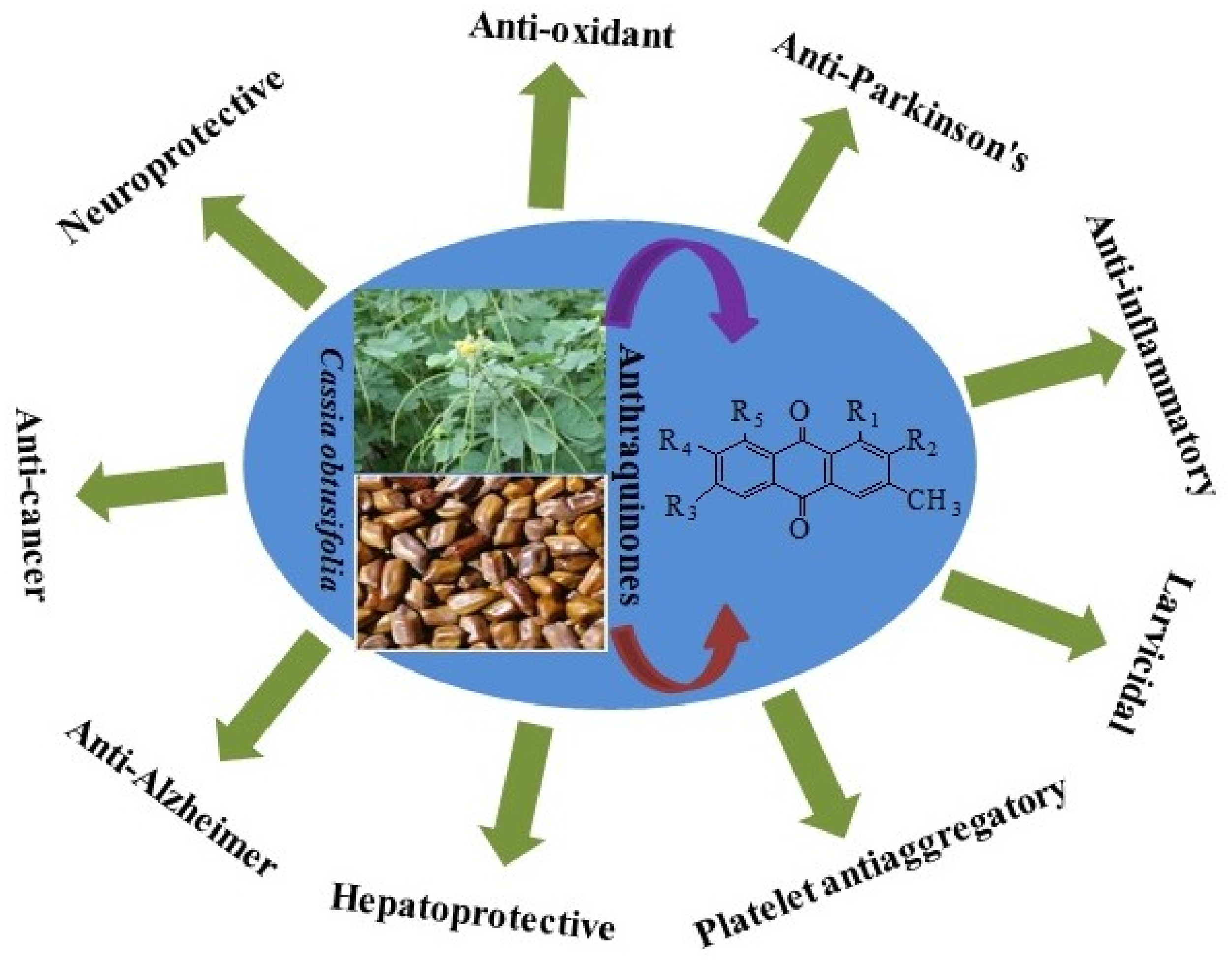
Phytochemistry, Ethnopharmacological Uses, Biological Activities, and Therapeutic Applications of Cassia obtusifolia L.: A Comprehensive Review
Abstract
1. Introduction
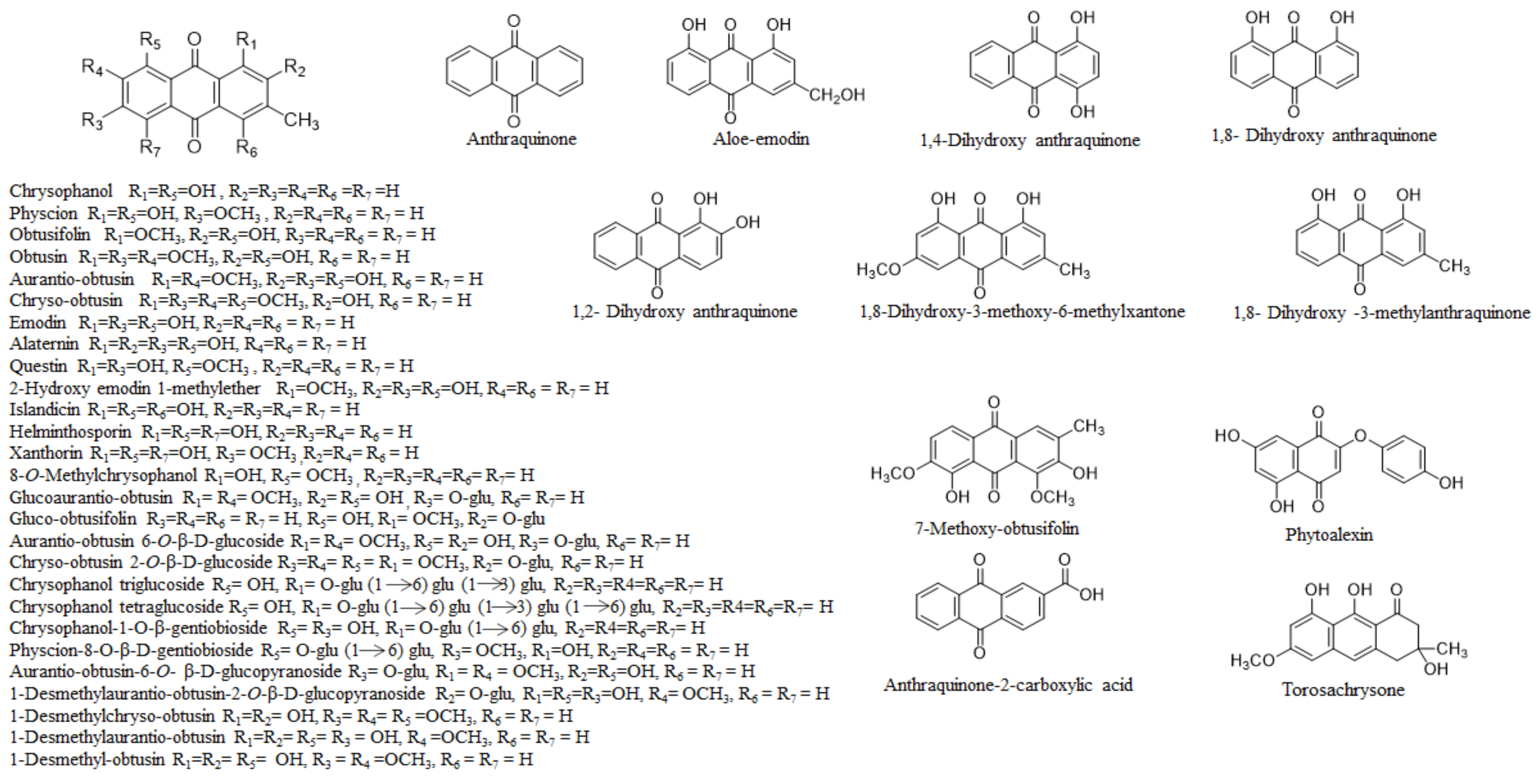
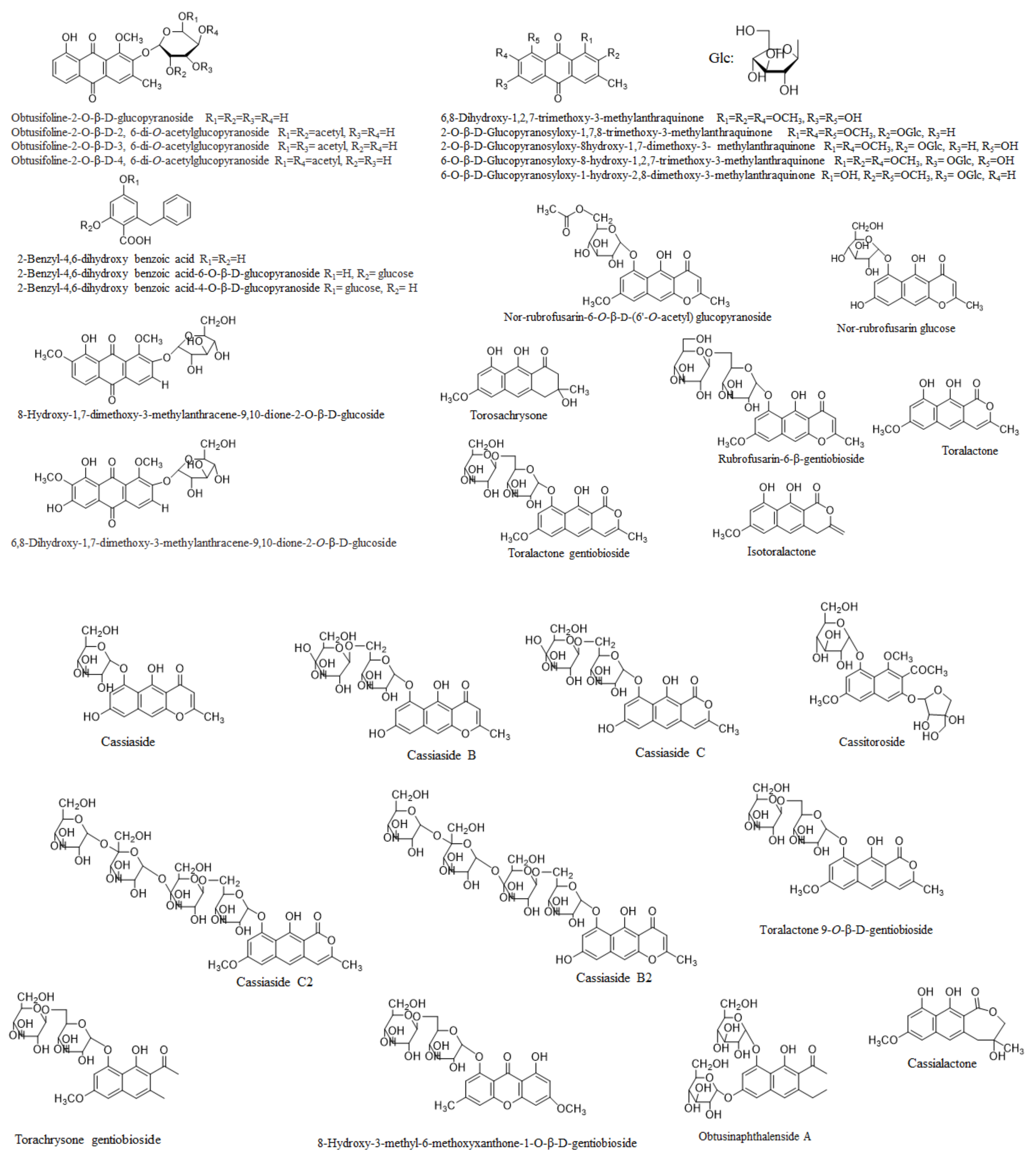
2. Phytochemistry
2.1. The Whole Plant
2.2. Seeds
2.3. Leaves
2.4. Roots
3. Bioactivity
3.1. Neuroprotective Activity
3.1.1. Anti-Alzheimer’s Disease Activity
3.1.2. Prevention and Treatment of Parkinson’s Disease
3.2. Hepatoprotective Activity
3.3. Anti-Inflammatory and Antioxidant Activity
3.4. Antimicrobial Activity
3.5. Antidiabetic Activity
3.6. Antiplatelet Aggregation Inhibitory Activity
3.7. Anticancer Activity
3.8. Larvicidal Activity
3.9. Other Activities
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sob, S.V.T.; Wabo, H.K.; Tchinda, A.T.; Tane, P.; Ngadjui, B.T.; Ye, Y. Anthraquinones, sterols, triterpenoids and xanthones from Cassia obtusifolia. Biochem. Syst. Ecol. 2010, 38, 342–345. [Google Scholar] [CrossRef]
- Tang, L.; Wu, H.; Zhou, X.; Xu, Y.; Zhou, G.; Wang, T.; Kou, Z.; Wang, Z. Discrimination of Semen cassiae from two re-lated species based on the multivariate analysis of high-performance liquid chromatography fingerprints. J. Sep. Sci. 2015, 38, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Yanjun, H.; Yuli, S.; Yuqing, Z. The advancement of the studies on the seeds of Cassia obtusifolia. Chin. Tradit. Herbal. Drugs. 2001, 32, 858–859. [Google Scholar]
- Li, Y.T.; Wang, Z.J.; Fu, M.H.; Yan, H.; Wei, H.W.; Lu, Q.H. A new anthraquinone glycoside from seeds of Cassia obtusifolia. Chin. Chem. Lett. 2008, 19, 1083–1085. [Google Scholar]
- Yun-Choi, H.S.; Kim, J.H.; Takido, M. Potential Inhibitors of Platelet Aggregation from Plant Sources, V. Anthraquinones from Seeds of Cassia obtusifolia and Related Compounds. J. Nat. Prod. 1990, 53, 630–633. [Google Scholar] [CrossRef]
- Kitanaka, S.; Nakayama, T.; Shibano, T.; Ohkoshi, E.; Takido, M. Antiallergic Agent from Natural Sources. Structures and Inhibitory Effect of Histamine Release of Naphthopyrone Glycosides from Seeds of Cassia obtusifolia L. Chem. Pharm. Bull. 1998, 46, 1650–1652. [Google Scholar] [CrossRef]
- Sung, B.K.; Kim, M.K.; Lee, W.H.; Lee, D.H.; Lee, H.S. Growth responses of Cassia obtusifolia toward human intestinal bac-teria. Fitoterapia 2004, 75, 505–509. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, B.H.; Kim, Y.-W.; Lee, S.; Shin, B.Y.; Jung, J.W.; Kim, H.J.; Lee, Y.S.; Choi, J.S.; Kim, S.Y.; et al. The seed extract of Cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J. Pharmacol. Sci. 2007, 105, 82–93. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, M.Y.; Choi, J.S. Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules 2017, 22, 28. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, M.Y.; Jung, H.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Inhibitory activities of major anthraquinones and other constituents from Cassia obtusifolia against β-secretase and cholinesterases. J. Ethnopharmacol. 2016, 191, 152–160. [Google Scholar] [CrossRef]
- Drever, B.D.; Anderson, W.G.; Riedel, G.; Kim, D.H.; Ryu, J.H.; Choi, D.-Y.; Platt, B. The seed extract of Cassia obtusifolia offers neuroprotection to mouse hippocampal cultures. J. Pharmacol. Sci. 2008, 107, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Song, J.-S.; Kim, Y.-M.; Jang, Y.P. Toralactone glycoside in Cassia obtusifolia mediates hepatoprotection via an Nrf2-dependent anti-oxidative mechanism. Food Res. Int. 2017, 97, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Doughari, J.H.; El-mahmood, A.M.; Tyoyina, I. Antimicrobial activity of leaf extracts of Senna obtusifolia (L). Afr. J. Pharm. Pharmacol. 2008, 2, 7–13. [Google Scholar]
- Vadivel, V.; Kunyanga, C.N.; Biesalski, H.K. Antioxidant Potential and Type II Diabetes-Related Enzyme Inhibition of Cassia obtusifolia L.: Effect of Indigenous Processing Methods. Food Bioprocess Technol. 2012, 5, 2687–2696. [Google Scholar] [CrossRef]
- Xie, Q.; Guo, F.F.; Zhou, W. Protective effects of cassia seed ethanol extract against carbon tetrachloride-induced liver in-jury in mice. Acta Biochem. Pol. 2012, 59, 265–270. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plant; Lalit Mohan Basu Press: Allahbad, India, 2006. [Google Scholar]
- Sob, S.V.T.; Wabo, H.K.; Tane, P.; Ngadjui, B.T.; Ma, D. A xanthone and a polyketide derivative from the leaves of Cassia obtusifolia (Leguminosae). Tetrahedron 2008, 64, 7999–8002. [Google Scholar] [CrossRef]
- Deshpande, S.R.; Naik, B.S. Evaluation of in vitro antimicrobial activity of extracts from Cassia obtusifolia L. and Senna so-phera (L.) Roxb against pathogenic organisms. J. Appl. Pharm. Sci. 2016, 6, 83–85. [Google Scholar] [CrossRef][Green Version]
- Paudel, P.; Seong, S.H.; Shrestha, S.; Jung, H.A.; Choi, J.S. In vitro and in silico human monoamine oxidase inhibitory po-tential of anthraquinones, naphthopyrones, and naphthalenic lactones from Cassia obtusifolia Linn seeds. ACS Omega 2019, 4, 16139–16152. [Google Scholar] [CrossRef]
- Dave, H.; Ledwani, L. A review on anthraquinones isolated from Cassia species and their applications. Indian, J. Nat. Prod. Resour. 2012, 3, 291–319. [Google Scholar]
- Harry-O’Kuru, R.E.; Mohamed, A. Processing scale-up of sciklepod (Senna obtusifolia L.) seed. J. Agri. Food Chem. 2009, 57, 2726–2731. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.V.; Abbott, T.P. Gum and protein enrichment from sciklepod (Cassia obtusifolia) seed by fine grinding and sieving. Ind. Crops. Prod. 2005, 21, 387–390. [Google Scholar] [CrossRef]
- Abbott, T.P.; Vaughu, S.F.; Dowd, P.F.; Mojtahedi, H.; Wilson, R.F. Potential uses ofsciklepod (Cassia obtusifolia). Ind. Crops. Prod. 1998, 8, 77–82. [Google Scholar] [CrossRef]
- Kitanaka, S.; Takido, M. Studies on the constituents of the seeds of Cassia obtusifolia: The structures of two new lactones, isotoralactone and cassialactone. Phytochemistry 1981, 20, 1951–1953. [Google Scholar] [CrossRef]
- Wu, X.-H.; Ruan, J.-L.; Cheng, C.-R.; Wu, Z.-Y.; Guan, S.-H.; Tao, S.-J.; Xu, P.-P.; Guo, D.-A. Benzyl-β-resorcylates from Cassia obtusifolia. Fitoter. 2010, 81, 617–620. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Liu, B.; Tu, G. Structure elucidation of a sodium salified anthraquinone from the seeds of Cassia obtusifolia by NMR technique assisted with acid-alkali titration. Magn. Reson. Chem. 2011, 49, 529–532. [Google Scholar] [CrossRef]
- Wu, X.-H.; Cai, J.-J.; Ruan, J.-L.; Lou, J.-S.; Duan, H.-Q.; Zhang, J.; Cheng, C.-R.; Guo, D.-A.; Wu, Z.-Y.; Zhang, Y.-W. Acetylated anthraquinone glycosides from Cassia obtusifolia. J. Asian Nat. Prod. Res. 2011, 13, 486–491. [Google Scholar] [CrossRef]
- Pang, X.; Li, N.-N.; Yu, H.-S.; Kang, L.-P.; Yu, H.-Y.; Song, X.-B.; Fan, G.-W.; Han, L.-F. Two new naphthalene glycosides from the seeds of Cassia obtusifolia. J. Asian Nat. Prod. Res. 2018, 21, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, L.M.; Zhang, Y.C.; Kang, L.P.; Yu, H.S.; Fan, G.W.; Han, L.F. New anthraquinone and eurotinone ana-logue from the seeds of Senna obtusifolia and their inhibitory effects on human organic anion transporters 1 and 3. Nat. Prod. Res. 2018, 4, 1–8. [Google Scholar]
- Feng, L.; Yin, J.Y.; Nie, S.P.; Wan, Y.Q.; Xiea, M.Y. Enzymatic purification and structure characterization of glucuronoxy-lan from water extract of Cassia obtusifolia seeds. Int. J. Biol. Macromol. 2018, 107, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Sharon, A.; Ghirlando, R.; Gressel, J. Isolation, Purification, and Identification of 2-(p-Hydroxyphenoxy)-5,7-Dihydroxychromone: A Fungal-Induced Phytoalexin from Cassia obtusifolia. Plant Physiol. 1992, 98, 303–308. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Z.; Yang, R.; Guo, D.; Zheng, J. Anthraquinones from hairy root cultures of Cassia obtusifolia. Phytochemistry 1998, 49, 1623–1625. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.; Jung, W.Y.; Park, S.J.; Park, D.H.; Kim, J.M.; Cheong, J.H.; Ryu, J.H. The neuroprotective effects of the seeds of Cassia obtusifolia on transient cerebral global ischemia in mice. Food Chem. Toxicol. 2009, 47, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.S.; Kim, H.G.; Choi, J.G.; Ryu, J.H.; Hur, J.; Kim, Y.J.; Oh, M.S. Cassiae semen, a seed of Cassia obtusifolia, has neu-roprotective effects in Parkinson’s disease models. Food Chem. Toxicol. 2010, 48, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Park, H.J.; Lee, S.; Jung, J.W.; Kim, B.C.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. Cassia obtusifolia seed ameliorates amyloid β-induced synaptic dysfunction through anti-inflammatory and Akt/GSK-3β pathways. J. Ethnopharmacol. 2016, 178, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.; Jeong, G.S.; An, R.B.; Li, B.; Lee, D.S.; Ko, E.K.; Yoon, K.H.; Kim, Y.C. Hepatoprotective compounds of Cassiae Semen on tacrine-induced cytotoxicity in HepG2 cells. Korean, J. Pharmacogn. 2007, 38, 400–402. [Google Scholar]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Min, B.-S.; Paudel, P.; Choi, J.S. Hepatoprotective effect of Cassia obtusifolia seed extract and constituents against oxidative damage induced by tert -butyl hydroperoxide in human hepatic HepG2 cells. J. Food Biochem. 2018, 42, e12439. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, Y.; Fang, N.; Guo, Y. Hepatoprotective effects of Cassia semen ethanol extract on non-alcoholic fatty liver disease in experimental rat. Pharm. Biol. 2019, 57, 98–104. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Tang, L.-Y.; Zhou, X.-D.; Zhou, G.-H.; Wang, Z.-J. Five new anthraquinones from the seed of Cassia obtusifolia. Arch. Pharmacal Res. 2014, 38, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, K.-W.; Kim, D.-S.; Kim, M.-C.; Jeon, Y.-D.; Kim, S.-G.; Jung, H.-J.; Jang, H.-J.; Lee, B.-C.; Chung, W.-S.; et al. The Protective Effect of Cassia obtusifolia on DSS-Induced Colitis. Am. J. Chin. Med. 2011, 39, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yin, J.; Nie, S.; Wan, Y.; Xie, M. Fractionation, physicochemical property and immunological activity of poly-saccharides from Cassia obtusifolia. Int. J. Biol. Macromol. 2016, 91, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Voukeng, I.K.; Beng, V.P.; Kuete, V. Antibacterial activity of six medicinal Cameroonian plants against Gram-positive and Gram-negative multidrug resistant phenotypes. BMC Complement. Altern. Med. 2016, 16, 388. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-S.; Baek, B.-R.; Yang, Y.-C.; Kim, M.-K.; Lee, H.-S. Larvicidal activity of leguminous seeds and grains against Aedes aegypti and Culex pipiens pallens. J. Am. Mosq. Control. Assoc. 2002, 18, 210–213. [Google Scholar]
- Yang, Y.C.; Lim, M.Y.; Lee, H.S. Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal activity against three mosquito species. J. Agri. Food. Chem. 2003, 51, 7629–7631. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.; Jebanesan, A. Larvicidal and oviposition activity of Cassia obtusifolia Linn (Family: Leguminosae) leaf ex-tract against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol. Res. 2009, 104, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, S.; Takido, M. Studies on the constituents in the roots of Cassia obtusifolia L. and the antimicrobial activities of aonstituents of the roots and the seeds. Yakugaku Zasshi 1986, 106, 302–306. [Google Scholar] [CrossRef]
- Yun-Choi, H.S.; Lee, J.R.; Kim, J.H.; Kim, Y.H.; Kim, T.H. Potential inhibitors oplatelet aggregation from plant sources, IV. Anthraquinones from seeds of Cassia obtusifolia and related compounds. Korean. J. Pharmacogn. 1987, 18, 203–206. [Google Scholar]
- Shin, B.Y.; Kim, D.H.; Hyun, S.K.; Jung, H.A.; Kim, J.M.; Park, S.J.; Kim, S.Y.; Cheong, J.H.; Choi, J.S.; Ryu, J.H. Alater-nin attenuates delayed neuronal cell death induced by transient cerebral hypoperfusion in mice. Food Chem. Toxicol. 2010, 48, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Hyun, S.K.; Yoon, B.H.; Seo, J.-H.; Lee, K.-T.; Cheong, J.H.; Jung, S.Y.; Jin, C.; Choi, J.S.; Ryu, J.H. Gluco-obtusifolin and its aglycon, obtusifolin, attenuate scopolamine-induced memory impairment. J. Pharmacol. Sci. 2009, 111, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.-Y.; Wu, M.-L.; Wei, P.-J.; Cao, Z.-P.; Xiao, P.; Li, C.-H. Changes in Plasma Lipid Levels and Antioxidant Activities in Rats after Supplementation of Obtusifolin. Planta Medica 2016, 82, 539–543. [Google Scholar] [CrossRef]
- He, Z.-W.; Wei, W.; Li, S.-P.; Ling, Q.; Liao, K.-J.; Wang, X. Anti-allodynic effects of obtusifolin and gluco-obtusifolin against inflammatory and neuropathic pain: Possible mechanism for neuroinflammation. Biol. Pharm. Bull. 2014, 37, 1606. [Google Scholar] [CrossRef]
- Shi, B.-J.; Zhang, W.-D.; Jiang, H.-F.; Zhu, Y.-Y.; Chen, L.; Zha, X.-M.; Lu, Y.-Y. A new anthraquinone from seed of Cassia obtusifolia. Nat. Prod. Res. 2016, 30, 35–41. [Google Scholar] [CrossRef]
- Vishnuprasad, C.N.; Tsuchiya, T.; Kanegasaki, S.; Kim, J.H.; Han, S.S. Aurantio-Obtusin stimulates chemotactic migra-tion and differentiation of MC3T3-E1 osteoblast cells. Planta Med. 2014, 80, 544–549. [Google Scholar]
- Hou, J.; Gu, Y.; Zhao, S.; Huo, M.; Wang, S.; Zhang, Y.; Qiao, Y.; Li, X. Anti-Inflammatory Effects of Aurantio-Obtusin from Seed of Cassia obtusifolia L. through Modulation of the NF-κB Pathway. Molecules 2018, 23, 3093. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Seong, S.H.; Paudel, P.; Jung, A.H.; Choi, J.S. Structure related inhibition of enzyme systems in cholinester-ases and BACE1 in vitro by naturally occurring naphthopyrone and its glycosides isolated from Cassia obtusifolia. Molecules 2018, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Kim, D.; Jeon, J.; Park, S.; Seong, S.; Jung, H.; Choi, J. Neuroprotective Effect of Aurantio-Obtusin, a Putative Vasopressin V1A Receptor Antagonist, on Transient Forebrain Ischemia Mice Model. Int. J. Mol. Sci. 2021, 22, 3335. [Google Scholar] [CrossRef]
- Paudel, P.; Jung, H.A.; Choi, J.S. Anthraquinone and naphthopyrone glycosides from Cassia obtusifolia seeds mediate hepatoprotection via Nrf2-mediated HO-1 activation and MAPK modulation. Arch. Pharm. Res. 2018, 41, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.S.; Lee, J.H.; So, K.S.; Park, B.K.; Lim, H.; Choi, J.S.; Kim, H.P. Aurantio-obtusin, an anthraquinone from cassiae semen, ameliorates lung inflammatory responses. Phytother. Res. 2018, 32, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhong, Z. Obtusifolin Treatment Improves Hyperlipidemia and Hyperglycemia: Possible Mechanism Involving Oxidative Stress. Cell Biophys. 2014, 70, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Paudel, P.; Seong, S.H.; Min, B.S.; Seo, E.K.; Jung, H.A.; Choi, J.S. Two new naphthalenic lactone glyco-sides from Cassia obtusifolia L. seeds. Arch. Pharm. Res. 2018, 41, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Shang, M.; Dong, Q.; Liao, W.; Xiao, F.; Ding, K. Structure and activities of a novel heteroxylan from Cassia ob-tusifolia seeds and its sulfated derivative. Carbohydr. Res. 2014, 393, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ruan, J.; Yang, V.C.; Wu, Z.; Lou, J.; Duan, H.; Zhang, Y.J.; Zhang, A.; Guo, D. Three new acetylated ben-zyl-beta-resorcylate glycosides from Cassia obtusifolia. Fitoterapia 2012, 83, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Chow, C.-J.; Tsai, Y.-H. Composition, characteristics, and in-vitro physiological effects of the water-soluble polysaccharides from Cassia seed. Food Chem. 2012, 134, 1967–1972. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Guo, X.; Sun, J.; Liu, L.; Wu, L. Simultaneous determination of seven anthraquinones in rat plasma by ultra-high-performance liquid chromatography–tandem mass spectrometry and pharmacokinetic study after oral admin-istration of semen cassiae extract. J. Ethnopharmacol. 2015, 169, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.C.; Shibamoto, T. Effects of Cassia obtusifolia (sicklepod) extracts and anthraquinones on muscle mitochondrial function. Toxicon 1989, 27, 519–529. [Google Scholar] [CrossRef]
- Liao, H.; Ren, W.; Kang, Z.; Jiang, J.-H.; Zhao, X.-J.; Du, L.-F. A trypsin inhibitor from Cassia obtusifolia seeds: Isolation, characterization and activity against Pieris rapae. Biotechnol. Lett. 2007, 29, 653–658. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Li, J.; Zhang, G.; Jiamahate, A.; Zhou, J.; Liao, H. Isolation, structure modeling and function characteri-zation of a trypsin inhibitor from Cassia obtusifolia. Biotechnol. Lett. 2015, 37, 863–869. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Yang, C.; Zhang, X.; Wang, W.; Du, X.; Wang, Q.; Ni, J. Cassiae semen: A review of its phyto-chemistry and pharmacology. Mol. Med. Rep. 2017, 16, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
| Sr. No. | Plant Part Used | Ethnomedicinal Use |
|---|---|---|
| 1 | Whole plant | In traditional Oriental medicine, the whole plant of C. obtusifolia has been used for treatment of Laxative, eye infections, diarrhea, urinary tract infections, gingivitis, fever, and cough remedy [13]. |
| 2 | Roots | Root is considered bitter, tonic, stomachic and is antidote against snake bite. Other uses are in treatment of fungal diseases, worm infection, abdominal tumors, bronchitis, and asthma. The roots of C. obtusifolia are also usually crushed, mixed with lime juice, and applied to ringworms [14]. |
| 3 | Seeds | The seeds of C. obtusifolia are used to treat dizziness and to benefit the eyes by anchoring and nourishing the liver. The dried and roasted seeds are also used as brew a tea. Seeds of C. obtusifolia were also used for the treatment of headache, ophthalmic diseases, constipation, hypertension, and hyperlipidemia. In Korea, the hot extract of seeds is taken orally for protection of liver [10,15]. |
| 4 | Leaves | C. obtusifolia leaves and pods have been widely used as purgatives and laxatives. In Indian traditional ayurveda system, the leaves and Pods are used as digestible, laxative, diuretic, stomachic, antipyretic, improves the appetite, biliousness, blood diseases, burning sensation, leprosy, bronchitis, piles, and leucorrhoea [16,17]. |
| 5 | Stem bark | In Indian traditional ayurveda system, Stem bark extract is used for various skin ailments, rheumatic diseases, and as laxative [18]. |
| 6 | Pods and fruits | Pods are used in dysentery, in eye diseases and pains in the joints. The unripe fruits are also cooked and eaten [14]. |
| Pharmacological Activity | Part of Plant | Type of Extract | In Vivo/ In Vitro | Model | Administration (In Vivo) | Dose Range | Active Concentration | Reference |
|---|---|---|---|---|---|---|---|---|
| Neuroprotective Activity | Seeds | 85% EtOH ext. | In vivo | Ameliorate scopolamine or 2VO-induced memory impairments by inhibiting AChE | Oral | 25–100 mg/kg | 50 mg/kg | [8] |
| Seeds | 85% EtOH ext. | In vivo | Neuroprotection by inhibition of pro-inflammatory genes iNOX, and COX-2, and increased neurotrophic factor expression of pCREB and BDNF | Oral | 10, 50 mg/kg | 50 mg/kg | [33] | |
| Seeds | 85% EtOH ext. | In vitro | Reduced Aβ toxicity and maintenance of Ca2+ dysregulation and excitotoxicity, mitochondrial dysfunction in primary hippocampal cultures | - | 0.1–10 µg/mL | 1, 10 µg/mL | [11] | |
| Seeds | EtOH ex. | In vivo | protected the dopaminergic cells against 6-OHDA- and MPP+-induced neurotoxicities in primary mesencephalic cultures and in a mouse model in PD | Intraperitoneal injection | 0.1–10 µg/mL for DA, 50 mg/kg mouse | 0.1, 1 µg/mL 50 mg/kg | [34] | |
| Seeds | EtOH ext. | In vitro | Inhibited cell loss against 6-OHDA-induced DA neural toxicity by an anti-oxidant and anti-mitochondrial-mediated apoptosis mechanism in PC12 cells. | - | 0.1–10 µg/mL 1000 µg/mL for DPPH, ABTS | 1 µg/mL ROS, 10 µg/mL GSH, 75% Casp-3, 92%-DPPH, 85% ABTS | [35] | |
| Seeds | MeOH ext. EtOAc fr. CH2Cl2 fr. BuOH fr. | In vitro | Inhibitory activity against MAO-A, and MAO-B | - | 0.25–120 µg/mL | EtOAc fr. exhibited greatest inhibitory IC50 = 20, and 56 µg/mL activity against MAO-A, and MAO-B | [36] | |
| Seeds | MeOH ext. EtOAc fr. CH2Cl2 fr. BuOH fr. H2O fr. | In vitro | Inhibitory activity against AChE, BChE, BACE1 | - | 0.4–120 µg/mL | IC50 = 9.45~29 µg/mL for AChE, IC50 = 7.58~49 µg/mL for BChE, IC50 = 26~96 µg/mL for BACE1 | [10] | |
| Seeds | 85% EtOH ext. | In vivo | Ameliorate Aβ-induced LTP impairment in the acute hippocampal slices and regulates GSK-3β, Akt signaling pathways through the inhibition of iNOS, COX expression | - | 1 and 10 µg/mL | 10 µg/mL | [35] | |
| Hepatoprotective Activity | Seeds | MeOH ext. | In vitro | Protection against tacrine-induced hepatotoxicity in HepG2 cells | - | 300 µg/mL | 300 µg/mL | [36] |
| Seeds | 70% EtOH ext. EtOAc, CH2Cl2, BuOH, H2O fr. | In vitro | Protective effect against t-BHP-induced hepatotoxicity in HepG2 cells | - | 10–100 µg/mL | EtOAc fr. showed most potent hepatoprotective activity (30 µg/mL) | [12] | |
| Seeds | EtOH ext. | In vivo | Hepatoprotective effects against CCl4-induced liver injury in mice | Intraperitoneal injection | 0.5, 1, 2 g/kg | Reduced ALT and AST, Ca2+, MDA, and increased GSH, SOD, GR, GPx, GST, CYP2E1 (2 g/kg) | [15] | |
| seeds | EtOAc fr. CH2Cl2 fr. BuOH fr. H2O fr. | In vitro | Protective effect against t-BHP-induced hepatotoxicity in HepG2 cells | - | 12.5–50 µg/mL | EtOAc fr. showed most potent hepatoprotective activity (50 µg/mL) | [37] | |
| Seeds | 70% EtOH ext. | In vivo | (a) Significantly decreased the levels of AST, ALT, TG, TC, TNF-a, IL-6, IL-8 and MDA; (b) Increased the levels of SOD and GSH; (c) Significantly increased the mRNA expression levels of LDL-R | Oral | 0.5–2 g/kg | (a) Dose-dependently decreased biomarkers at 0.5–2 g/kg; (b) Dose-dependently decreased at 0.5–2 g/kg; (c) Significantly increased the levels of LDL-R at 2 g/kg | [38] | |
| Anti-diabetic Activity | Seeds | MeOH ext. EtOAc fr. CH2Cl2 fr. BuOH fr. H2O fr. | In vitro | Inhibitory activity against PTP1B and α-glucosidase | - | 0.4–400 µg/mL for PTP1B, 0.16–400 µg/mL for α-glucosidase | MeOH ext. (IC50 = 14 µg/mL) and EtOAc fr. (IC50 = 74 µg/mL) exhibited greatest inhibitory activity against PTP1B and α-glucosidase | [9] |
| Seeds | EtOH ext. | In vitro | Inhibitory activity against α-glucosidase | - | 1000 µg/mL | 20% inhibition of α-glucosidase (1000 µg/mL) | [39] | |
| Anti-inflammatory, Antioxidant, and Immune-modulatory Activities | Roasted seeds | Hot H2O ext. | In vivo | Protection against dextran sulfate sodium (DSS)-induced colitis through the inhibition of (IL)-6, COX-2, NF-κB | Oral | 1 g/kg | Significantly reduced clinical signs and the levels of inflammatory mediators (at concentration 1 g/kg) | [40] |
| Seeds | H2O soluble polysaccharide fr. | In vitro | Increased immune-modulatory activity by promoting phagocytosis and stimulating the production of NO and cytokines TNF- and IL-6 on macrophage cell line RAW264.7 | - | 62.5–500 µg/mL | Stimulates NO, TNF- and IL-6 expression (250 µg/mL) and promotes phagocytic activity (500 µg/mL) | [41] | |
| Seeds | MeOH ext. | In vitro | DPPH, Fe [II], superoxide radicals scavenging activity and inhibit ß-carotene degradation | - | 1 mg/mL | Inhibition 65.79% DPPH, 50.78% superoxide radical, 49.92% inhibit ß-carotene degradation,1292 mM Fe [II] inhibited (at 1 mg/mL) | [14] | |
| Antimicrobial Activity | Seeds | MeOH ext. Hexane fr. EtOAc fr. CH2Cl2 fr. BuOH fr.H2O fr. | In vitro | Bifidobacterium adolescentis, B. bifidum, B. longum, B. breve, Clostridium perfringens, Escherichia coli, Lactobacillus casei | - | 5 mg discs−1 | CH2Cl2 fr, MeOH ext. and Hexane fr. exhibited the greatest antibacterial activity | [7] |
| Leaf | Pet ether ext. EtOH ext. Chloroform ext. | In vitro | Aspergilus fumigatus, Staphylococcus aureus, Enterococcus faecalis, E. coli, Klebsiella sp., Candia albicans | - | 0.6–1 mg/mL | Pet ether, chloroform ext. active against C. albicans (MIC 0.3524, and 0.4239 mg/mL), ethanol E. faecalis (MIC 0.2738 mg/mL) | [18] | |
| stem | Pet ether ext. EtOH ext. Chloroform ext. | In vitro | Aspergilus fumigatus, Staphylococcus aureus, Enterococcus faecalis, E. coli, Klebsiella sp., Candia albicans | 0.6–1 mg/mL | Ethanol, pet ether, chloroform ext. was more active against E.faecalis (MIC 0.298, 0.254, and 0.589 mg/mL, respectively) | [18] | ||
| Whole plant | MeOH ext. | In vitro | E. coli, P. aeruginosa, Enterobacter aerogenes Providencia stuartii, K.pneumoniae, Enterobacter cloacae, S. aureus | - | 256 µg/mL | inhibition of S. aureus, E. coli, P. aeruginosa, E. aerogenes, K. pneumoniae (MIC ranges of 64–289 μg/mL | [42] | |
| Larvicidal Activity | Seeds | MeOH ext. | In vitro | Larvicidal activity against Aedes aegypti and Culex pipiens pallens | - | 10–300 ppm | 40 ppm | [43] |
| Seeds | Chloroform fr. | In vitro | Larvicidal activity against A. aegypti, Aedes togoi, and Cx. pipiens | - | 25 mg/L | 100% Mortality (at concentration 25 mg/L) | [44] | |
| Leaf | EtOH ext. | In vitro | Larvicidal activity against Anopheles stephensi | - | 25–125 mg/L | LC50 = 52.2 mg/L, LC90 = 108.7 mg/L (at concentration 25 mg/L) | [45] | |
| Leaf | EtOH ext. | In vitro | Anti-oviposition activity against Anopheles stephensi | - | 100–400 mg/L | 92.5% for 400 mg/L 87.2% for 300 mg/L 83.0% for 200 mg/L | [45] |
| Compounds | Biological Activity | In Vivo/ In Vitro | Model | Administration (In Vivo) | Dose Range | Active Concentration | Reference |
|---|---|---|---|---|---|---|---|
| Anthraquinones | |||||||
| Emodin | Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 9.17µg/mL (b) IC50 = 157 µg/mL (c) IC50 = 4.48 µg/mL | [10] |
| Antimicrobial activity | In vitro | Antibacterial activity against (a) Staphylococcus aureus 209P (b) Escherichia coli NIHJ | - | 0–1 mg/mL | MIC (a) 4.5 µg/mL (b) 25 µg/mL | [46] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity (c) Stimulation of glucose uptake in HepG2 cells | - | (a) 0–100 µg/mL (b) 0–400 µg/mL (c) 3.12–12.5 µM | (a) IC50 = 3.51 µg/mL (b) IC50 = 1.02 µg/mL (c) glucose uptake | [9] | |
| Platelet anti-aggregatory activity | In vitro | (a) Adenosine 5′-diphosphate inhibitory activity (b) Arachidonic-acid inhibitory activity (c) Collagen inhibitory activity | - | 0–1 mg/mL | 1 mg/mL | [47] | |
| Larvicidal activity | In vitro | Larvicidal activity against (a) Culex pipiens pallens (b), Aedes aegypti (c) Aedes togoi | - | 1–20 mg/L | (a) LC50 = 1.4 mg/L (b) LC50 = 1.9 mg/L (c) LC50 = 2.2 mg/L | [44] | |
| Hepatoprotective activity | In vitro | Protection against t-BHP-induced hepatotoxicity in HepG2 cells | - | 25 µM | protect cells damage | [37] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 25 µM | (a) IC50 = 23 µM (b) IC50 = 54 µM | [19] | |
| Alaternin | Neuroprotective activity | In vivo | Prevented nitrotyrosine and lipid peroxidation, as well as BCCAO induced-iNOS expression and significantly reduced microglial activation | Orally | 1, 10 mg/kg | 10 mg/kg | [48] |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity (c) Stimulation of glucose uptake in HepG2 cells | - | (a) 0–100 µg/mL (b) 0–400 µg/mL (c) 12.5–50 µM | (a) IC50 = 1.22 µg/mL (b) IC50 = 0.99 µg/mL (c) glucose uptake | [9] | |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 6.29 µg/mL (b) IC50 = 113 µg/mL (c) IC50 = 0.94 µg/mL | [10] | |
| Hepatoprotective activity | In vitro | Protection against t-BHP-induced hepatotoxicity in HepG2 cells | - | 50, 100 µM | (a) protect cells damage (b) increased GSH level and reduce ROS level | [37] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 10 µM | (a) IC50 = 5.35 µM (b) IC50 = 4.55 µM | [19] | |
| Obtusifolin | Neuroprotective activity | In vivo | Significantly reversed scopolamine-induced cognitive impairments in the passive avoidance test, improved escape latencies, swimming times in the target quadrant, and crossing numbers in the zone in Morris water maze test | Orally | 0.25–2 mg/kg | 0.5 mg/kg | [49] |
| Hyperlipidemia and antioxidant activity | In vivo | Reduced body weight, TC, TG, LDL-C and increased HDL-C levels, as well as increased SOD and NO, and reduced MDA levels in hyperlipidemic rats. | Orally | 5 and 20 mg/kg | 20 mg/kg | [50] | |
| Neuropathic and anti-inflammatory activity | In vivo | Inhibition of TNF-α, IL-1β, IL-6 and NF-kB up-regulation in the spinal cord in mice and rat models | Intraperitoneal injection | 0.25–2 mg/kg | 1 and 2 mg/kg | [51] | |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 18.5 µg/mL (b) IC50 = 284 µg/mL (c) IC50 = 64.8 µg/mL | [10] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 35.2 µg/mL (b) IC50 = 142 µg/mL | [9] | |
| Hepatoprotective activity | In vitro | Protection against tacrine-induced hepatotoxicity in HepG2 cells | - | 160 µM | Protection ratio value 41.2% at 160 µM | [36] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity; (b) MAO-B inhibitory activity | - | 100 µM | (a) IC50 = 31 µM (b) IC50 ≥ 400 µM | [19] | |
| Gluco-obtusifolin | Neuropathic and anti-inflammatory activity | In vivo | Inhibition of TNF-α, IL-1β, IL-6 and NF-kB up-regulation in the spinal cord in mice and rat models | Intraperitoneal injection | 0.25–2 mg/kg | 1 and 2 mg/kg | [51] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–400 µg/mL | (a) IC50 = 37.2 µg/mL (b) IC50 = 172 µg/mL (c) IC50 = 41.1 µg/mL | [10] | |
| Neuroprotective activity | In vivo | Significantly reversed scopolamine-induced cognitive impairments in the passive avoidance test, improved escape latencies, swimming times in the target quadrant, and crossing numbers in the zone in the Morris water maze test | Orally | 0.25–2 mg/kg | 0.5 mg/kg | [49] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 53.35 µg/mL (b) IC50 = 23.77 µg/mL | [9] | |
| Platelet anti-aggregatory activity | In vitro | (a) Adenosine 5′-diphosphate inhibitory activity (b) Arachidonic-acid inhibitory activity (c) Collagen inhibitory activity | - | 0–1 mg/mL | (a) IC50 = 0.25 µg/mL (b) IC50 = 0.05 µg/mL (c) IC50 = 0.1 µg/mL | [5] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 500 µM | (a) IC50 ≥ 400 µM (b) IC50 ≥ 400 µM | [19] | |
| Aurantio-obtusin | Hepatoprotective activity | In vitro | Protection against tacrine-induced hepatotoxicity in HepG2 cells | - | 160 µM | Protection ratio value 55.3% at 160 µM | [36] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 92.1 µg/mL (b) IC50 = 314 µg/mL (c) IC50 = 67.9 µg/mL | [10] | |
| Platelet anti-aggregatory activity | In vitro | (a) Adenosine 5′-diphosphate inhibitory activity (b) Arachidonic-acid inhibitory activity (c) Collagen inhibitory activity | - | 0–1 mg/mL | 1 mg/mL | [48] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 27.19 µg/mL (b) IC50 = 41.20 µg/mL | [9] | |
| Anti-cancer activity | In vitro | Cytotoxicity against (a) HCT-116, (b) A549, (c) SGC7901 and (d) LO2 cell lines | - | 0.4–50 µg/mL | (a) IC50 = 18.9 µg/mL (b) IC50 = 20.1 µg/mL (c) IC50 = 22.0 µg/mL (d) IC50 = 23.1 µg/mL | [52] | |
| Prevention of bone disease | In vitro | Stimulates osteoblast migration, differentiation, and mineralization in a dose-dependent manner in MC3T3-E1 osteoblast cells | - | 0.1–100 µM | 10 µM | [53] | |
| Anti-inflammatory activity | In vitro | (a) Significantly decreased the production of NO, PGE2, and inhibited the iNOS, COX-2, TNF-α and IL-6. (b) Reduced the LPS-induced activation of nuclear factor-κB in RAW264.7 cells. | - | 6.12–100 µM | 6.12–100 µM | [54] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 200 µM | (a) IC50 = 27.23 µM (b) IC50 = 174.40 µM | [19] | |
| Obtusin | Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 6.44 µg/mL (b) IC50 = 20.92 µg/mL | [9] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 82 µg/mL (b) IC50 = 287 µg/mL (c) IC50 = 61.9 µg/mL | [10] | |
| Anti-cancer activity | In vitro | Cytotoxicity against (a) HCT-116, (b) A549, and (c) SGC7901 cell lines | - | 0.4–50 µg/mL | (a) IC50 = 13.1 µg/mL (b) IC50 = 29.2 µg/mL (c) IC50 = 15.2 µg/mL | [52] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 400 µM | (a) IC50 = 11.12 µM (b) IC50 ≥ 400 µM | [19] | |
| Chryso-obtusin | Anti-cancer activity | In vitro | Cytotoxicity against (a) HCT-116, (b) A549, (c) SGC7901 and (d) LO2 cell lines | - | 0.4–50 µg/mL | (a) IC50 = 10.5 µg/mL (b) IC50 = 14.6 µg/mL (c) IC50 = 12.0 µg/mL (d) IC50 = 15.8 µg/mL | [52] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 68.6 µg/mL (b) IC50 = 287 µg/mL (c) IC50 = 49.9 µg/mL | [10] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 14.88 µg/mL (b) IC50 = 36.1 µg/mL | [9] | |
| Platelet anti-aggregatory activity | In vitro | (a) Adenosine 5′-diphosphate inhibitory activity (b) Arachidonic-acid inhibitory activity (c) Collagen inhibitory activity | - | 0–1 mg/mL | 1 mg/mL | [47] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 400 µM | (a) IC50 = 327.67 µM (b) IC50 ≥ 400 µM | [19] | |
| Questin | Antimicrobial activity | In vitro | Antibacterial activity against (a) Staphylococcus aureus 209P and (b) Escherichia coli NIHJ | - | 0–100 µg/mL | MIC (a) 25 µg/mL (b) 50 µg/mL | [48] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 34.0 µg/mL (b) IC50 = 138 µg/mL (c) IC50 = 32.8 µg/mL | [10] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 5.69 µg/mL (b) IC50 = 136.1 µg/mL | [9] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 20 µM | (a) IC50 = 0.17 µM (b) IC50 = 10.58 µM | [19] | |
| Gluco-aurantio-obtusin | Platelet anti-aggregatory activity | In vitro | (a) Adenosine 5′-diphosphate inhibitory activity (b) Arachidonic-acid inhibitory activity (c) Collagen inhibitory activity | - | 0–1 mg/mL | (a) IC50 = 0.25 µg/mL (b) IC50 = 0.05 µg/mL (c) IC50 = 0.1 µg/mL | [5] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 109 µg/mL (b) IC50 = 50.9 µg/mL | [10] | |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 31.3 µg/mL (b) IC50 = 142.1 µg/mL | [9] | |
| Hepatoprotective activity | In vitro | Hepatoprotective efficacy against t-BHP-induced cell death in HepG2 cells | - | 20 µM | Protection ratio value 49.7% at 20 µM | [12] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 400 µM | (a) IC50 = 39.55 µM (b) IC50 = 180.76 µM | [19] | |
| Chrysophanol; Aloe-emodin; Physcion; Chrysophanol tri, Tetraglucoside; 2-hydroxyemodin-1methylether; Chryso-obtusin-2-O-β-d-glucoside | Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 5~103 µg/mL (b) IC50 = 5~228 µg/mL | [9] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–400 µg/mL | (a) IC50 = 14~71 µg/mL (b) IC50 ≥ 100 µg/mL (c) IC50 = 13~59 µg/mL | [10] | |
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 400 µM | (a) IC50 = 2.47~400 µM (b) IC50 ≥ 400 µM | [19] | |
| Dihydroxyanthraquinone | Bacterial growth promoting and inhibiting activity | In vitro | (a) Growth promoting activity against Bifidobacterium bifidum (b) Growth inhibiting activity against Clostridium perfringens and Escherichia coli | - | (a) 0.05–0.5 mg/d (b) 0.1–5 mg/d | (a) GIR > 2.0 at 0.5 mg/disk (b) Inhibitory zone diameter > 30 mm | [7] |
| Naphthopyrone | |||||||
| Cassiaside | Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 18.1 µg/mL (b) IC50 = 177 µg/mL (c) IC50 = 1.85 µg/mL | [10] |
| Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 48.55 µg/mL (b) IC50 = 129.2 µg/mL | [9] | |
| Hepatoprotective activity | In vitro | Hepatoprotective efficacy against t-BHP-induced cell death in HepG2 cells | 25 µM | (a) protect cells damage (b) increased GSH level and reduce ROS level | [37] | ||
| Parkinson’s disease activity | In vitro | (a) MAO-A inhibitory activity (b) MAO-B inhibitory activity | - | 400 µM | (a) IC50 = 11.26 µM (b) IC50 ≥ 400 µM | [19] | |
| Isotoralactone; Toralactone | Antimicrobial activity | In vitro | Antibacterial activity against (a) Staphylococcus aureus 209P and (b) Escherichia coli NIHJ | - | 0–100 µg/mL | MIC (a) 2–3 µg/Ml (b) 5.5–12 µg/mL | [46] |
| Cassiaside B2, Cassiaside C2 | Antiallergic activity | In vitro | Inhibition of histamine release in rat peritoneal mast cells | - | 100 µM | Cassiaside B2 inhibit 17.2%; Cassiaside C2 Inhibit 53.9% | [6] |
| Toralactone Gentiobioside | Antidiabetic activity | In vitro | (a) PTP 1B inhibitory activity (b) α-glucosidase inhibitory activity | - | (a) 0–100 µg/mL (b) 0–400 µg/mL | (a) IC50 = 81.1µg/mL (b) IC50 = 37.60 µg/mL | [9] |
| Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) Butyrylcholinesterase inhibitory activity (c) β-secretase inhibitory activity | - | 0–100 µg/mL | (a) IC50 = 91.3 µg/mL (b) IC50 = 117 µg/mL (c) IC50 = 69.0 µg/mL | [10] | |
| Hepatoprotective activity | In vitro | Hepatoprotective efficacy against t-BHP-induced cell death in HepG2 cells | - | 20 µM | Increased in Nrf2/ARE-luciferase activity, and upregulated NQO1, GLC, HO-1 levels | [12] | |
| rubrofusarin, Rubrofusarin 6-O-β-d-glucopyranoside, Rubrofusarin 6-O-β-d-gentiobioside, Nor-rubrofusarin 6-O-β-d-glucoside | Anti-Alzheimer’s activity | In vitro | (a) Acetylcholinesterase inhibitory activity (b) β-secretase inhibitory activity | - | (a) 0–100 µM (b) 0–750 µM | (a)15.95–148 µM (b) 14.0–190 µM | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Park, S.; Chang, M. Phytochemistry, Ethnopharmacological Uses, Biological Activities, and Therapeutic Applications of Cassia obtusifolia L.: A Comprehensive Review. Molecules 2021, 26, 6252. https://doi.org/10.3390/molecules26206252
Ali MY, Park S, Chang M. Phytochemistry, Ethnopharmacological Uses, Biological Activities, and Therapeutic Applications of Cassia obtusifolia L.: A Comprehensive Review. Molecules. 2021; 26(20):6252. https://doi.org/10.3390/molecules26206252
Chicago/Turabian StyleAli, Md Yousof, Seongkyu Park, and Munseog Chang. 2021. "Phytochemistry, Ethnopharmacological Uses, Biological Activities, and Therapeutic Applications of Cassia obtusifolia L.: A Comprehensive Review" Molecules 26, no. 20: 6252. https://doi.org/10.3390/molecules26206252
APA StyleAli, M. Y., Park, S., & Chang, M. (2021). Phytochemistry, Ethnopharmacological Uses, Biological Activities, and Therapeutic Applications of Cassia obtusifolia L.: A Comprehensive Review. Molecules, 26(20), 6252. https://doi.org/10.3390/molecules26206252






