La-Doped ZnTiO3/TiO2 Nanocomposite Supported on Ecuadorian Diatomaceous Earth as a Highly Efficient Photocatalyst Driven by Solar Light
Abstract
1. Introduction
2. Results
2.1. Characterization of the Samples
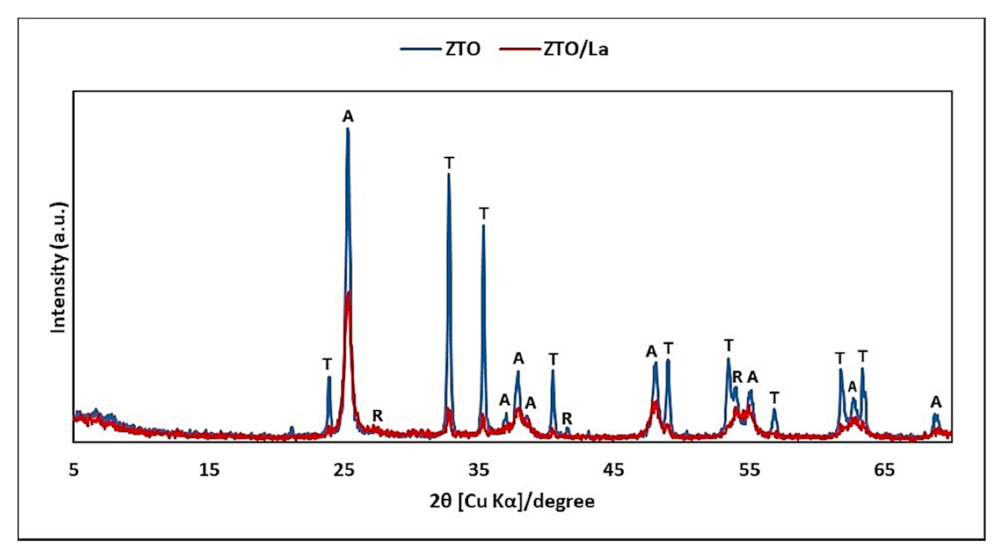
2.1.1. XRD and XRF Analysis
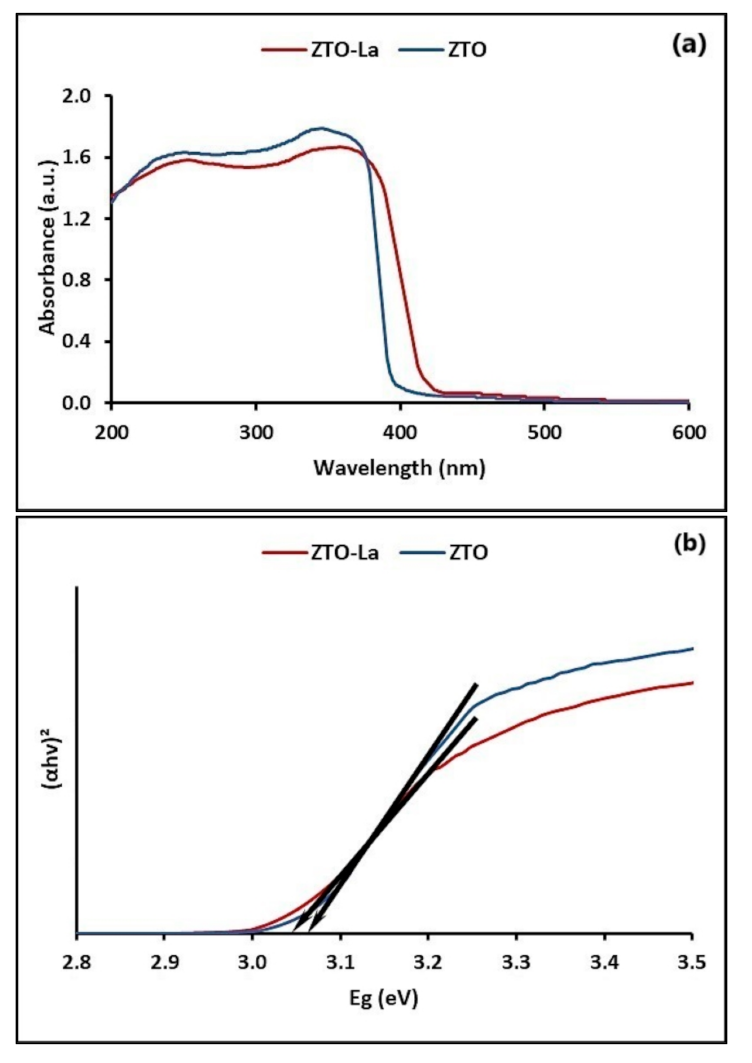
2.1.2. Optical and Photoelectric Properties
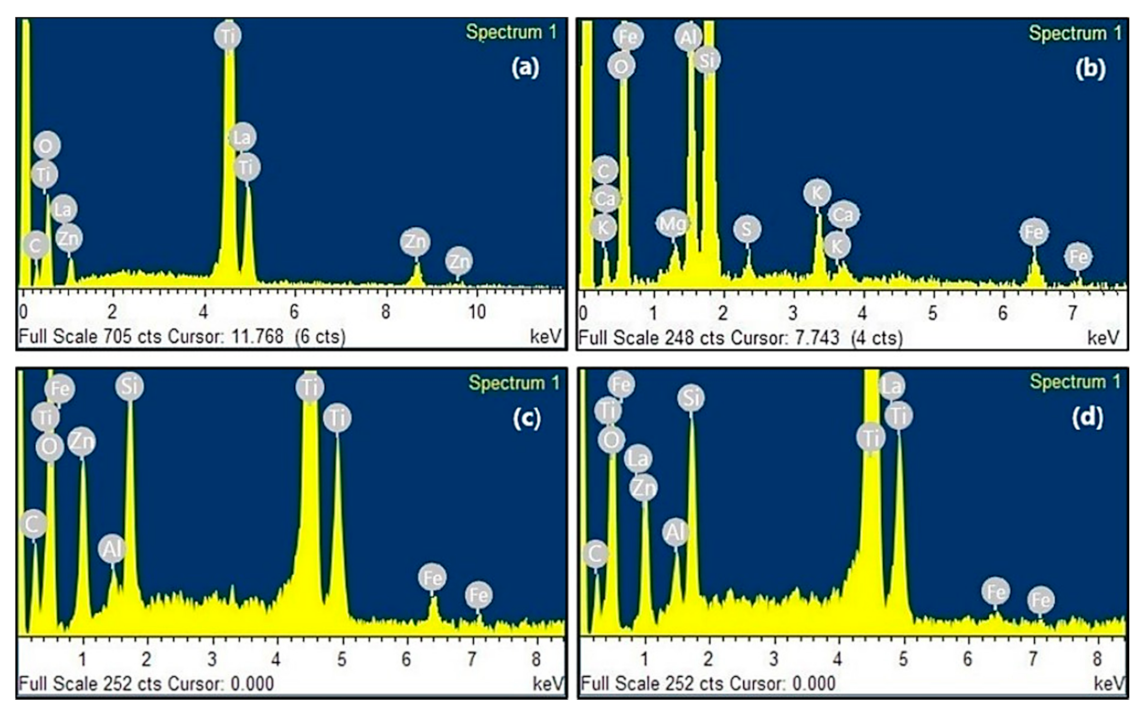
2.1.3. SEM and EDS Analysis
2.1.4. Specific Surface Area (SSA) Analysis
2.2. MB Adsorption
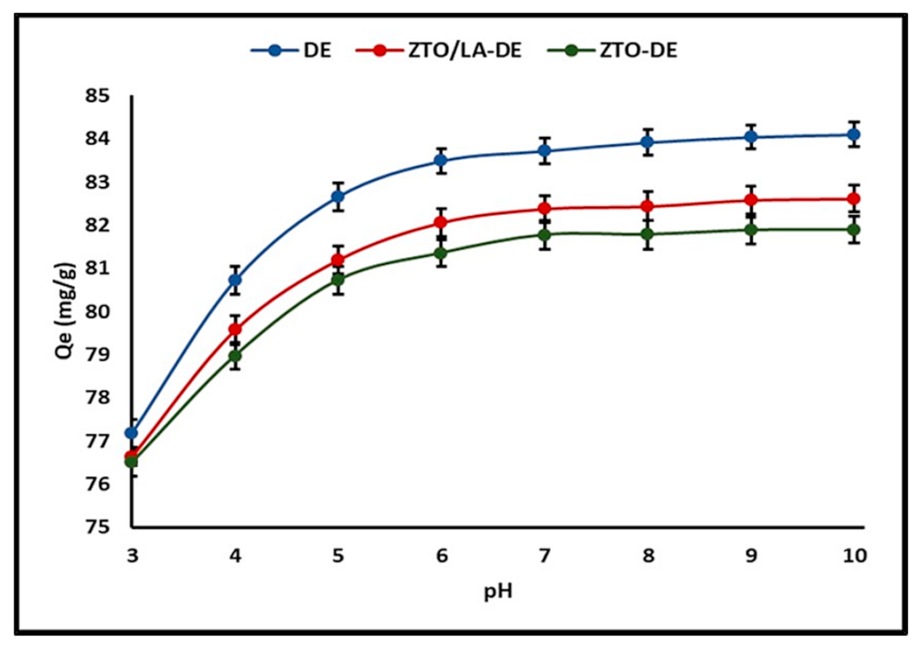
2.2.1. Effect of pH
2.2.2. Adsorption Isotherm
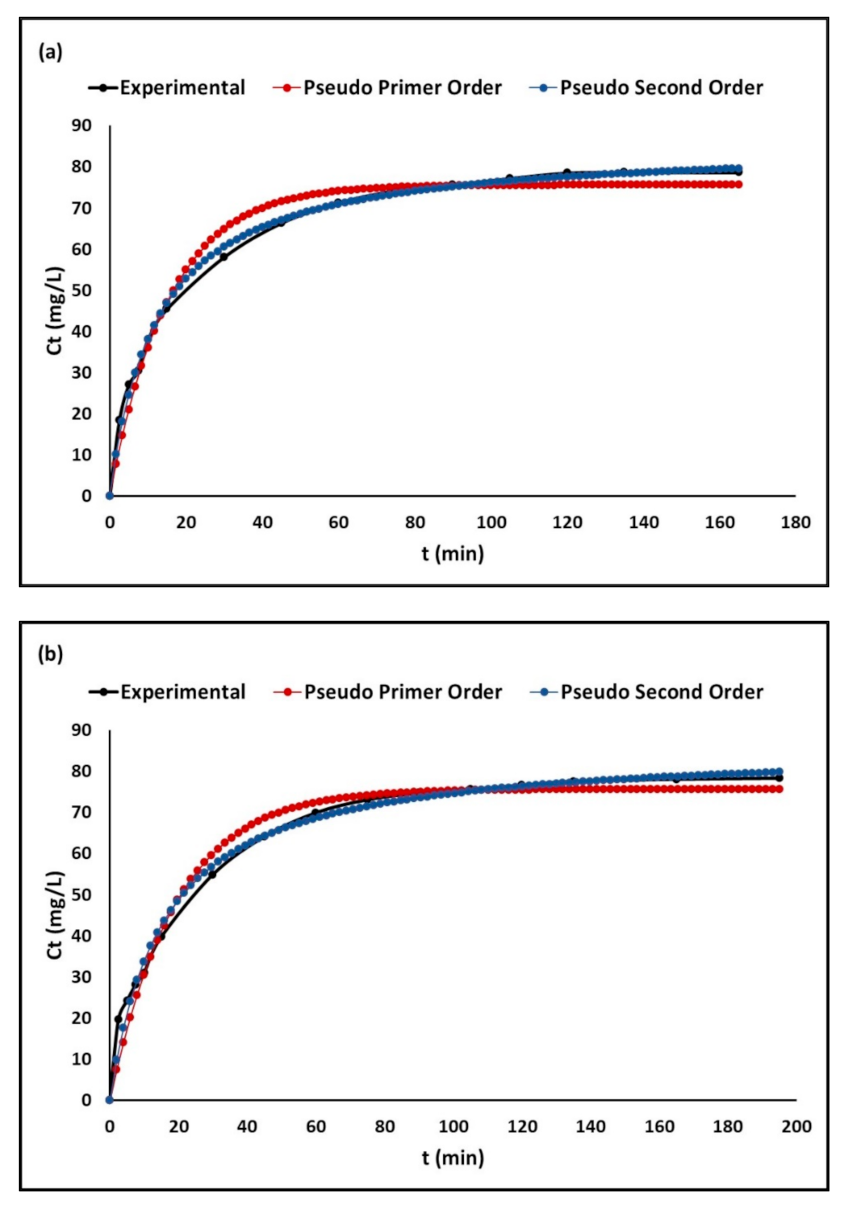
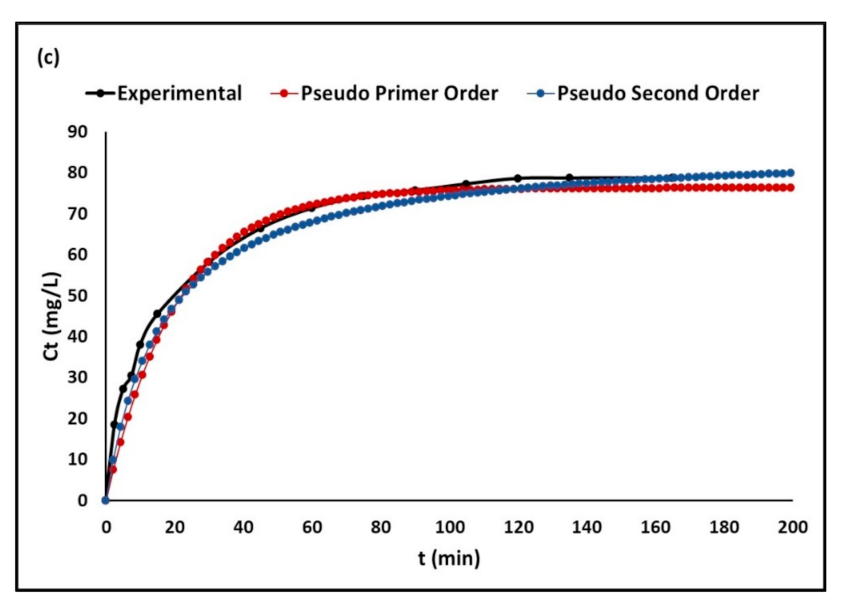
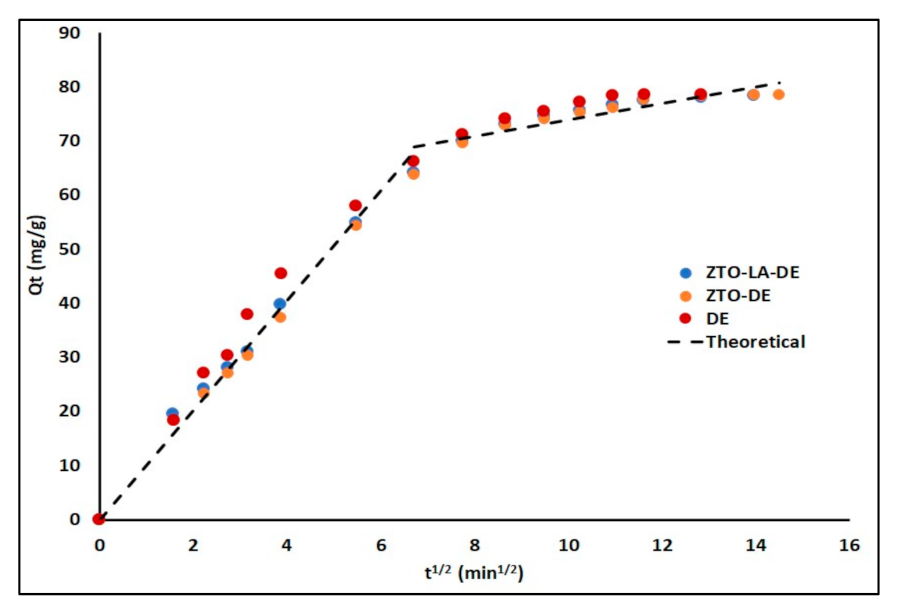
2.2.3. Adsorption Kinetics
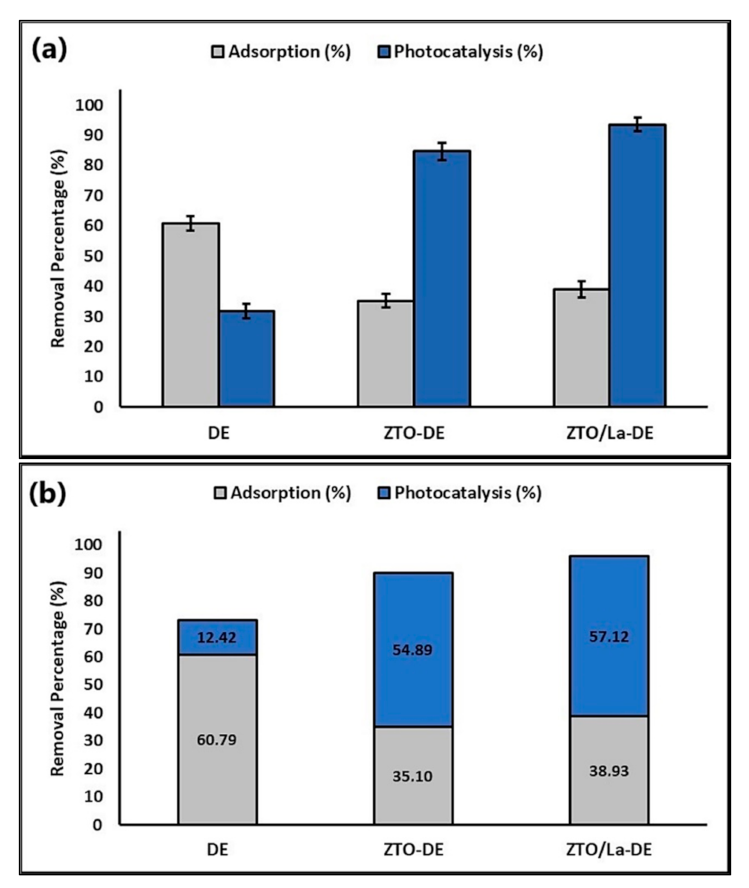
2.3. Photocatalytic Degradation of MB
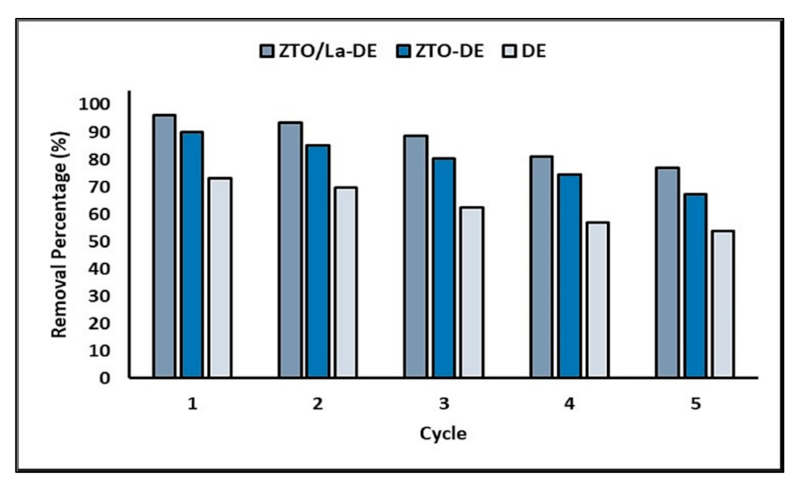
2.4. Reuse of the Composites
3. Discussion
3.1. Characterization of the Samples
3.1.1. XRD and XRF Analysis
3.1.2. Optical and Photoelectric Properties
3.1.3. SEM and EDS Analysis
3.1.4. Specific Surface Area (SSA) Analysis
3.2. MB Adsorption
3.2.1. Effect of pH
3.2.2. Adsorption Isotherm
3.2.3. Adsorption Kinetics
3.3. Photocatalytic Degradation of MB
3.4. Reuse of the Composites
3.5. MB Adsorption Capacity and Photocatalytic Activity of the Synthesized Materials Compared to Other Materials Described in the Literature
4. Material and Methods
4.1. Materials
4.2. Diatomaceous Earth Purification
4.3. Synthesis of the DE-Supported Nanocomposites
4.4. Structuring of the DE-Supported Nanocomposites
4.5. Characterization
4.6. Adsorption Studies
4.6.1. Effect of pH
4.6.2. Isotherm Models
4.6.3. Kinetic Models
4.7. Photocatalytic Degradation
4.8. Reuse of the Supported Photocatalysts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total. Environ. 2020, 706, 136026. [Google Scholar] [CrossRef]
- Wang, B.; De Godoi, F.C.; Zheng, S.; Gentle, I.; Li, C. Enhanced photocatalytic properties of reusable TiO2-loaded natural porous minerals in dye wastewater purification. Powder Technol. 2016, 302, 426–433. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Kader, S.; Islam, M.; Khan, M. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Omer, O.S.; Hussein, M.A.; Hussein, B.; Mgaidi, A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.R.; Sadjadi, M.S.; Farhadyar, N.; Sadr, M.H. Improving the photocatalytic performance of a perovskite ZnTiO3 through ZnTiO3@S nanocomposites for degradation of Crystal violet and Rhodamine B pollutants under sunlight. Inorg. Chem. Commun. 2020, 119, 108091. [Google Scholar] [CrossRef]
- Wu, A.; Wang, D.; Wei, C.; Zhang, X.; Liu, Z.; Feng, P.; Ou, X.; Qiang, Y.; Garcia, H.; Niu, J. A comparative photocatalytic study of TiO2 loaded on three natural clays with different morphologies. Appl. Clay Sci. 2019, 183, 105352. [Google Scholar] [CrossRef]
- Ahmed, M.; El-Katori, E.E.; Gharni, Z.H. Photocatalytic degradation of methylene blue dye using Fe2O3/TiO2 nanoparticles prepared by sol–gel method. J. Alloys Compd. 2013, 553, 19–29. [Google Scholar] [CrossRef]
- Shahid, M.; El Saliby, I.; McDonagh, A.; Chekli, L.; Tijing, L.D.; Kim, J.-H.; Shon, H.K. Adsorption and Photocatalytic Degradation of Methylene Blue Using Potassium Polytitanate and Solar Simulator. J. Nanosci. Nanotechnol. 2016, 16, 4342–4349. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ismail, A.A.; Mohamed, R.M. Enhancement of titania by doping rare earth for photodegradation of organic dye (Direct Blue). J. Hazard. Mater. 2009, 166, 138–143. [Google Scholar] [CrossRef]
- Mazierski, P.; Lisowski, W.; Grzyb, T.; Winiarski, M.; Klimczuk, T.; Mikołajczyk, A.; Flisikowski, J.; Hirsch, A.; Kołakowska, A.; Puzyn, T.; et al. Enhanced photocatalytic properties of lanthanide-TiO2 nanotubes: An experimental and theoretical study. Appl. Catal. B Environ. 2017, 205, 376–385. [Google Scholar] [CrossRef]
- Cai, H.; Chen, X.; Li, Q.; He, B.; Tang, Q. Enhanced photocatalytic activity from Gd, La codoped TiO2 nanotube array photocatalysts under visible-light irradiation. Appl. Surf. Sci. 2013, 284, 837–842. [Google Scholar] [CrossRef]
- Abirami, R.; Kalaiselvi, C.; Kungumadevi, L.; Senthil, T.; Kang, M. Synthesis and characterization of ZnTiO3 and Ag doped ZnTiO3 perovskite nanoparticles and their enhanced photocatalytic and antibacterial activity. J. Solid State Chem. 2020, 281, 121019. [Google Scholar] [CrossRef]
- El-Sharkawy, E.; Soliman, A.Y.; Al-Amer, K.M. Comparative study for the removal of methylene blue via adsorption and photocatalytic degradation. J. Colloid Interface Sci. 2007, 310, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Mahamadi, C.; Mawere, E. Kinetic Modeling of Methylene Blue and Crystal Violet Dyes Adsorption on Alginate-Fixed Water Hyacinth in Single and Binary Systems. Am. J. Anal. Chem. 2013, 04, 17–24. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Ghiasvand, A.; Noorimotlagh, Z. Removal of methylene blue and acid orange 7 from aqueous solutions by activated carbon coated with zinc oxide (ZnO) nanoparticles: Equilibrium, kinetic, and thermodynamic study. Desalination Water Treat. 2015, 55, 252–262. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Z.; Wang, D.; Kang, J.; Qi, H. Effective photocatalytic degradation and physical adsorption of methylene blue using cellulose/GO/TiO2 hydrogels. RSC Adv. 2020, 10, 23936–23943. [Google Scholar] [CrossRef]
- Salazar-Rábago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Perez, R.O.; Cerino-Cordova, F. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Abdullah, N.; Lau, W.J.; Ng, B.C.; Ismail, A.F. Adsorption and photocatalytic degradation of methylene blue using high surface area titanate nanotubes (TNT) synthesized via hydrothermal method. J. Nanopart. Res. 2017, 19, 220. [Google Scholar] [CrossRef]
- Laysandra, L.; Sari, M.W.M.K.; Soetaredjo, F.E.; Foe, K.; Putro, J.N.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Adsorption and photocatalytic performance of bentonite-titanium dioxide composites for methylene blue and rhodamine B decoloration. Heliyon 2017, 3, e00488. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T.; Lai, C.-W.; Hsien, K.-J. Characterization and adsorption properties of diatomaceous earth modified by hydrofluoric acid etching. J. Colloid Interface Sci. 2006, 297, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Jia, Y.; Han, W.; Xiong, G.; Yang, W. Diatomite as high performance and environmental friendly catalysts for phenol hydroxylation with H2O. Sci. Technol. Adv. Mater. 2007, 8, 106–109. [Google Scholar] [CrossRef]
- Tokarský, J.; Matějka, V.; Neuwirthová, L.; Vontorová, J.; Kutláková, K.M.; Kukutschová, J.; Čapková, P. A low-cost photoactive composite quartz sand/TiO. Chem. Eng. J. 2013, 222, 488–497. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Selim, A.Q. Heat Treatment of Natural Diatomite; Physicochem. Probl. Miner. Process.: Wrocław, Poland, 2012; Volume 48, pp. 413–424. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2019, 160, 154–163. [Google Scholar] [CrossRef]
- Irani, M.; Mohammadi, T.; Mohebbi, S. Photocatalytic Degradation of Methylene Blue with ZnO Nanoparticles; a Joint Experimental and Theoretical Study. J. Mex. Chem. Soc. 2017, 60, 218–225. [Google Scholar] [CrossRef]
- Ozturk, B.; Soylu, G.S.P. Promoting role of transition metal oxide on ZnTiO3–TiO2 nanocomposites for the photocatalytic activity under solar light irradiation. Ceram. Int. 2016, 42, 11184–11192. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiaka, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef]
- Belver, C.; Hinojosa, M.; Bedia, J.; Tobajas, M.; Alvarez, M.A.; Rodríguez-González, V.; Rodriguez, J.J. Ag-Coated Heterostructures of ZnO-TiO2/Delaminated Montmorillonite as Solar Photocatalysts. Materials 2017, 10, 960. [Google Scholar] [CrossRef]
- Nešić, J.; Manojlović, D.D.; Anđelković, I.; Dojčinović, B.P.; Vulić, P.J.; Krstić, J.; Roglić, G.M. Preparation, characterization and photocatalytic activity of lanthanum and vanadium co-doped mesoporous TiO2 for azo-dye degradation. J. Mol. Catal. A Chem. 2013, 378, 67–75. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Lin, L.; He, D. Gd–La codoped TiO2 nanoparticles as solar photocatalysts. Prog. Nat. Sci. 2015, 25, 6–11. [Google Scholar] [CrossRef]
- Sridevi, A.; Ramji, B.; Venkatesan, G.P.; Sugumaran, V.; Selvakumar, P. A facile synthesis of TiO2/BiOCl and TiO2/BiOCl/La2O3 heterostructure photocatalyst for enhanced charge separation efficiency with improved UV-light catalytic activity towards Rhodamine B and Reactive Yellow. Inorg. Chem. Commun. 2021, 130, 108715. [Google Scholar] [CrossRef]
- Daou, I.; Zegaoui, O.; Elghazouani, A. Physicochemical and photocatalytic properties of the ZnO particles synthesized by two different methods using three different precursors. Comptes Rendus Chim. 2017, 20, 47–54. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Shwetharani, R.; Sakar, M.; Chandan, H.; Balakrishna, R.G. Observation of simultaneous photocatalytic degradation and hydrogen evolution on the lanthanum modified TiO2 nanostructures. Mater. Lett. 2018, 218, 262–265. [Google Scholar] [CrossRef]
- Yu, L.; Yang, X.; He, J.; He, Y.; Wang, D. A fluorine free method to synthesize nitrogen and lanthanum co-doped TiO2 nanocrystals with exposed {001} facets for enhancing visible-light photocatalytic activity. J. Mol. Catal. A Chem. 2015, 399, 42–47. [Google Scholar] [CrossRef]
- Nie, J.; Mo, Y.; Zheng, B.; Yuan, H.; Xiao, D. Electrochemical fabrication of lanthanum-doped TiO2 nanotube array electrode and investigation of its photoelectrochemical capability. Electrochimica Acta 2013, 90, 589–596. [Google Scholar] [CrossRef]
- Peng, H.; Cui, J.; Zhan, H.; Zhang, X. Improved photodegradation and detoxification of 2,4,6-trichlorophenol by lanthanum doped magnetic TiO. Chem. Eng. J. 2015, 264, 316–321. [Google Scholar] [CrossRef]
- Dal’Toé, A.T.; Colpani, G.L.; Padoin, N.; Fiori, M.A.; Soares, C. Lanthanum doped titania decorated with silver plasmonic nanoparticles with enhanced photocatalytic activity under UV-visible light. Appl. Surf. Sci. 2018, 441, 1057–1071. [Google Scholar] [CrossRef]
- Du, J.; Li, B.; Huang, J.; Zhang, W.; Peng, H.; Zou, J. Hydrophilic and photocatalytic performances of lanthanum doped titanium dioxide thin films. J. Rare Earths 2013, 31, 992–996. [Google Scholar] [CrossRef]
- Nasir, M.; Xi, Z.; Xing, M.; Zhang, J.; Chen, F.; Tian, B.; Bagwasi, S. Study of Synergistic Effect of Ce- and S-Codoping on the Enhancement of Visible-Light Photocatalytic Activity of TiO. J. Phys. Chem. C 2013, 117, 9520–9528. [Google Scholar] [CrossRef]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A review of advances in multifunctional XTiO3 perovskite-type oxides as piezo-photocatalysts for environmental remediation and energy production. J. Hazard. Mater. 2021, 421, 126792. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.D.; Thangappan, R.; Jayavel, R. Synthesis and characterization of LaFeO3/TiO2 nanocomposites for visible light photocatalytic activity. J. Phys. Chem. Solids 2017, 101, 25–33. [Google Scholar] [CrossRef]
- Ruzimuradov, O.; Hojamberdiev, M.; Fasel, C.; Riedel, R. Fabrication of lanthanum and nitrogen—Co-doped SrTiO3—TiO2 heterostructured macroporous monolithic materials for photocatalytic degradation of organic dyes under visible light. J. Alloys Compd. 2017, 699, 144–150. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Tan, A.L.; Young, D.J. La modified TiO2 photoanode and its effect on DSSC performance: A comparative study of doping and surface treatment on deep and surface charge trapping. Mater. Chem. Phys. 2016, 172, 105–112. [Google Scholar] [CrossRef]
- Jaimy, K.B.; Ghosh, S.; Warrier, K.G. Enhanced visible light activity of nano-titanium dioxide doped with multiple ions: Effect of crystal defects. J. Solid State Chem. 2012, 196, 465–470. [Google Scholar] [CrossRef]
- Umar, K.; Haque, M.; Muneer, M.; Harada, T.; Matsumura, M. Mo, Mn and La doped TiO2: Synthesis, characterization and photocatalytic activity for the decolourization of three different chromophoric dyes. J. Alloys Compd. 2013, 578, 431–438. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Feng, X.; Zhou, J.; Zhou, S. Preparation of a La/N co-doped TiO2 film electrode with visible light response and its photoelectrocatalytic activity on a Ni substrate. Dye. Pigment. 2016, 125, 375–383. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Cui, Z.; Zhang, K.; Feng, Y.; Meng, S. Influence of difference quantity La-doped TiO2 photoanodes on the performance of dye-sensitized solar cells: A strategy for choosing an appropriate doping quantity. J. Solid State Chem. 2016, 237, 242–247. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.; Muneer, M.; Fleisch, M.; Hakki, A.; Bahnemann, D.B.D. Photocatalytic degradation of different chromophoric dyes in aqueous phase using La and Mo doped TiO2 hybrid carbon spheres. J. Alloys Compd. 2015, 632, 837–844. [Google Scholar] [CrossRef]
- Chai, Y.; Lin, L.; Zhang, K.; Zhao, B.; He, D. Efficient visible-light photocatalysts from Gd–La codoped TiO2 nanotubes. Ceram. Int. 2014, 40, 2691–2696. [Google Scholar] [CrossRef]
- Rafieh, A.I.; Ekanayake, P.; Tan, A.L.; Lim, C.M. Effects of ionic radii of co-dopants (Mg, Ca, Al and La) in TiO2 on performance of dye-sensitized solar cells. Sol. Energy 2017, 141, 249–255. [Google Scholar] [CrossRef]
- Armaković, S.J.; Grujić-Brojčin, M.; Šćepanović, M.; Armaković, S.; Golubović, A.; Babić, B.; Abramović, B.F. Efficiency of La-doped TiO2 calcined at different temperatures in photocatalytic degradation of β-blockers. Arab. J. Chem. 2019, 12, 5355–5369. [Google Scholar] [CrossRef]
- Grujić-Brojčin, M.; Armaković, S.; Tomic, N.; Abramović, B.; Golubović, A.; Stojadinović, B.; Kremenovic, A.; Babić, B.; Dohčević-Mitrović, Z.; Šćepanović, M. Surface modification of sol–gel synthesized TiO2 nanoparticles induced by La-doping. Mater. Charact. 2014, 88, 30–41. [Google Scholar] [CrossRef]
- Lan, X.; Wang, L.; Zhang, B.; Tian, B.; Zhang, J. Preparation of lanthanum and boron co-doped TiO2 by modified sol–gel method and study their photocatalytic activity. Catal. Today 2014, 224, 163–170. [Google Scholar] [CrossRef]
- Elsellami, L.; Lachheb, H.; Houas, A. Synthesis, characterization and photocatalytic activity of Li-, Cd-, and La-doped TiO. Mater. Sci. Semicond. Process. 2015, 36, 103–114. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Weng, W.; Zheng, Z.; Wang, D. Adsorption behavior of Congo red from aqueous solution on La2O3-doped TiO2 nanotubes. J. Ind. Eng. Chem. 2014, 20, 3081–3088. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Xu, Y.; Zhang, H.; Wang, Y. The effect of lanthanide on the degradation of RB in nanocrystalline Ln/TiO2 aqueous solution. J. Photochem. Photobiol. A Chem. 2005, 170, 279–285. [Google Scholar] [CrossRef]
- Coelho, L.L.; Hotza, D.; Estrella, A.S.; de Amorim, S.M.; Puma, G.L.; Moreira, R.D.F.P.M. Modulating the photocatalytic activity of TiO2 (P25) with lanthanum and graphene oxide. J. Photochem. Photobiol. A Chem. 2019, 372, 1–10. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, T.; Wang, H. Preparation and photocatalytic activity of La3+ and Eu3+ co-doped TiO2 nanoparticles: Photo-assisted degradation of methylene blue. J. Rare Earths 2011, 29, 746–752. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.-Y.; Wang, X.; Yu, T.; Guan, J.; Yu, Z.; Li, Z.; Zou, Z. Increasing the Oxygen Vacancy Density on the TiO2 Surface by La-Doping for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 18396–18400. [Google Scholar] [CrossRef]
- Hafez, H.; Wu, J.; Lan, Z.; Li, Q.; Xie, G.; Lin, J.; Huang, M.; Huang, Y.; Abdel-Mottaleb, M.S. Enhancing the photoelectrical performance of dye-sensitized solar cells using TiO2:Eu3+ nanorods. Nanotechnology 2010, 21, 415201. [Google Scholar] [CrossRef] [PubMed]
- Saif, M. Luminescence based on energy transfer in silica doped with lanthanide titania (Gd2Ti2O7:Ln3+) [Ln3+ = Eu3+ or Dy3+]. J. Photochem. Photobiol. A Chem. 2009, 205, 145–150. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S.; Frost, R.L. A comparative study about the influence of metal ions (Ce, La and V) doping on the solar-light-induced photodegradation toward rhodamine B. J. Environ. Chem. Eng. 2015, 3, 1444–1451. [Google Scholar] [CrossRef]
- Priyanka, K.; Revathy, V.; Rosmin, P.; Thrivedu, B.; Elsa, K.; Nimmymol, J.; Balakrishna, K.; Varghese, T. Influence of La doping on structural and optical properties of TiO2 nanocrystals. Mater. Charact. 2016, 113, 144–151. [Google Scholar] [CrossRef]
- Khalid, N.; Ahmed, E.; Hong, Z.; Ahmad, M. Synthesis and photocatalytic properties of visible light responsive La/TiO2-graphene composites. Appl. Surf. Sci. 2012, 263, 254–259. [Google Scholar] [CrossRef]
- Li, H.; Feng, B. Visible-light-driven composite La2O3/TiO2 nanotube arrays: Synthesis and improved photocatalytic activity. Mater. Sci. Semicond. Process. 2016, 43, 55–59. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikołajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B Environ. 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Tanyi, A.R.; Rafieh, A.I.; Ekaneyaka, P.; Tan, A.L.; Young, D.; Zheng, Z.; Chellappan, V.; Subramanian, G.S.; Chandrakanthi, R. Enhanced efficiency of dye-sensitized solar cells based on Mg and La co-doped TiO2 photoanodes. Electrochimica Acta 2015, 178, 240–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Xu, Y.; Wang, Y. Significant effect of lanthanide doping on the texture and properties of nanocrystalline mesoporous TiO. J. Solid State Chem. 2004, 177, 3490–3498. [Google Scholar] [CrossRef]
- Xu, A.-W.; Gao, Y.; Liu, H.-Q. The Preparation, Characterization, and their Photocatalytic Activities of Rare-Earth-Doped TiO2 Nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Willner, I.; Bossmann, A.S.H.; Braun, A.M. Lanthanide Oxide-Doped Titanium Dioxide Photocatalysts: Novel Photocatalysts for the Enhanced Degradation ofp-Chlorophenoxyacetic Acid. Environ. Sci. Technol. 2001, 35, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n–p–n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef]
- Fu, R.; Wang, Q.; Gao, S.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. Effect of different processes and Ti/Zn molar ratios on the structure, morphology, and enhanced photoelectrochemical and photocatalytic performance of Ti3+ self-doped titanium–zinc hybrid oxides. J. Power Sources 2015, 285, 449–459. [Google Scholar] [CrossRef]
- Pengkalsinan, K.; Tio, Z.; Melalui, F. Effect of Calcination Temperature on ZnO/TiO2 Composite in Photocatalytic Treatment of Phenol under Visible Light; Malaysian Analytical Sciences Society: Selangor, Malaysia, 2017; Volume 21, pp. 173–181. [Google Scholar]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Enhanced UV–Visible photocatalytic activity of Cu-doped ZnO/TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 5480–5495. [Google Scholar] [CrossRef]
- Khang, K.C.L.; Hatta, M.H.M.; Lee, S.L.; Yuliati, L. Photocatalytic removal of phenol over mesoporous ZnO/TiO2 composites. J. Tekno. 2018, 80, 153–160. [Google Scholar] [CrossRef][Green Version]
- Chorfi, H.; Saadoun, M.; Bousselmi, L.; Bessaïs, B. TiO2-ITO and TiO2-ZnO nanocomposites: Application on water treatment. In Proceedings of the EPJ Web of Conferences, Sousse, Tunisie, 6–10 September 2011; EDP Sciences: Paris, France, 2012; Volume 29, p. 00015. [Google Scholar]
- Jaramillo-Fierro, X.V.; Zambrano, C.; Fernández, F.; Saenz-Puche, R.; Costa, C.; Guerrero, V.; Gonzalez, S. Synthesis, characterization and theoretical calculations of Cu(I) complex of trithiocyanuric acid [Cu(ttc)3]. Univ. Sci. 2018, 23, 241–266. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Montesdeoca-Mendoza, F.; Medina, F. Structuring of ZnTiO3/TiO2 Adsorbents for the Removal of Methylene Blue, Using Zeolite Precursor Clays as Natural Additives. Nanomaterials 2021, 11, 898. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Capa, L.; Medina, F.; González, S. DFT Study of Methylene Blue Adsorption on ZnTiO3 and TiO2 Surfaces (101). Molecules 2021, 26, 3780. [Google Scholar] [CrossRef]
- Wang, R.; An, S.; Zhang, J.; Song, J.; Wang, F. Existence form of lathanum and its improving mechanism of visible-light-driven La-F co-doped TiO. J. Rare Earths 2020, 38, 39–45. [Google Scholar] [CrossRef]
- Ke, S.; Cheng, X.; Wang, Q.; Wang, Y.; Pan, Z. Preparation of a photocatalytic TiO2/ZnTiO3 coating on glazed ceramic tiles. Ceram. Int. 2014, 40, 8891–8895. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Mehrabi, M.; Javanbakht, V. Photocatalytic degradation of cationic and anionic dyes by a novel nanophotocatalyst of TiO2/ZnTiO3/αFe2O3 by ultraviolet light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 9908–9919. [Google Scholar] [CrossRef]
- García-Ramírez, E.; Mondragón-Chaparro, M.; Zelaya-Angel, O. Band gap coupling in photocatalytic activity in ZnO–TiO2 thin films. Appl. Phys. A 2012, 108, 291–297. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Pérez, S.G.; Jaramillo, X.; Cabello, F.M. Synthesis of the ZnTiO3/TiO2 Nanocomposite Supported in Ecuadorian Clays for the Adsorption and Photocatalytic Removal of Methylene Blue Dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Gil, A.; Assis, F.; Albeniz, S.; Korili, S. Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 2011, 168, 1032–1040. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, J.S.; Li, W.; Oh, S.H. Electronic Band Structure and Photocatalytic Activity of Ln2Ti2O7 (Ln = La, Pr, Nd). J. Phys. Chem. B 2003, 107, 4963–4970. [Google Scholar] [CrossRef]
- Li, L.; Zhuang, H.; Bu, D. Characterization and activity of visible-light-driven TiO2 photocatalyst codoped with lanthanum and iodine. Appl. Surf. Sci. 2011, 257, 9221–9225. [Google Scholar] [CrossRef]
- Smitha, V.S.; Manjumol, K.A.; Baiju, K.V.; Ghosh, S.; Perumal, P.; Warrier, K.G.K. Sol–gel route to synthesize titania-silica nano precursors for photoactive particulates and coatings. J. Sol-Gel Sci. Technol. 2010, 54, 203–211. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of Single Metal-Ion Doping on the Visible-Light Photoreactivity of TiO. J. Phys. Chem. C 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Yang, P.; Lu, C.; Hua, N.; Du, Y. Titanium dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater. Lett. 2002, 57, 794–801. [Google Scholar] [CrossRef]
- Sanabria, N.; Avila, P.; Yates, M.; Rasmussen, S.; Molina, R.; Moreno, S. Mechanical and textural properties of extruded materials manufactured with AlFe and AlCeFe pillared bentonites. Appl. Clay Sci. 2010, 47, 283–289. [Google Scholar] [CrossRef]
- Grande, C.; Águeda, V.I.; Spjelkavik, A.; Blom, R. An efficient recipe for formulation of metal-organic Frameworks. Chem. Eng. Sci. 2015, 124, 154–158. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Fernández, A.G.; Cabeza, L.F.; Gómez, M.A. Influence of thermal treatments on the absorption and thermal properties of a clay mineral support used for shape-stabilization of fatty acids. J. Energy Storage 2021, 36, 102427. [Google Scholar] [CrossRef]
- El-Kousy, S.M.; El-Shorbagy, H.G.; El-Ghaffar, M.A. Chitosan/montmorillonite composites for fast removal of methylene blue from aqueous solutions. Mater. Chem. Phys. 2020, 254, 123236. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Huan, Y.; Hao, C.; Chen, W. Acrylic acid functionalized graphene oxide: High-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 2020, 261, 127736. [Google Scholar] [CrossRef] [PubMed]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- An, F.; Liu, J.; Xu, Z.; Zheng, S. Efficient removal of three dyes using porous covalent triazine frameworks: Adsorption mechanism and role of pore distribution. Water Sci. Technol. 2020, 82, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Sarici-Ozdemir, C. Adsorption and desorption kinetics behaviour of methylene blue onto activated carbon. Physicochem. Probl. Miner. Process. 2012, 48, 441–454. [Google Scholar] [CrossRef]
- Malatji, N.; Makhado, E.; Ramohlola, K.E.; Modibane, K.D.; Maponya, T.C.; Monama, G.R.; Hato, M.J. Synthesis and characterization of magnetic clay-based carboxymethyl cellulose-acrylic acid hydrogel nanocomposite for methylene blue dye removal from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 44089–44105. [Google Scholar] [CrossRef]
- Al-Degs, Y.; El-Barghouthi, M.; El-Sheikh, A.; Walker, G. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Khraisheh, M.; Allen, S.; Ahmad, M. The removal of dyes from textile wastewater: A study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J. Environ. Manag. 2003, 69, 229–238. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, M.; Nishioka, H. Adsorption of methylene blue dye from aqueous solution by novel biomassEucalyptus sheathianabark: Equilibrium, kinetics, thermodynamics and mechanism. Desalination Water Treat. 2016, 57, 5858–5878. [Google Scholar] [CrossRef]
- Hosseini, S.; Khan, M.A.; Malekbala, M.R.; Cheah, W.; Choong, T.S. Carbon coated monolith, a mesoporous material for the removal of methyl orange from aqueous phase: Adsorption and desorption studies. Chem. Eng. J. 2011, 171, 1124–1131. [Google Scholar] [CrossRef]
- Shi, J.-W.; Chen, S.-H.; Wang, S.-M.; Ye, Z.-L.; Wu, P.; Xu, B. Favorable recycling photocatalyst TiO2/CFA: Effects of calcination temperature on the structural property and photocatalytic activity. J. Mol. Catal. A Chem. 2010, 330, 41–48. [Google Scholar] [CrossRef]
- Hu, X.-S.; Liang, R.; Sun, G. Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Varmazyar, A.; Sedaghat, S.; Khalaj, M. Highly efficient removal of methylene blue by a synthesized TiO2/montmorillonite-albumin nanocomposite: Kinetic and isothermal analysis in water. RSC Adv. 2017, 7, 37214–37219. [Google Scholar] [CrossRef]
- Marsiezade, N.; Javanbakht, V. Novel hollow beads of carboxymethyl cellulose/ZSM-5/ZIF-8 for dye removal from aqueous solution in batch and continuous fixed bed systems. Int. J. Biol. Macromol. 2020, 162, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xu, F.; Wei, W.; Gao, H.; Zhang, K.; Zhang, G.; Xu, Y.; Zhang, P. Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J. Solid State Chem. 2021, 295, 121917. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Guo, D.-X.; Li, S.-F.; Cao, J.-P.; Wei, X.-Y. Removal of methylene blue by NaX zeolites synthesized from coal gasification fly ash using an alkali fusion-hydrothermal method. Desalination Water Treat. 2020, 185, 355–363. [Google Scholar] [CrossRef]
- Xu, R.; Mao, J.; Peng, N.; Luo, X.; Chang, C. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohydr. Polym. 2018, 188, 143–150. [Google Scholar] [CrossRef]
- Bée, A.; Obeid, L.; Mbolantenaina, R.; Welschbillig, M.; Talbot, D. Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 2017, 421, 59–64. [Google Scholar] [CrossRef]
- Marrakchi, F.; Bouaziz, M.; Hameed, B. Activated carbon–clay composite as an effective adsorbent from the spent bleaching sorbent of olive pomace oil: Process optimization and adsorption of acid blue 29 and methylene blue. Chem. Eng. Res. Des. 2017, 128, 221–230. [Google Scholar] [CrossRef]
- Woolard, C.; Strong, P.J.; Erasmus, C. Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams. Appl. Geochem. 2002, 17, 1159–1164. [Google Scholar] [CrossRef]
- El-Mekkawi, D.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Ge, S.; Geng, W.; He, X.; Zhao, J.; Zhou, B.; Duan, L.; Wu, Y.; Zhang, Q. Effect of framework structure, pore size and surface modification on the adsorption performance of methylene blue and Cu2+ in mesoporous silica. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 154–162. [Google Scholar] [CrossRef]
- Sahoo, S.; Uma; Banerjee, S.; Sharma, Y.C. Application of natural clay as a potential adsorbent for the removal of a toxic dye from aqueous solutions. Desalination Water Treat. 2013, 52, 6703–6711. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.; Dai, S.; Dong, X.; Li, F. Methylene blue adsorption properties of mechanochemistry modified coal fly ash. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 2133–2141. [Google Scholar] [CrossRef]
- Nayeri, D.; Mousavi, S.A.; Fatahi, M.; Almasi, A.; Khodadoost, F. Dataset on adsorption of methylene blue from aqueous solution onto activated carbon obtained from low cost wastes by chemical-thermal activation—Modelling using response surface methodology. Data Brief 2019, 25, 104036. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Shen, F. Preparation and characterization of TiO2 photocatalysts co-doped with iron (III) and lanthanum for the degradation of organic pollutants. Appl. Surf. Sci. 2011, 257, 7671–7677. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D.; Chen, M.; Li, D.; Zhu, J.; Lü, X.; Yan, C. Preparation and characterization of monodisperse Ce-doped TiO2 microspheres with visible light photocatalytic activity. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 372, 107–114. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+-TiO2 Photocatalysts toward Visible Photooxidation for Water and Wastewater Treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef]
- Moon, J.; Takagi, H.; Fujishiro, Y.; Awano, M. Preparation and characterization of the Sb-doped TiO2 photocatalysts. J. Mater. Sci. 2001, 36, 949–955. [Google Scholar] [CrossRef]
- Rattanakam, R.; Supothina, S. Visible-light-sensitive N-doped TiO2 photocatalysts prepared by a mechanochemical method: Effect of a nitrogen source. Res. Chem. Intermed. 2009, 35, 263–269. [Google Scholar] [CrossRef]
- Wen, C.; Zhu, Y.-J.; Kanbara, T.; Zhu, H.-Z.; Xiao, C.-F. Effects of I and F codoped TiO2 on the photocatalytic degradation of methylene blue. Desalination 2009, 249, 621–625. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Wang, Q.; Yang, D. Carbon and Nitrogen Co-doped TiO2 with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping. J. Catal. 2008, 260, 128–133. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Zhu, J.; Yang, D. A facile method to synthesize nitrogen and fluorine co-doped TiO2 nanoparticles by pyrolysis of (NH4)2TiF6. J. Nanopart. Res. 2009, 11, 303–313. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, F. Characterization, activity and mechanisms of a visible light driven photocatalyst: Manganese and iron co-modified TiO2 nanoparticles. Russ. J. Phys. Chem. A 2011, 85, 1825–1831. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Labis, J.P.; Al-Assiri, M. A novel Ag/PANI/ZnTiO3 ternary nanocomposite as a highly efficient visible-light-driven photocatalyst. Sep. Purif. Technol. 2021, 256, 117847. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Benkacem, T.; Hamdi, B.; Chamayou, A.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–507. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Bello, M.O.; Abdus-Salam, N.; Adekola, F.A.; Pal, U. Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem. Data Collect. 2021, 31, 100607. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, N.; Yang, L.; Lin, Q. Sorption behavior of nano-TiO2 for the removal of selenium ions from aqueous solution. J. Hazard. Mater. 2009, 170, 1197–1203. [Google Scholar] [CrossRef]
- Milanovic, M.; Nikolic, L.M. Modification of TiO2 nanoparticles through lanthanum doping and peg templating. Process. Appl. Ceram. 2014, 8, 195–202. [Google Scholar] [CrossRef]
- Ambigadevi, J.; Kumar, P.S.; Vo, D.-V.N.; Haran, S.H.; Raghavan, T.S. Recent developments in photocatalytic remediation of textile effluent using semiconductor based nanostructured catalyst: A review. J. Environ. Chem. Eng. 2021, 9, 104881. [Google Scholar] [CrossRef]





| Al2O3 | SiO2 | S | P2O5 | K2O | CaO | TiO2 | MgO | Fe2O3 | Co3O4 | SnO2 | CeO2 | WO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12.10 (±0.72) | 61.00 (±0.80) | 0.71 (±0.03) | 0.26 (±0.09) | 1.19 (±0.02) | 0.53 (±0.01) | 0.29 (±0.01) | 0.06 (±0.00) | 1.63 (±0.01) | 0.42 (±0.01) | 0.16 (±0.04) | 0.04 (±0.01) | 0.01 (±0.00) |
| Adsorbent | Form | SSA (m2/g) |
|---|---|---|
| ZTO/La | Powder | 126.45 |
| ZTO | Powder | 105.84 |
| DE | Powder | 89.84 |
| DE | Extrudate | 48.89 |
| ZTO/La-DE | Powder | 93.24 |
| ZTO/La-DE | Extrudate | 67.38 |
| ZTO-DE | Powder | 72.21 |
| ZTO-DE | Extrudate | 40.36 |
| Isotherm Parameters | ZTO-DE | ZTO/La-DE | DE | |
|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 37.32 (±1.21) | 40.44 (±1.06) | 77.05 (±2.33) |
| KL (L mg−1) | 0.63 (±0.10) | 0.99 (±0.14) | 0.56 (±0.06) | |
| RL | 0.03 | 0.02 | 0.06 | |
| χ2 | 2.27 | 2.31 | 2.30 | |
| R2 | 0.99 | 0.99 | 0.99 | |
| Freundlich | KF (L mg−1) | 13.38 (±1.21) | 17.24 (±1.51) | 20.82 (±2.22) |
| n | 2.85 (±0.90) | 3.26 (±0.37) | 2.23 (±0.24) | |
| 1/n | 0.35 | 0.31 | 0.45 | |
| χ2 | 6.62 | 10.52 | 10.42 | |
| R2 | 0.97 | 0.96 | 0.96 | |
| Kinetic Parameters | ZTO-DE | ZTO/LA-DE | DE | |
|---|---|---|---|---|
| Pseudo-first order | qmax (mg g−1) | 76.21 (±1.37) | 75.62 (±1.53) | 75.60 (±1.50) |
| k1 (min−1) | 0.05 (±3.91 × 10−3) | 0.05 (±4.61 × 10−3) | 0.06 (±5.49 × 10−3) | |
| χ2 | 14.69 | 16.83 | 15.87 | |
| R2 | 0.98 | 0.98 | 0.98 | |
| Pseudo-second order | qmax (mg g−1) | 86.35 (±4.40) | 85.96 (±1.53) | 85.57 (±0.99) |
| k2 (g mg−1 min−1) | 7.11 × 10−4 (±6.14 × 10−5) | 7.63 × 10−4 (±7.06 × 10−5) | 9.41 × 10−4 (±5.68 × 10−5) | |
| χ2 | 6.29 | 6.79 | 2.89 | |
| R2 | 0.99 | 0.99 | 1.00 | |
| Intraparticle diffusion | k3 (mg g−1 min−1/2) | 5.37 (±0.51) | 5.66 (±0.52) | 6.05 (±0.56) |
| A | 15.31 (±4.37) | 14.68 (±4.21) | 15.28 (±4.23) | |
| R2 | 0.87 | 0.89 | 0.89 | |
| External-film diffusion | Df (m2 min−1) | 1.32 × 10−11 | 1.27 × 10−11 | 1.37 × 10−11 |
| R2 | 0.97 | 0.98 | 0.93 | |
| Internal-pore diffusion | Dp (m2 min−1) | 1.20 × 10−17 | 1.24 × 10−17 | 2.00 × 10−17 |
| R2 | 0.99 | 0.99 | 0.90 | |
| Material | qe (mg/g) | References |
|---|---|---|
| Activated lignin–chitosan composite extrudates | 36.25 | [114] |
| TiO2/montmorillonite–albumin nanocomposite | 18.18 | [115] |
| Carboxymethyl cellulose/ZSM-5/ZIF-8 | 10.49 | [116] |
| ZSM-5 zeolite | 105.82 | [117] |
| NaX zeolite | 127.13 | [118] |
| Chitosan/clay microspheres | 152.20 | [119] |
| Magnetic chitosan/clay beads | 82.00 | [120] |
| Activated carbon–clay composite | 178.64 | [121] |
| Hydroxysodalite | 10.82 | [122] |
| Kaolin | 21.41 | [123] |
| Nonporous silica | 91.10 | [124] |
| a-TiO2/ZnTiO3 | 16.00 | [86] |
| a-TiO2 | 15.00 | [86] |
| Natural clay | 15.40 | [125] |
| Raw coal fly ash | 5.06 | [126] |
| Activated carbon | 6.43 | [127] |
| DE | 77.05 | This study |
| ZnTiO3/TiO2/DE | 37.32 | This study |
| ZnTiO3/TiO2/La-DE | 40.11 | This study |
| Type of Dopant | MB (mg/L) | Type of Light | Reaction Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| TiO2/La | 0.1 | UV irradiation | 120 | 85 | [128] |
| TiO2/Fe | 0.1 | UV irradiation | 120 | 75 | [128] |
| TiO2/La | 0.1 | Visible irradiation | 120 | 20 | [128] |
| TiO2/Fe | 0.1 | Visible irradiation | 120 | 26 | [128] |
| TiO2/Ce | 32 | Visible irradiation | 180 | 90 | [129] |
| TiO2/Au | 12 | Visible irradiation | 48 | 92 | [130] |
| TiO2/Sb | 100 | Visible irradiation | 60 | 100 | [131] |
| TiO2/N | 10 | Solar light | 120 | 97 | [132] |
| TiO2/I | 8 | Solar light | 120 | 45 | [133] |
| TiO2/F | 10 | Solar light | 120 | 55 | [133] |
| TiO2/C | 28.5 | Visible irradiation | 420 | 70 | [134] |
| TiO2/Fe/La | 0.1 | Visible irradiation | 120 | 91 | [128] |
| TiO2/C/N | 10 | Visible irradiation | 180 | 85 | [135] |
| TiO2/N/F | 5.74 | Visible irradiation | 140 | 16 | [136] |
| TiO2/Mn/Fe | 10 | Visible irradiation | 150 | 85 | [137] |
| ZnTiO3/PANI/Ag | 10 | Visible irradiation | 25 | 96 | [138] |
| ZnTiO3/Ag | 10 | UV irradiation | 150 | 93 | [14] |
| ZnTiO3/TiO2/La | 20 | Solar light | 150 | 100 | This study |
| ZnTiO3/TiO2 (not doped) | 20 | Solar light | 150 | 87 | This study |
| ZnTiO3/TiO2/La-DE | 20 | Solar light | 150 | 93 | This study |
| ZnTiO3/TiO2-DE (not doped) | 20 | Solar light | 150 | 85 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; González, S.; Medina, F. La-Doped ZnTiO3/TiO2 Nanocomposite Supported on Ecuadorian Diatomaceous Earth as a Highly Efficient Photocatalyst Driven by Solar Light. Molecules 2021, 26, 6232. https://doi.org/10.3390/molecules26206232
Jaramillo-Fierro X, González S, Medina F. La-Doped ZnTiO3/TiO2 Nanocomposite Supported on Ecuadorian Diatomaceous Earth as a Highly Efficient Photocatalyst Driven by Solar Light. Molecules. 2021; 26(20):6232. https://doi.org/10.3390/molecules26206232
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Silvia González, and Francesc Medina. 2021. "La-Doped ZnTiO3/TiO2 Nanocomposite Supported on Ecuadorian Diatomaceous Earth as a Highly Efficient Photocatalyst Driven by Solar Light" Molecules 26, no. 20: 6232. https://doi.org/10.3390/molecules26206232
APA StyleJaramillo-Fierro, X., González, S., & Medina, F. (2021). La-Doped ZnTiO3/TiO2 Nanocomposite Supported on Ecuadorian Diatomaceous Earth as a Highly Efficient Photocatalyst Driven by Solar Light. Molecules, 26(20), 6232. https://doi.org/10.3390/molecules26206232








