Novel Orange Color Pigments Based on La3LiMnO7
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Powder Diffraction (XRD) and Scanning Electron Microscopy (SEM) Image
2.2. X-ray Fluorescence Analysis (XRF)
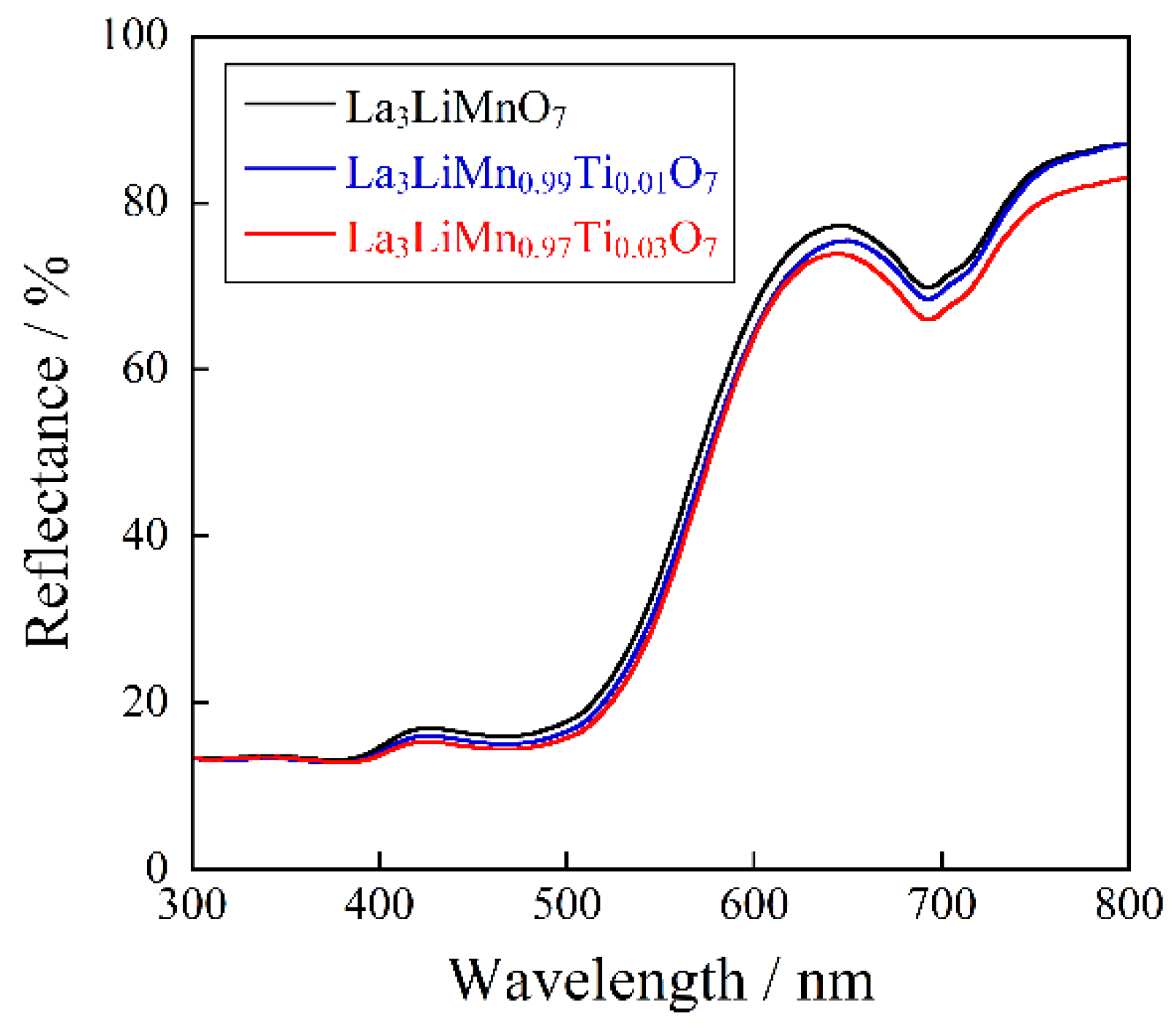
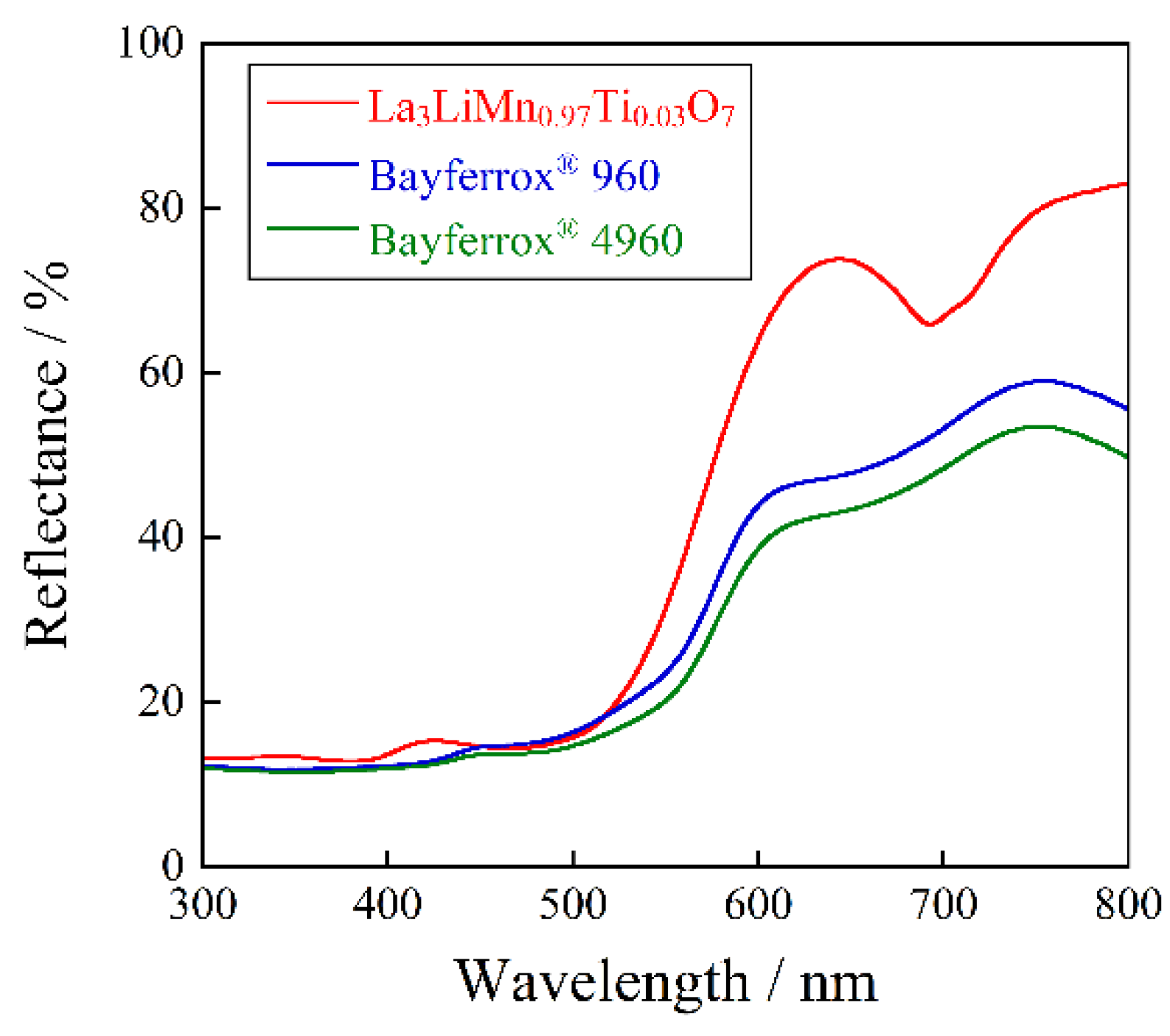
2.3. Optical Reflectance Spectra
2.4. Color Properties
2.5. Chemical Stability Test
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Faulkner, E.B.; Schwartz, R.J. High Performance Pigments, 2nd ed.; Wiley-VCH: Weinhein, Germany, 2009. [Google Scholar]
- Fortuño-Morte, M.; Beltrán-Mir, H.; Cordoncillo, E. Study of the role of praseodymium and iron in an environment-friendly reddish orange pigment based on Fe doped Pr2Zr2O7: A multifunctional material. J. Alloys Compd. 2020, 845, 155841. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Sreena, T.S. Color tunable pigments with high NIR reflectance in terbium-doped cerate systems for sustainable energy saving applications. ACS Sustain. Chem. Eng. 2019, 7, 8804–8815. [Google Scholar] [CrossRef]
- Šulcová, P.; Trojan, M. Thermal synthesis of the (Bi2O3)1–x(Er2O3)x pigments. J. Therm. Anal. Calorim. 2007, 88, 111–113. [Google Scholar] [CrossRef]
- Šulcová, P.; Trojan, M. Thermal synthesis and properties of the (Bi2O3)1–x(Ho2O3)x pigments. J. Therm. Anal. Calorim. 2006, 83, 557–559. [Google Scholar] [CrossRef]
- Schildhammer, D.; Fuhrmann, G.; Petschnig, L.; Schottenberger, H.; Huppertz, H. Synthesis and optical properties of new highly NIR reflective inorganic pigments RE6Mo2O15 (RE = Tb, Dy, Ho, Er). Dyes Pigm. 2017, 140, 22–28. [Google Scholar] [CrossRef]
- Oka, R.; Kosaya, T.; Masui, T. Novel environmentally friendly inorganic orange pigments based on Ca14Al10Zn6O35. Chem. Lett. 2018, 47, 1522–1525. [Google Scholar] [CrossRef]
- Oka, R.; Shobu, Y.; Masui, T. Synthesis and color evaluation of Ta5+-doped Bi2O3. ACS Omega 2019, 4, 7581–7585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Li, J.; Sleight, A.W.; Subramanian, M.A. New oxides showing an intense orange color based on Fe3+ trigonal-bipyramidal coordination. Inorg. Chem. 2011, 50, 5858–5860. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, K. Synthesis of Bi2O3–ZrO2 solid solutions for environment-friendly orange pigments. J. Ceram. Soc. Jpn. 2017, 125, 396–398. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.G.; Divya, D. Synthesis and characterization of orange pigments from rare earth metal ions. Asian J. Chem. 2017, 29, 1673–1676. [Google Scholar] [CrossRef]
- Giampaoli, G.; Li, J.; Hermann, R.P.; Stalick, J.K.; Subramanian, M.A. Tuning color through sulfur and fluorine substitutions in the defect tin (II, IV) niobate pyrochlores. Solid State Sci. 2018, 81, 32–42. [Google Scholar] [CrossRef]
- Kusumoto, K. Synthesis of Bi2O3–Nb2O5 solid solutions for environmental-friendly reddish yellow pigments. J. Ceram. Soc. Jpn. 2016, 124, 926–928. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xiao, Y.; Huang, B.; Sun, X. Sustainable cool pigments based on iron and tungsten co-doped lanthanum cerium oxide with high NIR reflectance for energy saving. Dyes Pigm. 2018, 154, 1–7. [Google Scholar] [CrossRef]
- Bae, B.; Takeuchi, N.; Tamura, S.; Imanaka, N. Environmentally friendly orange pigments based on hexagonal perovskite-type compounds and their high NIR reflectivity. Dyes Pigm. 2017, 147, 523–528. [Google Scholar] [CrossRef]
- Schildhammer, D.; Fuhrmann, G.; Petschnig, L.; Weinberger, N.; Schottenberger, H.; Huppertz, H. Synthesis and characterization of a new high NIR reflective ytterbium molybdenum oxide and related doped pigments. Dyes Pigm. 2017, 138, 90–99. [Google Scholar] [CrossRef]
- Llusar, M.; García, E.; García, M.T.; Gargori, C.; Badenes, J.A.; Monrós, G. Synthesis, stability and coloring properties of yellow–orange pigments based on Ni-doped karrooite (Ni,Mg)Ti2O5. J. Eur. Ceram. Soc. 2015, 35, 357–376. [Google Scholar] [CrossRef]
- Yang, F.; Qiao, L.; Ren, H.; Yan, F. Luminescence analysis of Mn4+,Zn2+: Li2TiO3 red phosphors. J. Lumin. 2018, 194, 179–184. [Google Scholar] [CrossRef]
- Takeda, Y.; Kato, H.; Kobayashi, M.; Kobayashi, H.; Kakihana, M. Photoluminescence properties of Mn4+-activated perovskite-type titanates, La2MTiO6:Mn4+ (M = Mg and Zn). Chem. Lett. 2015, 44, 1541–1543. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yang, L.; Ji, C.; Xu, D.; Zhang, C.; Bu, H.; Tan, X.; Yun, X.; Sun, J. Studies on luminescence properties of double perovskite deep red phosphor La2ZnTiO6:Mn4+ for indoor plant growth LED applications. J. Alloys Compd. 2019, 802, 628–635. [Google Scholar] [CrossRef]
- Cao, R.; Ye, Y.; Peng, Q.; Zheng, G.; Ao, H.; Fu, J.; Guo, Y.; Guo, B. Synthesis and luminescence characteristics of novel red-emitting Ba2TiGe2O8:Mn4+ phosphor. Dyes Pigm. 2017, 146, 14–19. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nishiwaki, Y.; Fujishiro, F.; Kamei, S.; Ueda, T. Quantitative determination of the effective Mn4+ concentration in a Li2TiO3:Mn4+ phosphor and its effect on the photoluminescence efficiency of deep red emission. ACS Omega 2019, 4, 19856–19862. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yuwen, M.; Liu, J.; Yu, C.; Xuan, T.; Li, H. Electrospinning, optical properties and white LED applications of one-dimensional CaAl12O19:Mn4+ nanofiber phosphors. Ceram. Int. 2017, 43, 5674–5679. [Google Scholar] [CrossRef]
- Adachi, S. Review—Mn4+-activated red and deep red-emitting phosphors. ECS J. Solid State Sci. Technol. 2020, 9, 016001. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Liang, J.; He, J.; Liu, Z.-Q.; Yin, Y. Localized charge accumulation driven by Li+ incorporation for efficient LED phosphors with tunable photoluminescence. Chem. Mater. 2020, 32, 9551–9559. [Google Scholar] [CrossRef]
- Oka, R.; Kusukami, K.; Masui, T. Effect of [MnO6] octahedra to the coloring mechanism of (Li1−xNax)2MnO3. ACS Omega 2019, 4, 19856–19862. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Laha, S.; Natarajan, S.; Gopalakrishnan, J. Li2MnO3: A rare red-coloured manganese (IV) oxide exhibiting tunable red–yellow–green emission. J. Mater. Chem. C 2015, 3, 4794–4800. [Google Scholar] [CrossRef]
- Gramm, G.; Fuhrmann, G.; Zimmerhofer, F.; Wieser, M.; Huppertz, H. Development of high NIR-reflective red Li2MnO3 pigments. Z. Anorg. Allg. Chem. 2020, 646, 1722–1729. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Found. Adv. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Battle, P.D.; Burley, J.C.; Gallon, D.J.; Grey, C.P.; Sloan, J. Magnetism and structural chemistry of the n = 2 Ruddlesden–Popper phase La3LiMnO7. J. Solid State Chem. 2004, 177, 119–125. [Google Scholar] [CrossRef]
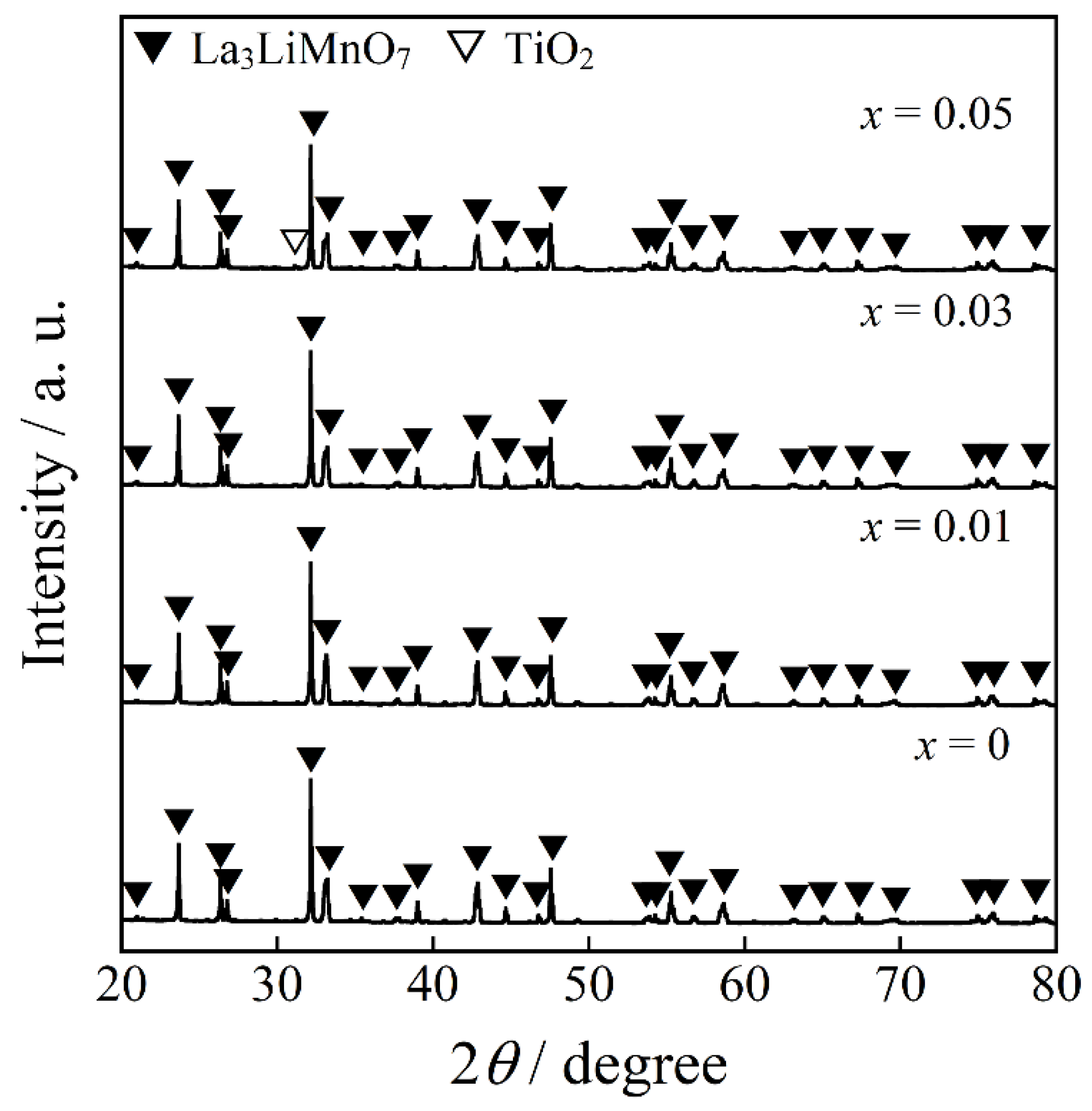

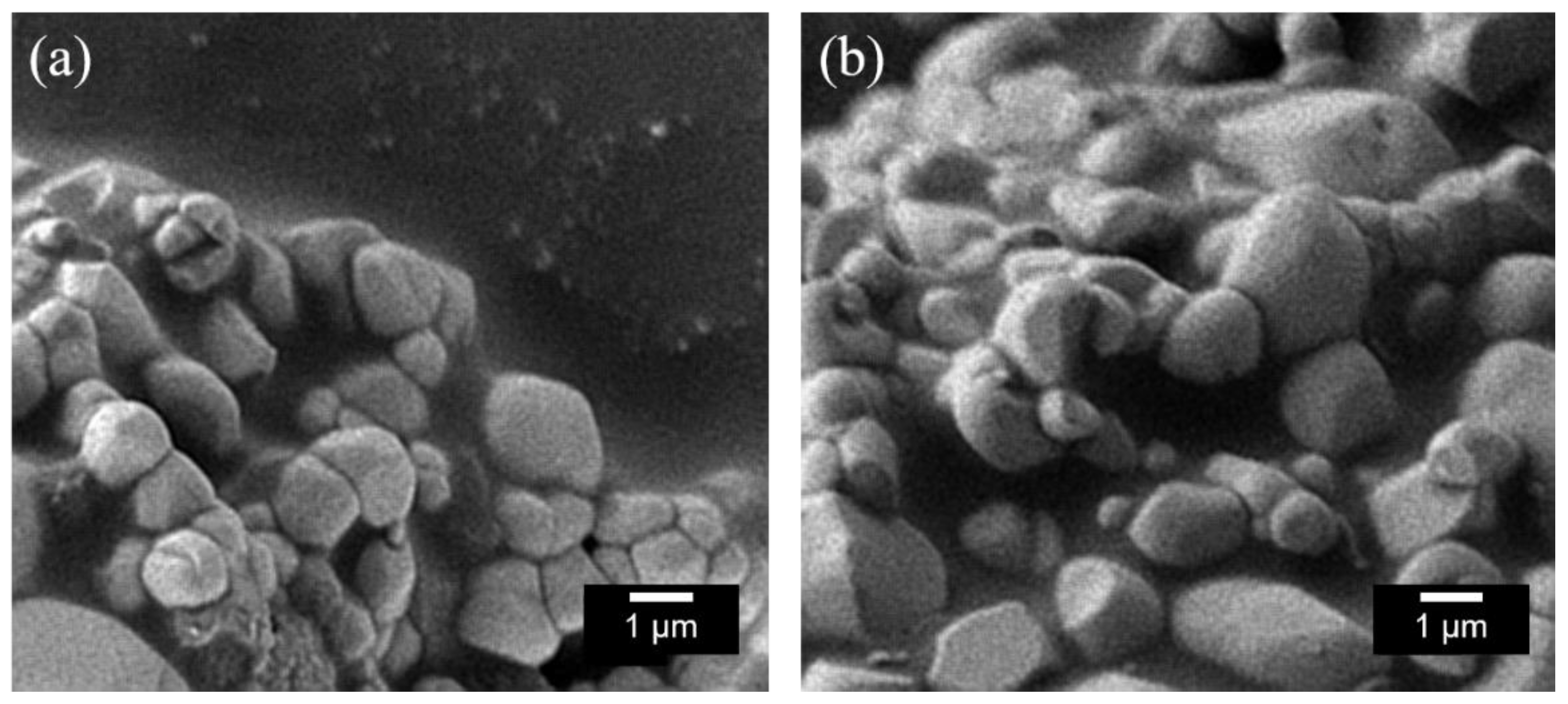


| x = 0 | x = 0.01 | x = 0.03 | x = 0.05 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2θ/deg. | FWHM | RII/% | 2θ/deg. | FWHM | RII/% | 2θ/deg. | FWHM | RII/% | 2θ/deg. | FWHM | RII/% |
| 21.00 | 0.12 | 4 | 20.99 | 0.48 | 6 | 20.98 | 0.40 | 11 | 20.99 | 0.08 | 4 |
| 23.67 | 0.06 | 51 | 23.67 | 0.07 | 50 | 23.67 | 0.07 | 51 | 23.67 | 0.07 | 50 |
| 26.36 | 0.07 | 28 | 26.36 | 0.07 | 25 | 26.37 | 0.07 | 27 | 26.36 | 0.07 | 25 |
| 26.78 | 0.07 | 15 | 26.77 | 0.07 | 15 | 26.77 | 0.07 | 15 | 26.77 | 0.07 | 14 |
| 32.16 | 0.07 | 100 | 32.16 | 0.07 | 100 | 32.16 | 0.07 | 100 | 32.16 | 0.07 | 100 |
| 33.07 | 0.19 | 47 | 33.11 | 0.22 | 69 | 33.04 | 0.17 | 39 | 33.22 | 0.23 | 54 |
| 35.41 | 0.05 | 2 | 35.38 | 0.11 | 3 | 35.41 | 0.08 | 2 | 35.39 | 0.06 | 2 |
| 37.70 | 0.24 | 7 | 37.66 | 0.20 | 8 | 37.69 | 0.27 | 7 | 37.61 | 0.19 | 6 |
| 38.99 | 0.07 | 15 | 38.99 | 0.08 | 15 | 38.99 | 0.08 | 15 | 38.99 | 0.08 | 16 |
| 42.76 | 0.14 | 30 | 42.82 | 0.18 | 55 | 42.73 | 0.13 | 27 | 42.73 | 0.13 | 30 |
| 44.66 | 0.07 | 11 | 44.66 | 0.08 | 10 | 44.67 | 0.08 | 10 | 44.66 | 0.08 | 10 |
| 46.77 | 0.08 | 5 | 46.77 | 0.09 | 5 | 46.77 | 0.09 | 5 | 46.77 | 0.08 | 5 |
| 47.54 | 0.08 | 42 | 47.53 | 0.09 | 42 | 47.52 | 0.08 | 41 | 47.52 | 0.08 | 42 |
| 53.72 | 0.29 | 10 | 53.76 | 0.30 | 13 | 53.67 | 0.27 | 11 | 53.67 | 0.31 | 10 |
| 54.25 | 0.08 | 5 | 54.24 | 0.08 | 5 | 54.25 | 0.08 | 6 | 54.25 | 0.08 | 5 |
| 55.17 | 0.08 | 17 | 55.16 | 0.09 | 16 | 55.15 | 0.09 | 19 | 55.15 | 0.10 | 19 |
| 55.29 | 0.09 | 21 | 55.29 | 0.09 | 21 | 55.29 | 0.09 | 20 | 55.29 | 0.08 | 18 |
| 56.76 | 0.21 | 10 | 56.71 | 0.17 | 9 | 56.77 | 0.21 | 9 | 56.65 | 0.13 | 5 |
| 58.49 | 0.22 | 21 | 58.60 | 0.27 | 36 | 58.40 | 0.26 | 27 | 58.41 | 0.27 | 26 |
| 63.21 | 0.32 | 7 | 63.14 | 0.29 | 8 | 63.15 | 0.39 | 9 | 63.22 | 0.25 | 5 |
| 65.07 | 0.20 | 9 | 65.05 | 0.17 | 9 | 65.01 | 0.21 | 10 | 65.03 | 0.22 | 9 |
| 67.27 | 0.11 | 9 | 67.26 | 0.10 | 10 | 67.25 | 0.11 | 10 | 67.26 | 0.11 | 10 |
| 69.44 | 0.72 | 11 | 69.40 | 0.29 | 6 | 69.27 | 0.51 | 8 | 69.28 | 0.63 | 9 |
| 74.99 | 0.13 | 9 | 74.97 | 0.13 | 9 | 74.94 | 0.13 | 10 | 74.94 | 0.12 | 9 |
| 75.96 | 0.32 | 24 | 75.87 | 0.31 | 23 | 75.74 | 0.37 | 22 | 75.68 | 0.21 | 10 |
| 78.64 | 0.12 | 7 | 78.62 | 0.12 | 7 | 78.62 | 0.14 | 7 | 78.60 | 0.13 | 7 |
| 79.07 | 0.37 | 7 | 79.10 | 0.39 | 13 | 79.16 | 0.48 | 10 | 78.97 | 0.32 | 6 |
| Samples | Stoichiometry (La:Mn:Ti) | Analyzed Ratio (La:Mn:Ti) |
|---|---|---|
| La3LiMnO7 | 3:1 | 3.03:0.97 |
| La3LiMn0.99Ti0.01O7 | 3:0.99:0.01 | 3.01:0.96:0.03 |
| La3LiMn0.97Ti0.03O7 | 3:0.97:0.03 | 3.01:0.94:0.05 |
| Pigment | L* | a* | b* | C | h° |
|---|---|---|---|---|---|
| La3LiMnO7 | 69.5 | +25.7 | +63.8 | 68.8 | 68.1 |
| La3LiMn0.99Ti0.01O7 | 68.7 | +26.1 | +64.4 | 69.5 | 67.9 |
| La3LiMn0.97Ti0.03O7 | 67.2 | +27.3 | +65.4 | 70.9 | 67.3 |
| Bayferrox® 960 | 59.0 | +21.0 | +47.5 | 51.9 | 66.1 |
| Bayferrox® 4960 | 55.9 | +23.5 | +47.3 | 52.8 | 63.6 |
| Treatment | L* | a* | b* | C | h° |
|---|---|---|---|---|---|
| As synthesized | 67.2 | +27.3 | +65.4 | 70.9 | 67.3 |
| 4% CH3COOH | 65.5 | +27.0 | +58.7 | 64.6 | 65.3 |
| 4% NH4HCO3 | 62.0 | +26.9 | +58.2 | 64.1 | 65.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oka, R.; Koyama, J.-i.; Morimoto, T.; Masui, T. Novel Orange Color Pigments Based on La3LiMnO7. Molecules 2021, 26, 6243. https://doi.org/10.3390/molecules26206243
Oka R, Koyama J-i, Morimoto T, Masui T. Novel Orange Color Pigments Based on La3LiMnO7. Molecules. 2021; 26(20):6243. https://doi.org/10.3390/molecules26206243
Chicago/Turabian StyleOka, Ryohei, Jun-ichi Koyama, Takuro Morimoto, and Toshiyuki Masui. 2021. "Novel Orange Color Pigments Based on La3LiMnO7" Molecules 26, no. 20: 6243. https://doi.org/10.3390/molecules26206243
APA StyleOka, R., Koyama, J.-i., Morimoto, T., & Masui, T. (2021). Novel Orange Color Pigments Based on La3LiMnO7. Molecules, 26(20), 6243. https://doi.org/10.3390/molecules26206243






