Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development
Abstract
1. Introduction
2. MAO-A’s Location and Function
3. MAO-A’s Role in Cancer
4. MAO-B’s Role in Cancer
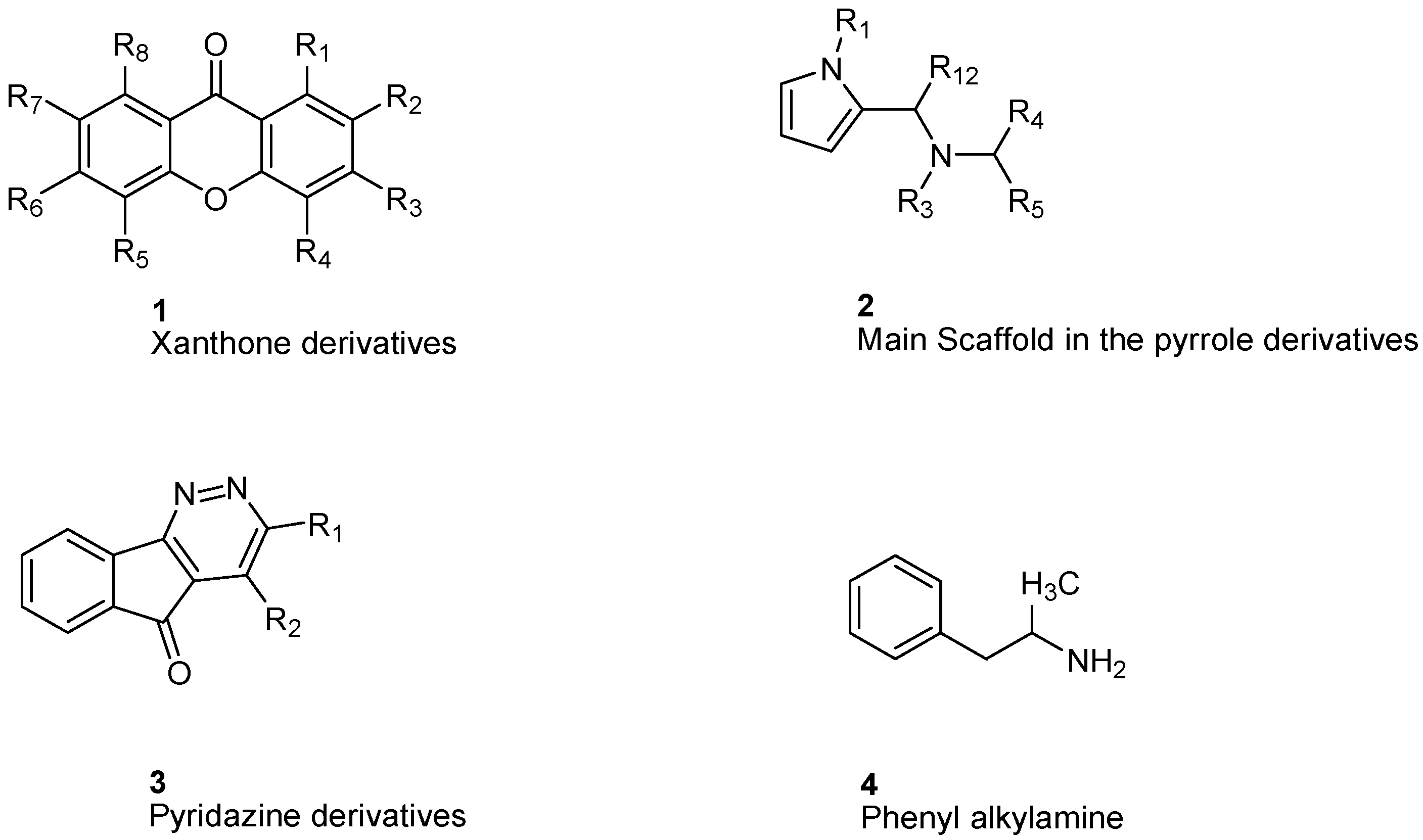
5. Structural Design of MAO-A Inhibitors
6. Xanthone Derivatives
7. Indole and Isatin Analogues
8. Pirlindole Analogues
9. Indolylmethylamine Derivatives
10. Phenethylamine Derivatives
11. Coumarin Derivatives
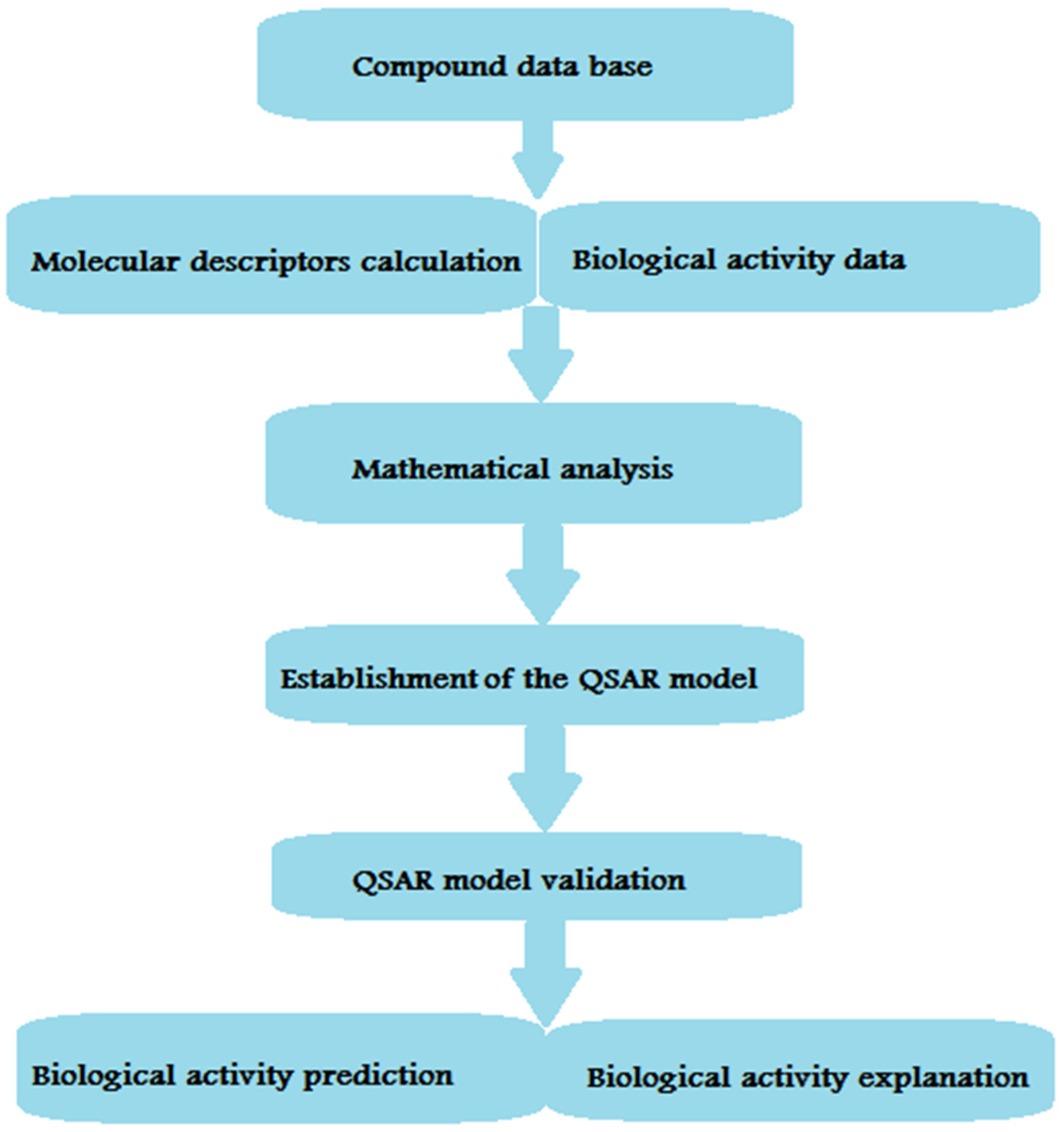
12. MAO Inhibitors
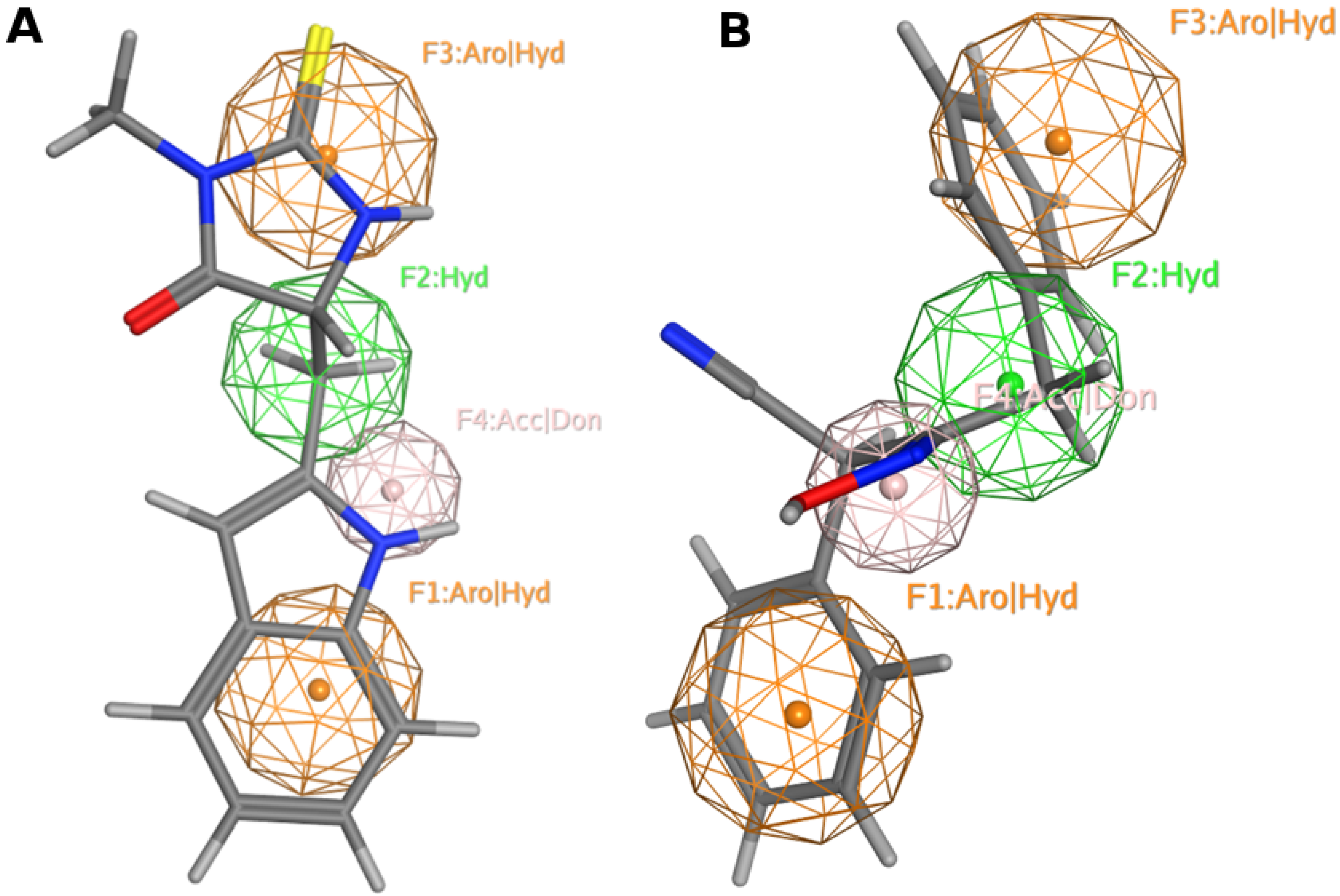
13. Pharmacophore Model Generation
14. Docking
15. MAO-A Inhibitors as Anticancer Agents
16. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
Abbreviations
| MAO | Monoamine Oxidase |
| ROS | Reactive Oxygen Species |
| FAD | Flavin Adenine Dinucleotide |
| PCa | Aggressive prostate cancer |
| NSCLC | None-small cell lung carcinoma |
| EMT | Epithelial to Mesenchymal Transition |
| QSAR | Quantitative structure–activity relationship |
| CoMFA | Comparative molecular field analysis |
| PLS | Partial least square |
| REST | Repressor element-1 silencing transcription factor |
| NED | Neuroendocrine differentiation |
| PCa | Prostate cancer |
References
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine oxidases (MAOs) as privileged molecular targets in neuroscience: Research literature analysis. Front. Mol. Neurosci. 2019, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.C.; Upadhyay, S.; Paliwal, S.; Saraf, S.K. Privileged scaffolds as MAO inhibitors: Retrospect and prospects. Eur. J. Med. Chem. 2018, 145, 445–497. [Google Scholar] [CrossRef]
- Manzoor, S.; Hoda, N. A Comprehensive Review of Monoamine Oxidase Inhibitors as Anti-Alzheimer’s Disease Agents: A Review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef]
- Shih, J.C.; Chen, K.; Ridd, M. Monoamine oxidase: From genes to behavior. Ann. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Singer, T.P.; Ramsay, R.R. Monoamine oxidases: Old friends hold many surprises. FASEB J. 1995, 9, 605–610. [Google Scholar] [CrossRef]
- Wouers, J. Structural aspects of monoamine oxidase and its reversible inhibition. Curr. Med. Chem. 1998, 5, 137–162. [Google Scholar]
- NS, S. Chemical aspects of amine oxidation by flavoprotein enzymes. Nat. Prod. Rep. 2004, 21, 722–730. [Google Scholar]
- Wang, C.C.; Borchert, A.; Ugun-Klusek, A.; Tang, L.Y.; Lui, W.T.; Chu, C.Y.; Billett, E.; Kuhn, H.; Ufer, C. Monoamine oxidase a expression is vital for embryonic brain development by modulating developmental apoptosis. J. Biol. Chem. 2011, 286, 28322–28330. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.K.; Bhattacharjee, A. Monoamine oxidase A (MAO-A): A signature marker of alternatively activated monocytes/macrophages. Inflamm. Cell Signal. 2014, 2014, 152–159. [Google Scholar]
- Meyer, J.H.; Ginovart, N.; Boovariwala, A.; Sagrati, S.; Hussey, D.; Garcia, A.; Young, T.; Praschak-Rieder, N.; Wilson, A.A.; Houle, S. Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry 2006, 63, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.G.; Nelen, M.; Breakefield, X.O.; Ropers, H.H.; van Oost, B. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 1993, 262, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.L. A neuroscientific update on monoamine oxidase and its inhibitors. CNS Spectr. 2013, 1, 25–32. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Akao, Y.; Yi, H.; Yamaoka, Y. Involvement of type A monoamine oxidase in neurodegeneration: Regulation of mitochondrial signaling leading to cell death or neuroprotection. J. Neural. Transm. Suppl. 2006, 71, 67–77. [Google Scholar]
- Kaludercic, N.; Carpi, A.; Menabo, R.; Di Lisa, F.; Paolocci, N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim. Biophys. Acta 2011, 1813, 1323–1332. [Google Scholar] [CrossRef]
- Coatrieux, C.; Sanson, M.; Negre-Salvayre, A.; Parini, A.; Hannun, Y.; Itohara, S.; Salvayre, R.; Auge, N. MAO-A-induced mitogenic signaling is mediated by reactive oxygen species, MMP-2, and the sphingolipid pathway. Free Radic. Biol. Med. 2007, 43, 80–89. [Google Scholar] [CrossRef]
- Bianchi, P.; Kunduzova, O.; Masini, E.; Cambon, C.; Bani, D.; Raimondi, L.; Seguelas, M.-H.; Nistri, S.; Colucci, W.; Leducq, N. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 2005, 112, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Gaweska, H.; Fitzpatrick, P.F. Structures and mechanism of the monoamine oxidase family. Biomol. Concepts 2011, 2, 365–377. [Google Scholar] [CrossRef]
- Finberg, J.P. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: Focus on modulation of CNS monoamine neurotransmitter release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C. Monoamine oxidase isoenzymes: Genes, functions and targets for behavior and cancer therapy. J. Neural. Transm. 2018, 125, 1553–1566. [Google Scholar] [CrossRef]
- Partin, A.W.; Kattan, M.W.; Subong, E.N.; Walsh, P.C.; Wojno, K.J.; Oesterling, J.E.; Pearson, J.D. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer: A multi-institutional update. JAMA 1997, 277, 1445–1451. [Google Scholar] [CrossRef]
- Wu, J.B.; Shao, C.; Li, X.; Li, Q.; Hu, P.; Shi, C.; Li, Y.; Chen, Y.-T.; Yin, F.; Liao, C.-P. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J. Clin. Investig. 2014, 124, 2891–2908. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endrocrinol. Metab. 2009, 20, 332–334. [Google Scholar] [CrossRef]
- Rybaczyk, L.; Bashaw, M.; Pathak, D.; Huang, K. An indicator of cancer: Downregulation of Monoamine Oxidase-A in multiple organs and species. BMC Genom. 2008, 9, 134. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.-M.; Wang, Y.-H.; Feng, M.-X.; Liu, X.-J.; Zhang, Y.-L.; Huang, S.; Wu, Z.; Xue, F.; Qin, W.-X. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J. Hepatol. 2014, 60, 1225–1234. [Google Scholar] [CrossRef]
- Frick, L.R.; Maximiliano, R. Antidepressants: Influence on cancer and immunity? Life Sci. 2013, 92, 525–532. [Google Scholar] [CrossRef]
- Lee, H.T.; Choi, M.R.; Doh, M.S.; Jung, K.H.; Chai, Y.G. Effects of the monoamine oxidase inhibitors pargyline and tranylcypromine on cellular proliferation in human prostate cancer cells. Oncol. Rep. 2013, 30, 1587–1592. [Google Scholar] [CrossRef]
- Satram-Maharaj, T.; Nyarko, J.N.K.; Kuski, K.; Fehr, K.; Pennington, P.R.; Truitt, L.; Freywald, A.; Lukong, K.E.; Anderson, D.H.; Mousseau, D.D. The monoamine oxidase-A inhibitor clorgyline promotes a mesenchymal-to-epithelial transition in the MDA-MB-231 breast cancer cell line. Cell. Signal. 2014, 26, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Long, Q.; Zhang, L.; Shi, Y.; Liu, X.; Li, X.; Guan, B.; Tian, Y.; Wang, X.; Li, L. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1alfa signaling. Int. J. Oncol. 2015, 47, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.W.; Lan, N.C.; Johnson, D.L.; Abell, C.W.; Bembenek, M.E.; Kwan, S.W.; Shih, J.C. cDNA cloning of human liver monoamine oxidase A and B: Molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. USA 1988, 85, 4934–4938. [Google Scholar] [CrossRef]
- Edmondson, D.; Bhattacharyya, A.; Walker, M. Spectral and kinetic studies of imine product formation in the oxidation of p-(N,N-dimethylamino) benzylamine analogues by monoamine oxidase B. Biochemistry 1993, 32, 5196–5202. [Google Scholar] [CrossRef] [PubMed]
- Bach, R.; Andres, J.; Su, M.-D.; McDouall, J.J. Theoretical model for electrophilic oxygen atom insertion into hydrocarbons. J. Am. Chem. Soc. 1993, 115, 5768–5775. [Google Scholar] [CrossRef]
- Johnston, J.P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem. Pharmacol. 1968, 17, 1285–1287. [Google Scholar] [CrossRef]
- Knoll, J. Citation Classic-Some Puzzling Pharmcological Effects of Monamine-oxidase inhibitors. Curr. Contents Clin. Med. 1982, 28, 20. [Google Scholar]
- Ozcan, A.; Metin, O. Biochemistry of reactive oxygen and nitrogen species. In Basic Principles and Clinical Significance of Oxidative Stress; Gowder, S.J.T., Ed.; IntechOpen: London, UK, 2015; Volume 3, pp. 37–58. [Google Scholar] [CrossRef]
- Bardaweel, S.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.; Bashatwah, R. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.; Sallam, R. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Hodorová, I.; Rybárová, S.; Vecanová, J.; Solár, P.; Domorákova, I.; Adamkov, M.; Mihalik, J. Comparison of expression pattern of monoamine oxidase A with histopathologic subtypes and tumour grade of renal cell carcinoma. Med. Sci. Monit. 2012, 18, BR482–BR486. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Kushal, S.; Wang, W.; Vaikari, V.P.; Kota, R.; Chen, K.; Yeh, T.S.; Jhaveri, N.; Groshen, S.L.; Olenyuk, B.Z.; Chen, T.C.; et al. Monoamine oxidase A (MAO A) inhibitors decrease glioma progression. Oncotarget 2016, 7, 13842–13853. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; Falcoz, P.; Olland, A.; Reeb, J.; Santelmo, N.; Massard, G. Mediastinal downstaging after induction treatment is not a significant prognostic factor to select patients who would benefit from surgery: The clinical value of the lymph node ratio. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 222–227. [Google Scholar] [CrossRef]
- Akthar, A.; Ferguson, M.; Koshy, M.; Vigneswaran, W.; Malik, R. Limitations of PET/CT in the detection of occult N1 metastasis in clinical stage I(T1-2aN0) non-small cell lung cancer for staging prior to stereotactic body radiotherapy. Technol. Cancer Res. Treat. 2017, 16, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Chen, Y.; He, B.; Chen, Q. Krüppel-like factor 4 enhances sensitivity of cisplatin to lung cancer cells and inhibits regulating epithelial-to-mesenchymal transition. Oncol. Res. 2016, 24, 81–87. [Google Scholar] [CrossRef]
- Yu, S.; Yan, C.; Yang, X.; He, S.; Liu, J.; Qin, C.; Jia, L. Pharmacoproteomic analysis reveals that metapristone (RU486 metabolite) intervenes E-cadherin and vimentin to realize cancer metastasis chemoprevention. Sci. Rep. 2016, 6, 22388. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, L.; Ma, Y.; Huang, B.; Xiu, Z.; Zhang, P.; Zhou, K.; Tang, X. Increased expression of monoamine oxidase A is associated with epithelial to mesenchymal transition and clinicopathological features in non-small cell lung cancer. Oncol. Lett. 2018, 15, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.P.; Zainer, C.M.; Kubat, C.K.; Mullen, N.K.; Windisch, A.K. The breast cancer epidemic: 10 facts. Linacre Q. 2014, 81, 244–277. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Dey, G.; Das, A.K.; Mandal, M. Differential expression of IL-6/IL-6R and MAO-A regulates invasion/angiogenesis in breast cancer. Br. J. Cancer 2018, 118, 1442–1452. [Google Scholar] [CrossRef]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Mandal, M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016, 375, 51–61. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Ojha, P.K.; Rajput, S.; Jaganathan, S.K.; Sen, R.; Mandal, M. Diacerein-mediated inhibition of IL-6/IL-6R signaling induces apoptotic effects on breast cancer. Oncogene 2015, 35, 3965–3975. [Google Scholar] [CrossRef]
- Santin, Y.; Resta, J.; Parini, A.; Mialet-Perez, J. Monoamine oxidases in age-associated diseases: New perspectives for old enzymes. Ageing Res. Rev. 2021, 66, 101256. [Google Scholar] [CrossRef]
- Go, P.H.; Klaassen, Z.; Meadows, M.C.; Chamberlain, R.S. Gastrointestinal cancer and brain metastasis: A rare and ominous sign. Cancer 2011, 117, 3630–3640. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chien, M.H.; Lai, T.C.; Su, C.Y.; Jan, Y.H.; Hsiao, M.; Chen, C.L. Monoamine Oxidase B Expression Correlates with a Poor Prognosis in Colorectal Cancer Patients and Is Significantly Associated with Epithelial-to-Mesenchymal Transition-Related Gene Signatures. Int. J. Mol. Sci. 2020, 21, 2813. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 14–27. [Google Scholar]
- Kondov, B.; Milenkovikj, Z.; Kondov, G.; Petrushevska, G.; Basheska, N.; Bogdanovska-Todorovska, M.; Ivkovski, L. Presentation of the molecular subtypes of breast cancer detected by immunohistochemistry in surgically treated patients. Open Access Maced. J. Med. Sci. 2018, 6, 961. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Choi, J.; Cha, Y.J.; Koo, J.S. Evaluation of the expression of amine oxidase proteins in breast cancer. Int. J. Mol. Sci. 2017, 18, 2775. [Google Scholar] [CrossRef]
- Bade, B.C.; Cruz, C.S.D. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kery, M.; Papandreou, I. Emerging strategies to target cancer metabolism and improve radiation therapy outcomes. Br. J. Radiol. 2020, 93, 20200067. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Yuan, C.; Luo, Y.; Li, Y.; Dai, P.; Xie, C. Establishment of the Prognostic Index Reflecting Tumor Immune Microenvironment of Lung Adenocarcinoma Based on Metabolism-Related Genes. J. Cancer 2020, 11, 7101. [Google Scholar] [CrossRef]
- Larsen, J.E.; Pavey, S.J.; Passmore, L.H.; Bowman, R.V.; Hayward, N.K.; Fong, K.M. Gene expression signature predicts recurrence in lung adenocarcinoma. Clin. Cancer Res. 2007, 13, 2946–2954. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Jun, S.Y.; Seo, H.; Youn, H.; Yang, H.J.; Kim, W.; Youn, B. Inhibitory effect of traditional oriental medicine-derived monoamine oxidase B inhibitor on radioresistance of non-small cell lung cancer. Sci. Rep. 2016, 6, 21986. [Google Scholar] [CrossRef] [PubMed]
- Sharif Siam, M.K.; Sarker, A.; Sayeem, M.M.S. In silico drug design and molecular docking studies targeting Akt1 (RAC-alpha serine/threonine-protein kinase) and Akt2 (RAC-beta serine/threonine-protein kinase) proteins and investigation of CYP (cytochrome P450) inhibitors against MAOB (monoamine oxidase B) for OSCC (oral squamous cell carcinoma) treatment. J. Biomol. Struct. Dyn. 2020, 17, 6467–6479. [Google Scholar]
- Oh, S.Y.; Kang, S.M.; Kang, S.H.; Lee, H.J.; Kwon, T.G.; Kim, J.W.; Hong, S.H. Potential salivary mRNA biomarkers for early detection of oral cancer. J. Clin. Med. 2020, 9, 243. [Google Scholar] [CrossRef]
- Marconi, G.D.; Gallorini, M.; Carradori, S.; Guglielmi, P.; Cataldi, A.; Zara, S. The up-regulation of oxidative stress as a potential mechanism of novel MAO-B inhibitors for glioblastoma treatment. Molecules 2019, 24, 2005. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Baskin, D.S. Monoamine oxidase B levels are highly expressed in human gliomas and are correlated with the expression of HiF-1α and with transcription factors Sp1 and Sp3. Oncotarget 2016, 7, 3379. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K. The pharmacology of selegiline. Int. Rev. Neurobiol. 2011, 1100, 65–84. [Google Scholar]
- Wang, Y.; Wang, S.; Yang, Q.; Li, J.; Yu, F.; Zhao, E. Norepinephrine Enhances Aerobic Glycolysis and May Act as a Predictive Factor for Immunotherapy in Gastric Cancer. J. Immunol. Res. 2021, 2021, 5580672. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Mahmood, Q.; Mariottini, G.L.; Chiang, T.A.; Lee, K.W. Adverse health effects of betel quid and the risk of oral and pharyngeal cancers. BioMed Res. Int. 2017, 2017, 3904098. [Google Scholar] [CrossRef]
- Chen, P.H.; Huang, B.; Shieh, T.Y.; Wang, Y.H.; Chen, Y.K.; Wu, J.H.; Lee, K.W. The influence of monoamine oxidase variants on the risk of betel quid-associated oral and pharyngeal cancer. Sci. World J. 2014, 2014, 183548. [Google Scholar] [CrossRef]
- Marin-Muller, C.; Li, D.; Bharadwaj, U.; Li, M.; Chen, C.; Hodges, S.E.; Yao, Q. A tumorigenic factor interactome connected through tumor suppressor microRNA-198 in human pancreatic cancer. Clin. Cancer Res. 2013, 19, 5901–5913. [Google Scholar] [CrossRef]
- Zhang, H.C.; Han, Y.Y.; Zhang, X.M.; Xiao, N.; Jiang, T.; Zhu, S.; Chen, C.B. miR-522 facilitates the prosperities of endometrial carcinoma cells by directly binding to monoamine oxidase B. Kaohsiung J. Med. Sci. 2019, 35, 598–606. [Google Scholar] [CrossRef]
- Hubálek, F.; Binda, C.; Khalil, A.; Li, M.; Mattevi, A.; Castagnoli, N.; Edmondson, D. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. J. Biol. Chem. 2005, 22, 15761–15766. [Google Scholar] [CrossRef]
- De Colibus, L.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.; Mattevi, A. Three-dimensional structure of human monoamine oxidase A (MAO A): Relation to the structures of rat MAO A and human MAO B. Proc. Natl. Acad. Sci. USA 2005, 102, 12684–12689. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Ferino, G.; Matos, M.J.o.; Vazquez-Rodreguez, S.; Delogu, G.; Vina, D.; Cadoni, E.; Santana, L.; Uriarte, E. Hydroxycoumarins as selective MAO-B inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Vina, D. MAO inhibitory activity modulation: 3-Phenylcoumarins versus 3-benzoylcoumarins. Bioorg. Med. Chem. Lett. 2011, 21, 4224–4227. [Google Scholar] [CrossRef]
- Cesura, A.M.; Pletscher, A. The new generation of monoamine oxidase inhibitors. In Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progres des Recherches Pharmaceutiques; Springer: Berlin/Heidelberg, Germany, 1992; pp. 171–297. [Google Scholar]
- Youdim, M.B. The advent of selective monoamine oxidase A inhibitor antidepressants devoid of the cheese reaction. Acta Psychiatr. Scand. Suppl. 1995, 91, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Newton-Vinson, P.; Hubálek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Mol. Biol. 2002, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L. Quantitative structure-activity studies on monoamine oxidase inhibitors. J. Med. Chem. 1976, 19, 600–605. [Google Scholar] [CrossRef]
- Mahmoudian, M. QSAR of inhibition of monoamine oxidase by substituted phenylalkylamines in vitro and in various neurons in vivo. Acta Pharm. Suec. 1988, 25, 151–162. [Google Scholar] [PubMed]
- Zhang, L.; Tsai, K.-C.; Du, L.; Fang, H.; Li, M.; Xu, W. How to generate reliable and predictive CoMFA models. Curr. Med. Chem. 2011, 18, 923–930. [Google Scholar] [CrossRef]
- Hong, R.; Li, X. Discovery of monoamine oxidase inhibitors by medicinal chemistry approaches. MedChemComm 2019, 10, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.A. The role of quantitative structure-activity relationships (QSAR) in biomolecular discovery. Brief. Bioinform. 2002, 3, 73–86. [Google Scholar] [CrossRef]
- Cramer, R.D.; Patterson, D.E.; Bunce, J.D. Comparative molecular field analysis (CoMFA) 1. Effect of shape on binding of steroids to carrier proteins. J. Am. Chem. Soc. 1988, 110, 5959–5967. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Kubinyi, H.; Folkers, G. Pharmacophores and Pharmacophore Searches; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 32. [Google Scholar]
- Park, K.; Kim, D. Binding similarity network of ligand. Proteins 2008, 71, 960–971. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, Y. 3D-QSAR method on indole and pyrrole inhibitors of monoamine oxidase type A. Mol. Simul. 2009, 35, 1242–1248. [Google Scholar] [CrossRef]
- Xie, H.; Chen, L.; Zhang, J.; Xie, X.; Qiu, K.; Fu, J. A Combined Pharmacophore Modeling, 3D QSAR and Virtual Screening Studies on Imidazopyridines as B-Raf Inhibitors. Int. J. Mol. Sci. 2015, 16, 12307–12323. [Google Scholar] [CrossRef] [PubMed]
- Yamaotsu, N.; Hirono, S. 3D-pharmacophore identification for κ-opioid agonists using ligand-based drug-design techniques. In Chemistry of Opioids; Springer: Berlin/Heidelberg, Germany, 2010; pp. 277–307. [Google Scholar]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197. [Google Scholar] [CrossRef]
- Santana, L.; González-Díaz, H.; Quezada, E.; Uriarte, E.; Yáñez, M.; Viña, D.; Orallo, F. Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors. J. Med. Chem. 2008, 51, 6740–6751. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Gnerre, C.; Thull, U.; Gaillard, P.; Carrupt, P.-A.; Testa, B.; Fernandes, E.; Silva, F.; Pinto, M.; Pinto, M.M.M.; Wolfender, J.-L. Natural and synthetic xanthones as monoamine oxidase inhibitors: Biological assay and 3D-QSAR. Helv. Chim. Acta 2001, 84, 552–570. [Google Scholar] [CrossRef]
- Moureau, F.; Wouters, J.; Vercauteren, D.P.; Collin, S.; Evrard, G.; Durant, F.o.; Ducrey, F.; Koenig, J.-J.; Jarreau, F.o.-X. A reversible monoamine oxidase inhibitor, Toloxatone: Spectrophotometric and molecular orbital studies of the interaction with flavin adenine dinucleotide (FAD). Eur. J. Med. Chem. 1994, 29, 269–277. [Google Scholar] [CrossRef]
- La Regina, G.; Silvestri, R.; Artico, M.; Lavecchia, A.; Novellino, E.; Befani, O.; Agostinelli, E. New pyrrole inhibitors of monoamine oxidase: Synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. J. Med. Chem. 2007, 50, 922–931. [Google Scholar] [CrossRef]
- Kumar, V.; Bansal, H. QSAR studies on estimation of monoamine oxidase-A inhibitory activity using topological descriptors. Med. Chem. Res. 2011, 20, 168–174. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics. 1. Alphabetical Listing; Wiley-VCH: Hoboken, NJ, USA, 2009. [Google Scholar]
- Vilar, S.; Ferino, G.; Quezada, E.; Santana, L.; Friedman, C. Predicting monoamine oxidase inhibitory activity through ligand-based models. Curr. Top. Med. Chem. 2012, 12, 2258–2274. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Cellamare, S.; Summo, L.; Catto, M.; Carotti, A.; Thull, U.; Carrupt, P.; Testa, B.; Stoeckli-Evans, H. Inhibition of monoamine oxidase-B by condensed pyridazines and pyrimidines: Effects of lipophilicity and structure-activity relationships. J. Med. Chem. 1998, 41, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Ivanov, A.; Kamyshanskaya, N.; Kirkel, A.; Moskvitina, T.; Gorkin, V.; Li, N.; Marshakov, V. Interaction of indole derivatives with monoamine oxidase A and B. Studies on the structure-inhibitory activity relationship. Biochem. Mol. Biol. Int. 1995, 36, 113–122. [Google Scholar]
- Norinder, U.; Florvall, L.; Ross, S.B. A PLS quantitative structure-activity relationship study of some monoamine oxidase inhibitors of the phenyl alkylamine type. Eur. J. Med. Chem. 1994, 29, 191–195. [Google Scholar] [CrossRef]
- Mabic, S.; Castagnoli, N. Assessment of structural requirements for the monoamine oxidase-B-catalyzed oxidation of 1,4-disubstituted-1,2,3,6-tetrahydropyridine derivatives related to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Med. Chem. 1996, 39, 3694–3700. [Google Scholar] [CrossRef] [PubMed]
- Mondov, B.; Agro, A.F. Structure and function of amine oxidases. In Structure and Function Relationships in Biochemical Systems; Springer: Berlin/Heidelberg, Germany, 1982; pp. 141–153. [Google Scholar]
- Harfenist, M.; Joyner, C.T.; Mize, P.D.; White, H.L. Selective inhibitors of monoamine oxidase. 2. Arylamide SAR. J. Med. Chem. 1994, 37, 2085–2089. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Veselovsky, A.V.; Shvedov, V.I.; Tikhonova, O.V.; Moskvitina, T.A.; Fedotova, O.A.; Axenova, L.N.; Kamyshanskaya, N.S.; Kirkel, A.Z.; Ivanov, A.S. Inhibition of monoamine oxidase by pirlindole analogues: 3D-QSAR and CoMFA analysis. J. Chem. Inf. Comput. Sci. 1998, 38, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Carrupt, P.A.; Gaillard, P.; El Tayar, N.; Testa, B.; Carotti, A. Quantitative structure-metabolism relationship analyses of MAO-mediated toxication of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and analogs. Chem. Res. Toxicol. 1992, 5, 366–375. [Google Scholar] [CrossRef]
- Thull, U.; Kneubuhler, S.; Gaillard, P.; Carrupt, P.-A.; Testa, B.; Altomare, C.; Carotti, A.; Jenner, P.; McNaught, K.S.P. Inhibition of monoamine oxidase by isoquinoline derivatives: Qualitative and 3D-quantitative structure-activity relationships. Biochem. Pharmacol. 1995, 50, 869–877. [Google Scholar] [CrossRef]
- Moron, J.A.; Campillo, M.; Perez, V.; Unzeta, M.; Pardo, L. Molecular determinants of MAO selectivity in a series of indolylmethylamine derivatives: Biological activities, 3D-QSAR/CoMFA analysis, and computational simulation of ligand recognition. J. Med. Chem. 2000, 43, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. Development and use of quantum mechanical molecular models. 76. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Tsugeno, Y.; Ito, A. A key amino acid responsible for substrate selectivity of monoamine oxidase A and B. J. Biol. Chem. 1997, 272, 14033–14036. [Google Scholar] [CrossRef]
- Gallardo-Godoy, A.; Fierro, A.; McLean, T.H.; Castillo, M.; Cassels, B.K.; Reyes-Parada, M.; Nichols, D.E. Sulfur-substituted alpha-alkyl phenethylamines as selective and reversible MAO-A inhibitors: Biological activities, CoMFA analysis, and active site modeling. J. Med. Chem. 2005, 48, 2407–2419. [Google Scholar] [CrossRef]
- Nichols, D.E.; Marona-Lewicka, D.; Huang, X.; Johnson, M.P. Novel serotonergic agents. Drug Des. Discov. 1993, 9, 299–312. [Google Scholar] [PubMed]
- Catto, M.; Nicolotti, O.; Leonetti, F.; Carotti, A.; Favia, A.D.; Soto-Otero, R.; Méndez-Álvarez, E.; Carotti, A. Structural insights into monoamine oxidase inhibitory potency and selectivity of 7-substituted coumarins from ligand-and target-based approaches. J. Med. Chem. 2006, 49, 4912–4925. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, P.; Carrupt, P.-A.; Testa, B.; Boudon, A. Molecular lipophilicity potential, a tool in 3D QSAR: Method and applications. J. Comput. Aided Mol. Des. 1994, 8, 83–96. [Google Scholar] [CrossRef]
- Gnerre, C.; Catto, M.; Leonetti, F.; Weber, P.; Carrupt, P.-A.; Altomare, C.; Carotti, A.; Testa, B. Inhibition of monoamine oxidases by functionalized coumarin derivatives: Biological activities, QSARs, and 3D-QSARs. J. Med. Chem. 2000, 43, 4747–4758. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Son, Y.K.; Kim, G.H.; Hwang, K.H. Xanthoangelol and 4-hydroxyderricin are the major active principles of the inhibitory activities against monoamine oxidases on Angelica keiskei K. Biomol. Ther. 2013, 21, 234–240. [Google Scholar] [CrossRef]
- Ministry of Education. The Molecular Operating, Environment Chemical Computing Group; Inc Montreal: Montreal, QC, Canada, 2016. [Google Scholar]
- NCI. Open Database Compounds Release 3; National Cancer Institute: Bethseda, MD, USA, 2013. Available online: http://cactus.nci.nih.gov/download/nci (accessed on 1 April 2020).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Jo, G.; Sung, S.H.; Lee, Y.; Kim, B.-G.; Yoon, J.; Lee, H.O.; Ji, S.Y.; Koh, D.; Ahn, J.-H.; Lim, Y. Discovery of monoamine oxidase A inhibitors derived from in silico docking. Bull. Korean. Chem. Soc 2012, 33, 3841–3844. [Google Scholar] [CrossRef]
- Yusufzai, S.K.; Khan, M.S.; Sulaiman, O.; Osman, H.; Lamjin, D.N. Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer’s disease. Chem. Cent. J. 2018, 12, 1–57. [Google Scholar] [CrossRef]
- Jung, H.A.; Roy, A.; Choi, J.S. In vitro monoamine oxidase A and B inhibitory activity and molecular docking simulations of fucoxanthin. Fish. Sci. 2017, 83, 123–132. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chang, Y.-T.; Campbell, M.; Lin, T.-P.; Pan, C.-C.; Lee, H.-C.; Shih, J.C.; Chang, P.-C. MAOA-a novel decision maker of apoptosis and autophagy in hormone refractory neuroendocrine prostate cancer cells. Sci. Rep. 2017, 7, 46338. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Mou, Y.; Wang, Y.; Wang, J.; Li, Y.; Kong, R.; Ding, M.; Wang, D.; Guo, C. Design, Synthesis, and Evaluation of Monoamine Oxidase a Inhibitors-Indocyanine Dyes Conjugates as Targeted Antitumor Agents. Molecules 2019, 24, 1400. [Google Scholar] [CrossRef]


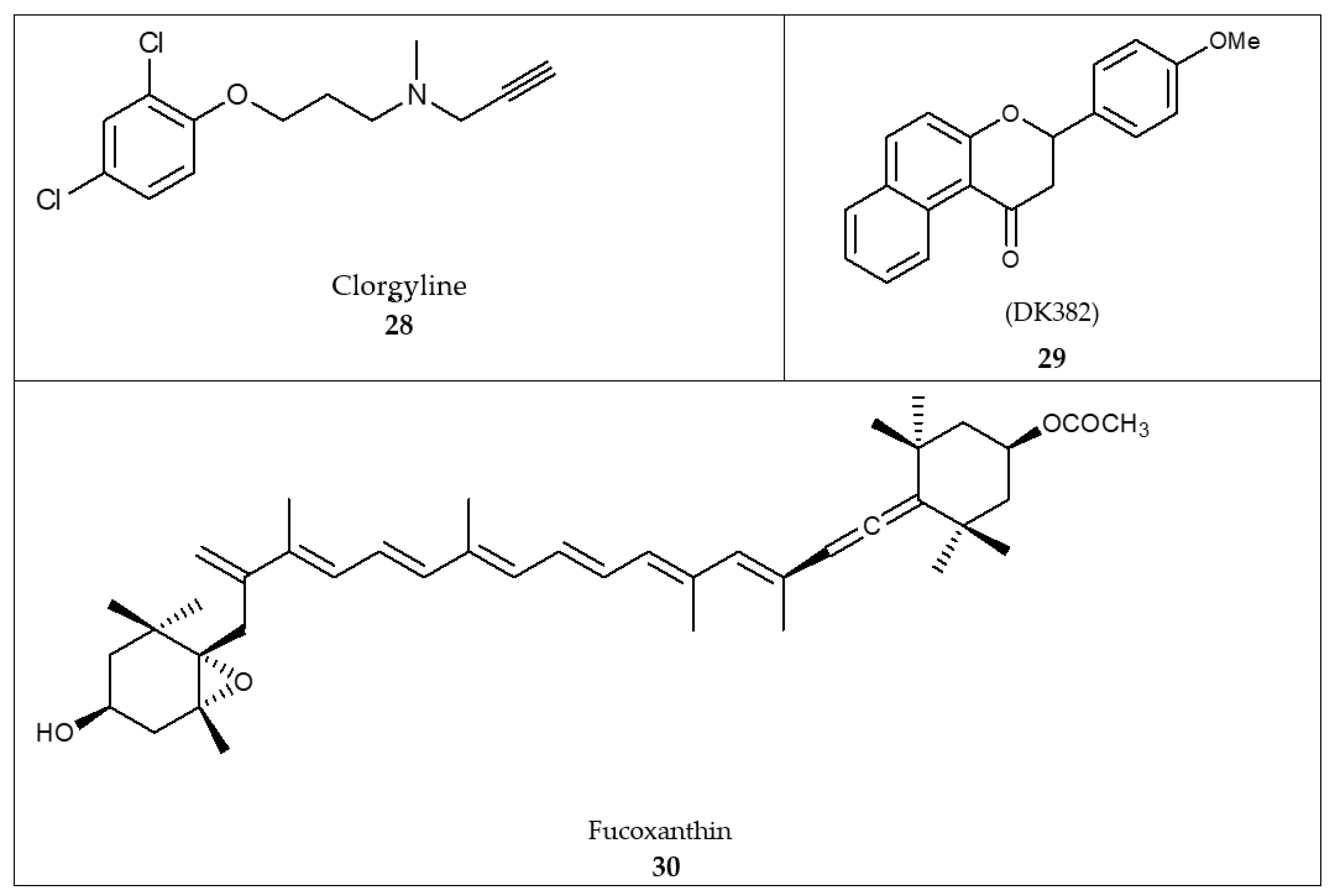
| Scaffold | Selectivity | IC50 MAO-A | IC50 MAO-B | Chemical Structure |
|---|---|---|---|---|
| Chalcones [119] | Nonselective | 43.4 µM | 43.9 µM | 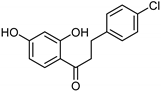 Xanthoangelol 13 |
| Flavonoids [119] | Selective | 1.23 µM | NA | 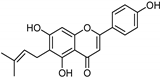 14 |
| Coumarins [119] | Nonselective | 8.9 nM | 8.9 nM |  15 |
| Xanthones [119] | Nonselective | 13.92 µM | 13.92 µM | 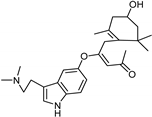 Desmodeleganine 16 |
| Nicotinamide [119] | Selective | 0.045 µM | 26 µM | 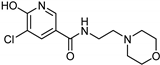 17 |
| Caffeine [119] | Selective | 34 µM | 0.148 µM |  18 |
| Indole alkaloids [119] | Selective | 0.07 µM | NA | 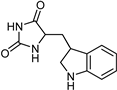 19 |
| Anthraquinone [119] | Selective | 2.5 µM | NA | 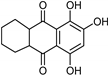 Purpurin 20 |
| Synthetic [95] | Selective | 5.5 nM | 150 nM | 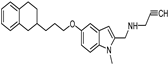 21 |
| Synthetic [96] | Selective | 0.49 µM | NA | 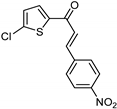 22 |
| Synthetic [96] | Selective | 0.14 mM | NA | 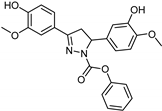 23 |
| Synthetic [96] | Selective | 0.06 µM | NA | 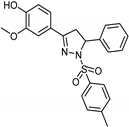 24 |
| Synthetic [96] | Selective | 0.01 µM | 2.15 | 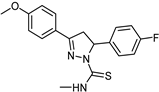 25 |
| Synthetic [96] | Selective | NA | 20 nm | 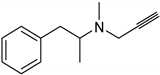 Selegiline 26 |
| Natural [96] | Selective | NA | 8.3 μM | 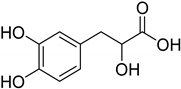 Danshensu 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljanabi, R.; Alsous, L.; Sabbah, D.A.; Gul, H.I.; Gul, M.; Bardaweel, S.K. Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development. Molecules 2021, 26, 6019. https://doi.org/10.3390/molecules26196019
Aljanabi R, Alsous L, Sabbah DA, Gul HI, Gul M, Bardaweel SK. Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development. Molecules. 2021; 26(19):6019. https://doi.org/10.3390/molecules26196019
Chicago/Turabian StyleAljanabi, Reem, Lina Alsous, Dima A. Sabbah, Halise Inci Gul, Mustafa Gul, and Sanaa K. Bardaweel. 2021. "Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development" Molecules 26, no. 19: 6019. https://doi.org/10.3390/molecules26196019
APA StyleAljanabi, R., Alsous, L., Sabbah, D. A., Gul, H. I., Gul, M., & Bardaweel, S. K. (2021). Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development. Molecules, 26(19), 6019. https://doi.org/10.3390/molecules26196019






