinberg, V.K.; Huang, Y.; Mi, J.T.; Formaker, C.G.; Small, E.J.; Harzstark, A.L.; Lin, A.M.; Fong, L.; Ryan, C.J. A phase II study of insulin-like growth factor receptor inhibition with nordihydroguaiaretic acid in men with non-metastatic hormone-sensitive prostate cancer. Oncol. Rep. 2012, 27, 3–9. [Google Scholar] [CrossRef][Green Version]
- Grossman, S.A.; Ye, X.; Peereboom, D.; Rosenfeld, M.R.; Mikkelsen, T.; Supko, J.G.; Desideri, S. Phase I study of terameprocol in patients with recurrent high-grade glioma. Neuro Oncol. 2012, 14, 511–517. [Google Scholar] [CrossRef]
- Li, P.; Tian, W.; Ma, X. Alpha-mangostin inhibits intracellular fatty acid synthase and induces apoptosis in breast cancer cells. Mol. Cancer 2014, 13, 138. [Google Scholar] [CrossRef]
- Kurose, H.; Shibata, M.A.; Linuma, M.; Otsuki, Y. Alterations in cell cycle and induction of apoptotic cell death in breast cancer cells treated with α-mangostin extracted from mangosteen pericarp. J. Biomed. Biotechnol. 2012, 2012, 672428. [Google Scholar] [CrossRef]
- Mizushina, Y.; Kuriyama, I.; Nakahara, T.; Kawashima, Y.; Yoshida, H. Inhibitory effects of α-mangostin on mammalian DNA polymerase, topoisomerase, and human cancer cell proliferation. Food Chem. Toxicol. 2013, 59, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Akao, Y.; Ohguchi, K.; Ito, T.; Tanaka, T.; Linuma, M.; Nozawa, T. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg. Med. Chem. 2005, 13, 6064–6069. [Google Scholar] [CrossRef]
- Lee, C.H.; Ying, T.H.; Chiou, H.L.; Hsieh, S.C.; Wen, S.H.; Chou, R.H.; Hsieh, Y.H. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017, 8, 47425–47439. [Google Scholar] [CrossRef]
- Kwak, H.H.; Kim, I.R.; Kim, H.J.; Park, B.S.; Yu, S.B. α-Mangostin induces apoptosis and cell cycle arrest in oral squamous cell carcinoma cell. Evid. Based Complement. Altern. Med. 2016, 2016, 5352412. [Google Scholar] [CrossRef] [PubMed]
- Zavodovskaya, M.; Campbell, M.J.; Maddux, B.A.; Shiry, L.; Allan, G.; Hodges, L.; Kushner, P.; Kener, J.A.; Youngren, J.F.; Goldfine, I.D. Nordihydroguaiaretic acid (NDGA), an inhibitor of the HER2 and IGF-1 receptor tyrosine kinases, blocks the growth of HER2-overexpressing human breast cancer cells. J. Cell. Biochem. 2008, 103, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.E.; Chesler, L.; Liu, D.; Gable, K.; Maddux, B.A.; Goldenberg, D.D.; Youngren, J.F.; Goldfine, I.D.; Weiss, W.A.; Matthay, K.K.; et al. Nordihydroguaiaretic acid inhibits insulin-like growth factor signaling, growth, and survival in human neuroblastoma cells. J. Cell. Biochem. 2007, 102, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.W.; Lin, Y.; Xu, C.R.; Yao, Y.L.; Cui, Y.H.; Zhang, X.; Bian, X.W. NDGA-P21, a novel derivative of nordihydroguaiaretic acid, inhibits glioma cell proliferation and stemness. Lab. Investig. 2017, 97, 1180–1187. [Google Scholar] [CrossRef]
- Meyer, A.N.; McAndrew, C.W.; Donoghue, D.J. Nordihydroguaiaretic acid inhibits an activated fibroblast growth factor receptor 3 mutant and blocks downstream signaling in multiple myeloma cells. Cancer Res. 2008, 68, 7362–7370. [Google Scholar] [CrossRef]
- Pérez-Rojas, J.M.; González-Macías, R.; González-Cortes, J.; Jurado, R.; Pedraza-Chaverri, J.; García-López, P. Synergic Effect of α-Mangostin on the Cytotoxicity of Cisplatin in a Cervical Cancer Model. Oxid. Med. Cell. Longev. 2016, 2016, 7981397. [Google Scholar] [CrossRef]
- Soriano, A.F.; Helfrich, B.; Chan, D.C.; Heasley, L.E.; Bunn, P.A.; Chou, T.C. Synergistic Effects of New Chemopreventive Agents and Conventional Cytotoxic Agents against Human Lung Cancer Cell Lines. Cancer Res. 1999, 59, 6178–6184. [Google Scholar]
- Nava, C.M.; Acero, G.; Pedraza-Chaverrì, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27. [Google Scholar] [CrossRef]
- Marquez-Valadez, B.; Maldonado, P.D.; Galvan-Arzate, S.; Mendez-Cuesta, L.A.; Perez de la Cruz, V.; Pedraza-Chaverri, J.; Chánez-Cárdenas, M.E.; Santamaría, A. Alpha mangostin induces changes in glutathione levels associated with glutathione peroxidase activity in rat brain synaptosomes. Nutr. Neurosci. 2012, 15, 13–19. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Meth. Enzymol. 1994, 233, 346–357. [Google Scholar]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Ochoa, A.; Córdova, E.J.; Carrillo-García, A.; Lizano, M.; Pedraza-Chaverri, J.; Patiño, N.; Cruz-Gregorio, A.; Osnaya, N. The Polyphenols α-Mangostin and Nordihydroguaiaretic Acid Induce Oxidative Stress, Cell Cycle Arrest, and Apoptosis in a Cellular Model of Medulloblastoma. Molecules 2021, 26, 7230. https://doi.org/10.3390/molecules26237230
Rojas-Ochoa A, Córdova EJ, Carrillo-García A, Lizano M, Pedraza-Chaverri J, Patiño N, Cruz-Gregorio A, Osnaya N. The Polyphenols α-Mangostin and Nordihydroguaiaretic Acid Induce Oxidative Stress, Cell Cycle Arrest, and Apoptosis in a Cellular Model of Medulloblastoma. Molecules. 2021; 26(23):7230. https://doi.org/10.3390/molecules26237230
Chicago/Turabian StyleRojas-Ochoa, Alberto, Emilio J. Córdova, Adela Carrillo-García, Marcela Lizano, José Pedraza-Chaverri, Nelly Patiño, Alfredo Cruz-Gregorio, and Norma Osnaya. 2021. "The Polyphenols α-Mangostin and Nordihydroguaiaretic Acid Induce Oxidative Stress, Cell Cycle Arrest, and Apoptosis in a Cellular Model of Medulloblastoma" Molecules 26, no. 23: 7230. https://doi.org/10.3390/molecules26237230
APA StyleRojas-Ochoa, A., Córdova, E. J., Carrillo-García, A., Lizano, M., Pedraza-Chaverri, J., Patiño, N., Cruz-Gregorio, A., & Osnaya, N. (2021). The Polyphenols α-Mangostin and Nordihydroguaiaretic Acid Induce Oxidative Stress, Cell Cycle Arrest, and Apoptosis in a Cellular Model of Medulloblastoma. Molecules, 26(23), 7230. https://doi.org/10.3390/molecules26237230







