Synthesis, Bioactivity, Pharmacokinetic and Biomimetic Properties of Multi-Substituted Coumarin Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antioxidant Activity
2.3. Lipoxygenase Inhibitory Activity
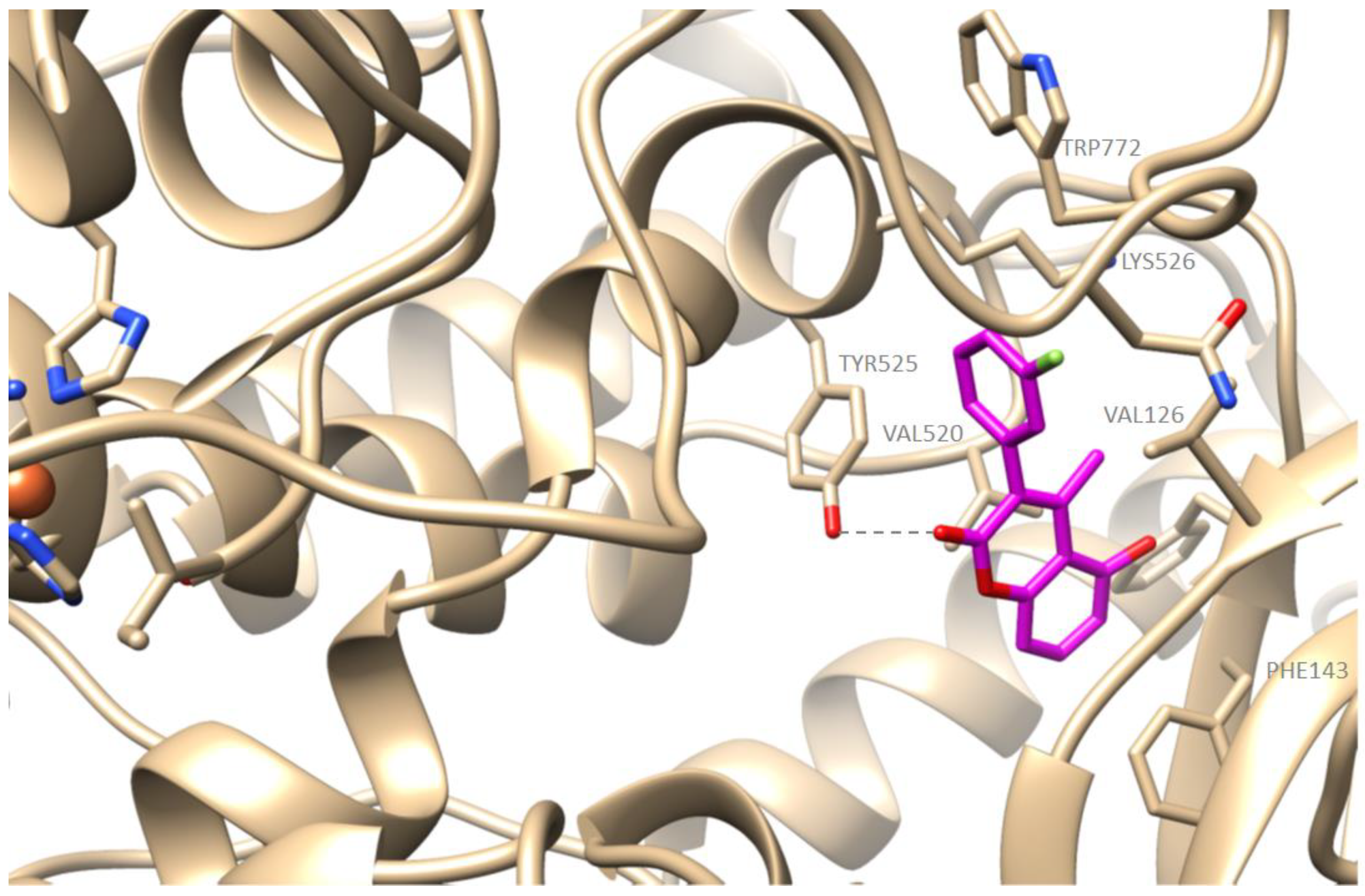
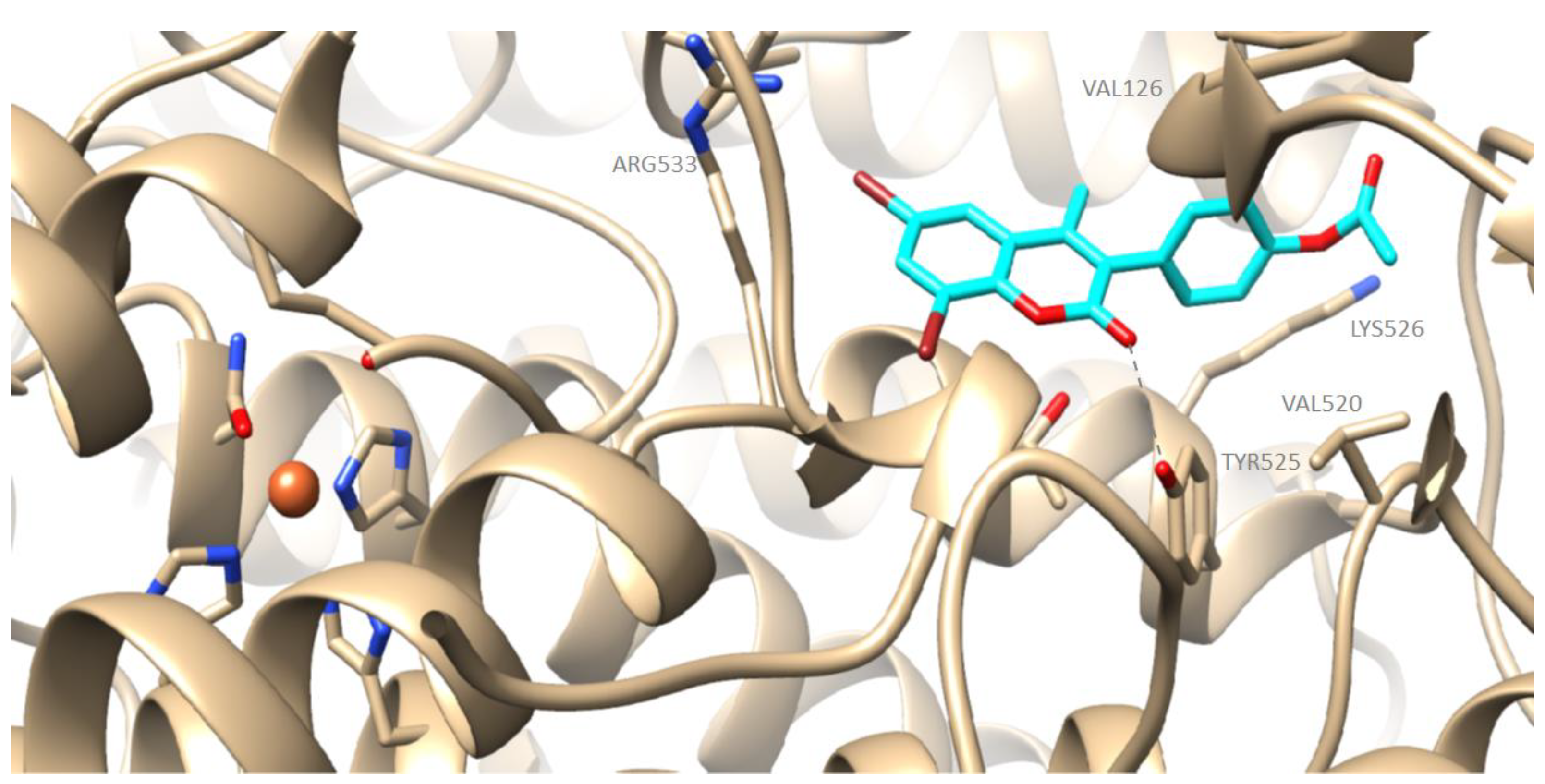
2.4. Computational Studies—Docking Simulations with Soybean Lipoxygenase
Molecular Modeling of the Synthesized Derivatives in Soybean LOX
2.5. Cell Viability in Human Epidermal Keratinocyte (HaCaT) Cell Line
2.6. Evaluation of Coumarin Compounds’ Cytotoxicity against Cancer Cell Lines
2.7. Physicochemical and Biomimetic Properties
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthesis and General Procedures
3.2.1. General Procedure for the Synthesis of Acetyloxy Coumarins (3a–3o):
3.2.2. General Procedure for the Synthesis of Hydroxy Coumarins (4a–4k):
3.3. Evaluation of In Vitro Biological Activity
3.3.1. ABTS Radical Scavenging Assay
3.3.2. Hydroxyl (HO•) Free Radical Scavenging Assay
3.3.3. Inhibition of AAPH Induced Linoleic Acid Oxidation
3.3.4. DCF-DA Assay Protocol
3.3.5. Inhibition of Soybean LOX
3.3.6. Cell Viability Assay
3.4. Computational Methods. Molecular Docking Studies on Soybean Lipoxygenase
3.5. IAM and HSA Chromatographic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Samples Availability
References
- Madhasu, M.; Doda, S.R.; Begari, P.K.; Dasari, K.R.; Thalari, G.; Kadari, S.; Yadav, J.S. Concise total synthesis of antiarrhythmic drug dronedarone via a conjugate addition followed intramolecular heck cyclization. J. Heterocycl. Chem. 2021, 58, 1861–1866. [Google Scholar] [CrossRef]
- Madhu, M.; Doda, S.R.; Begari, P.K.; Dasari, K.R.; Thalari, G.; Kadari, S.; Yadav, J.S. Enantioselective epoxidation by the chiral auxiliary approach: Asymmetric total synthesis of (+)-Ambrisentan. J. Heterocycl. Chem. 2021, 58, 942–946. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kutateladze, A.G. Photoinitiated Cascade for Rapid Access to Pyrroloquinazolinone Core of Vasicinone, Luotonins, and Related Alkaloids. Org. Lett. 2019, 21, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef] [PubMed]
- Al-Majedy, Y.K.; Al-Duhaidahawi, D.L.; Al-Azawi, K.F.; Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Coumarins as Potential Antioxidant Agents Complemented with Suggested Mechanisms and Approved by Molecular Modeling Studies. Molecules 2016, 21, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and Antiinflammatory Activity of Coumarin Derivatives. J. Med. Chem. 2005, 48, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Kapp, E.; Visser, H.; Sampson, S.L.; Malan, S.F.; Streicher, E.M.; Foka, G.B.; Warner, D.F.; Omoruyi, S.I.; Enogieru, A.B.; Ekpo, O.E.; et al. Versatility of 7-Substituted Coumarin Molecules as Antimycobacterial Agents, Neuronal Enzyme Inhibitors and Neuroprotective Agents. Molecules 2017, 22, 1644. [Google Scholar] [CrossRef] [Green Version]
- Gkionis, L.; Kavetsou, E.; Kalospyros, A.; Manousakis, D.; Garzon Sanz, M.; Butterworth, S.; Detsi, A.; Tirella, A. Investigation of the cytotoxicity of bioinspired coumarin analogues towards human breast cancer cells. Mol. Divers. 2020, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending topics on coumarin and its derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef] [PubMed]
- Roussaki, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Hamilakis, S.; Detsi, A. A novel synthesis of 3-aryl coumarins and evaluation of their antioxidant and lipoxygenase inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3889–3892. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.; Alkuwayti, M.A.; Aldayel, M.F.; Abdallah, B.M. Coumarin derivative, 5′-hydroxy-auraptene, extracted from Lotus lalambensis, displays antifungal and anti-aflatoxigenic activities against Aspergillus flavus. J. King Saud Univ.-Sci. 2021, 33, 101216. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as Modulators of the Keap1/Nrf2/ARE Signaling Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, I.; Diassakou, A.; Kavetsou, E.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.; Detsi, A. Novel quinolinone–pyrazoline hybrids: Synthesis and evaluation of antioxidant and lipoxygenase inhibitory activity. Mol. Divers. 2021, 25, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Orafaie, A.; Matin, M.M.; Sadeghian, H. The importance of 15-lipoxygenase inhibitors in cancer treatment. Cancer Metastasis Rev. 2018, 37, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Kavetsou, E.; Katopodi, A.; Argyri, L.; Chainoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D.; Chroni, A.; Detsi, A. Novel 3-aryl-5-substituted-coumarin analogues: Synthesis and bioactivity profile. Drug Dev. Res. 2020, 81, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Multi-target cinnamic acids for oxidative stress and inflammation: Design, synthesis, biological evaluation and modeling studies. Molecules 2019, 24, 12. [Google Scholar] [CrossRef] [Green Version]
- Zerangnasrabad, S.; Jabbari, A.; Moghadam, E.K.; Sadeghian, H.; Seyedi, S.M. Design, synthesis, and structure–activity relationship study of O-prenylated 3-acetylcoumarins as potent inhibitors of soybean 15-lipoxygenase. Drug Dev. Res. 2021, 82, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Kim, H.P. Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents. Arch. Pharmacal. Res. 2011, 34, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Hadjipavlou-Litina, D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU 2009, 17, 99. [Google Scholar]
- Önder, A. Anticancer activity of natural coumarins for biological targets. Stud. Nat. Prod. Chem. 2020, 64, 85–109. [Google Scholar] [CrossRef]
- Gala, U.H.; Miller, D.A.; Williams, R.O. Harnessing the therapeutic potential of anticancer drugs through amorphous solid dispersions. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188319. [Google Scholar] [CrossRef] [PubMed]
- Tsopelas, F.; Vallianatou, T.; Tsantili-Kakoulidou, A. Advances in immobilized artificial membrane (IAM) chromatography for novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 473–488. [Google Scholar] [CrossRef]
- Tsopelas, F.; Giaginis, C.; Tsantili-Kakoulidou, A. Lipophilicity and biomimetic properties to support drug discovery. Expert Opin. Drug Discov. 2017, 12, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Tsopelas, F.; Vallianatou, T.; Tsantili-Kakoulidou, A. The potential of immobilized artificial membrane chromatography to predict human oral absorption. Eur. J. Pharm. Sci. 2016, 81, 82–93. [Google Scholar] [CrossRef]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, P.; Malik, N.; Khatkar, A.; Kulharia, M. Antioxidant, Xanthine Oxidase and Monoamine Oxidase Inhibitory Potential of Coumarins: A Review. Curr. Org. Chem. 2017, 21, 294–304. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yoshida, Y.; Itoh, N.; Saito, Y.; Hayakawa, M.; Niki, E. Application of water-soluble radical initiator, 2,2′-azobis [2-(2-imidazolin-2-yl)propane] dihydrochloride, to a study of oxidative stress. Free Radic. Res. 2004, 38, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Bridi, R.; Giordano, A.; Peñailillo, M.F.; Montenegro, G. Antioxidant Effect of Extracts from Native Chilean Plants on the Lipoperoxidation and Protein Oxidation of Bovine Muscle. Molecules 2019, 24, 3264. [Google Scholar] [CrossRef] [Green Version]
- Li, W.B.; Qiao, X.P.; Wang, Z.X.; Wang, S.; Chen, S.W. Synthesis and antioxidant activity of conjugates of hydroxytyrosol and coumarin. Bioorg. Chem. 2020, 105, 104427. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, W.; Sun, J.; Zhang, B.; Lu, F.; Lai, R.; Zou, Y.; Yedid, G. Antioxidant and antitumor activities of 4-arylcoumarins and 4-aryl-3,4-dihydrocoumarins. Biochimie 2014, 107, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.M.; Cohen, G.; Cederbaum, A.I. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 2002, 20, 6006–6012. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Deligiannidou, G.E.; Karamani, V.; Hadjipavlou-Litina, D.; Lazari, D.; Papadopoulos, A. Antioxidant Profile of Home Prepared Taraxacum Officinale Weber Ex Wigg Beverage. Curr. Nutraceuticals 2020, 1, 64–72. [Google Scholar] [CrossRef]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Pavić, V.; Šubarić, D.; Rastija, V.; Molnar, M. Lipoxygenase Inhibition Activity of Coumarin Derivatives—QSAR and Molecular Docking Study. Pharmaceuticals 2020, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- The Biopharmaceutics Classification System (BCS) Guidance|FDA. Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/biopharmaceutics-classification-system-bcs-guidance (accessed on 2 August 2021).
- Kontogiorgis, C.; Deligiannidou, G.E.; Hadjipavlou-Litina, D.; Lazari, D.; Papadopoulos, A. Antioxidant protection: The contribution of proper preparation of fennel (Foeniculum vulgare Mill.) beverage. Ind. Crops Prod. 2016, 79, 57–62. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, E.S. Fluorescence-Based Detection and Quantification of Features of Cellular Senescence. Methods Cell Biol. 2011, 103, 149–188. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Fiser, A.; Šali, A. Modeller: Generation and Refinement of Homology-Based Protein Structure Models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—Antechamber python parser interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Bioinforma. 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsopelas, F.; Ochsenkühn-Petropoulou, M.; Tsantili-Kakoulidou, A. Void volume markers in reversed-phase and biomimetic liquid chromatography. J. Chromatogr. A 2010, 1217, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
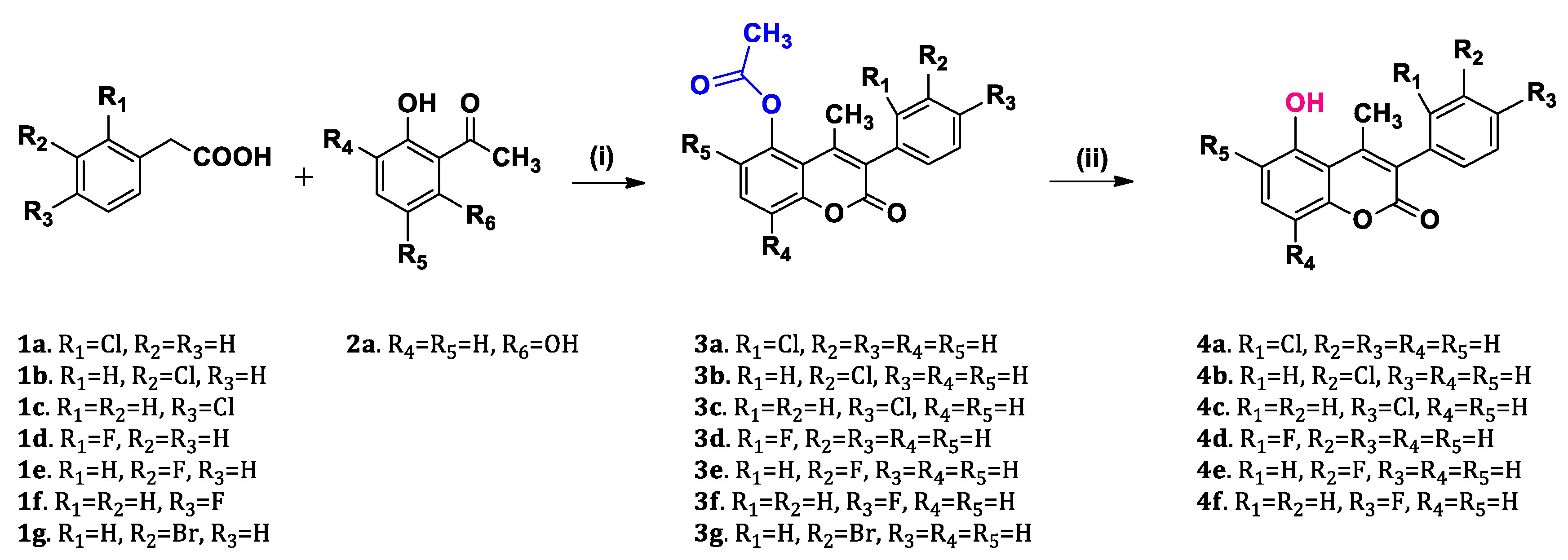
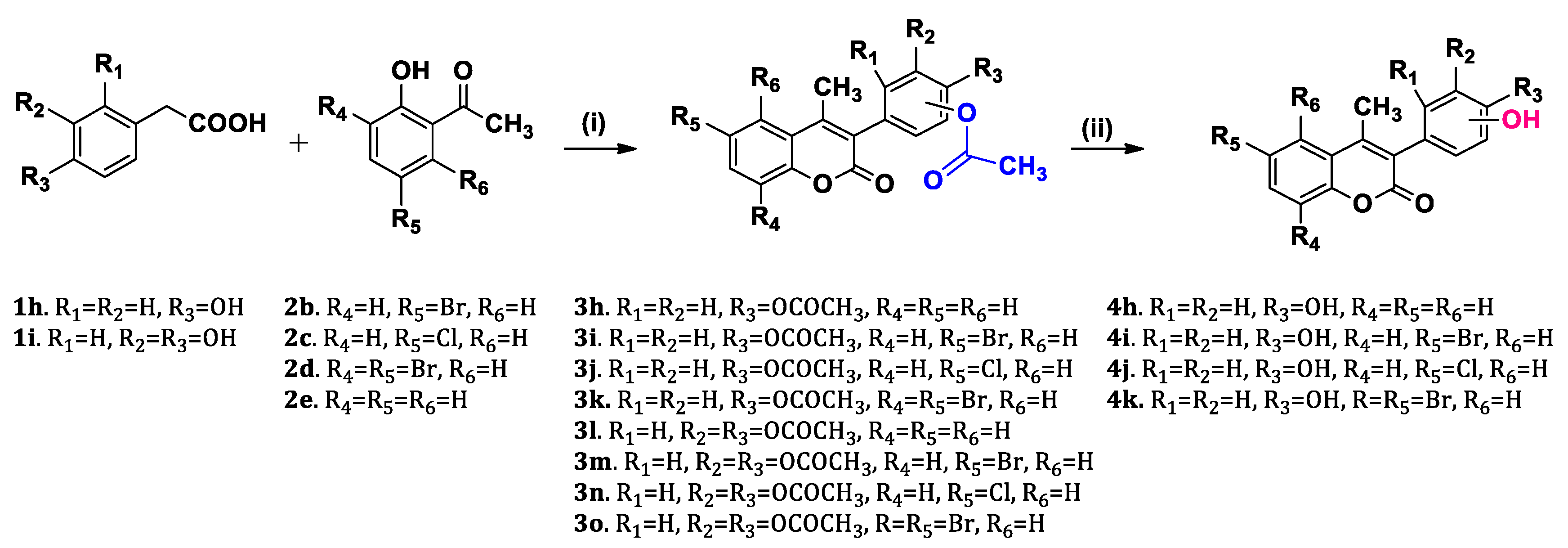
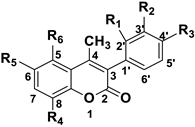 | ||||||
|---|---|---|---|---|---|---|
| Coumarin scaffold structure. | ||||||
| Compound | R1 | R2 | R3 | R4 | R5 | R6 |
| 3a | Cl | H | H | H | H | OCOCH3 |
| 3b | H | Cl | H | H | H | OCOCH3 |
| 3c | H | H | Cl | H | H | OCOCH3 |
| 3d | F | H | H | H | H | OCOCH3 |
| 3e | H | F | H | H | H | OCOCH3 |
| 3f | H | H | F | H | H | OCOCH3 |
| 3g | H | Br | H | H | H | OCOCH3 |
| 3h | H | H | OCOCH3 | H | H | H |
| 3i | H | H | OCOCH3 | H | Br | H |
| 3j | H | H | OCOCH3 | H | Cl | H |
| 3k | H | H | OCOCH3 | Br | Br | H |
| 3l | H | OCOCH3 | OCOCH3 | H | H | H |
| 3m | H | OCOCH3 | OCOCH3 | H | Br | H |
| 3n | H | OCOCH3 | OCOCH3 | H | Cl | H |
| 3o | H | OCOCH3 | OCOCH3 | Br | Br | H |
| 4a | Cl | H | H | H | H | OH |
| 4b | H | Cl | H | H | H | OH |
| 4c | H | H | Cl | H | H | OH |
| 4d | F | H | H | H | H | OH |
| 4e | H | F | H | H | H | OH |
| 4f | H | H | F | H | H | OH |
| 4h | H | H | OH | H | H | H |
| 4i | H | H | OH | H | Br | H |
| 4j | H | H | OH | H | Cl | H |
| 4k | H | H | OH | Br | Br | H |
| Compound | ABTS•+ % (100 μΜ) | HO• % (100 μΜ) | AAPH IC50 (μΜ)/% (100 μΜ) | DCFDA Assay, % (100 μΜ) |
|---|---|---|---|---|
| 3a | no | 97.0 | 71.9 | 33.8 |
| 3b | 1.9 | 96.4 | 91.5 | 56.9 |
| 3c | 15.0 | 30.8 | 75.1 | no |
| 3d | no | 100 | 42.6% | 22.4 |
| 3e | 2.0 | 85.2 | no | no |
| 3f | no | 71.6 | 36.8% | 100.0 |
| 3g | 2.8 | 42.0 | 50.7 | 45.5 |
| 3h | no | 100 | 70.7 | no |
| 3i | no | 64.5 | 70.7 | 80.9 |
| 3j | no | 26.6 | no | 9.0 |
| 3k | no | 92.9 | no | no |
| 3l | 2.8 | 10.7 | 85.5 | no |
| 3m | no | 46.7 | 37.1 | no |
| 3n | no | 65.7 | 84.3 | no |
| 3o | no | 52.1 | 77.2 | 25.7 |
| 4a | 56.5 | 37.3 | no | 0.0 |
| 4b | 49.2 | 100 | no | 27.4 |
| 4c | 65.2 | 48.5 | 86.6 | no |
| 4d | 73.3 | 21.9 | 0.0% | no |
| 4e | 69.2 | 100 | 31.0% | no |
| 4f | 64.2 | 99.4 | 86.8 | no |
| 4h | 8.6 | 72.2 | no | no |
| 4i | no | 100 | 70.7 | 100 |
| 4j | no | 100 | 36.1% | 2.1 |
| 4k | 27.8 | 100 | 36.9 | no |
| Ascorbic acid | 99.1 | - | - | 87.0 |
| Trolox | 92.0 | 85.0 | 92% | - |
| Compound | IC50 (μM) / % (100 μΜ) |
|---|---|
| 3a | 17% |
| 3b | 31.6 |
| 3c | no |
| 3d | 11.6% |
| 3e | 11.4 |
| 3f | 20.1% |
| 3g | 40.5 |
| 3h | 33.8% |
| 3i | 46.5% |
| 3j | 49.5 |
| 3k | 8.7 |
| 3l | 31.4% |
| 3m | 48.0% |
| 3n | 56.5 |
| 3o | no |
| 4a | 10 |
| 4b | 47.3% |
| 4c | 0.9% |
| 4d | 31.6 |
| 4e | 4.1 |
| 4f | 39.2% |
| 4h | 27.2% |
| 4i | 19.3% |
| 4j | 35.0% |
| 4k | 33.4% |
| NGDA | 91% |
| Caffeic acid | 600 |
| Compound | Cell Viability % (100 μΜ) |
|---|---|
| 3a | 100 |
| 3b | 100 |
| 3c | 100 |
| 3d | 100 |
| 3e | 96.5 |
| 3f | 100 |
| 3g | 100 |
| 3h | 96.2 |
| 3i | 100 |
| 3j | 90.5 |
| 3k | 100 |
| 3l | 100 |
| 3m | 100 |
| 3n | 100 |
| 3o | 100 |
| 4a | 100 |
| 4b | 100 |
| 4c | 63.7 |
| 4d | 100 |
| 4e | 92.4 |
| 4f | 100 |
| 4h | 100 |
| 4i | 100 |
| 4j | 73 |
| 4k | 100 |
| Silibinin | 35.7 |
| Compound | Cell Toxicity A549 % (100 μΜ) | Cell Toxicity A375 % (100 μΜ) |
|---|---|---|
| 3a | no | 38.7 |
| 3b | 24.6 | 72.2 |
| 3c | no | 24.8 |
| 3d | 7.5 | 28.5 |
| 3e | 8.9 | 60.7 |
| 3f | no | 35.8 |
| 3g | no | 7.02 |
| 3h | no | 43.2 |
| 3i | no | 51 |
| 3j | no | 54.6 |
| 3k | no | 28.8 |
| 3l | no | 40.4 |
| 3m | no | 33.3 |
| 3n | no | 11.5 |
| 3o | no | 45.6 |
| 4a | 64.7 | 33.1 |
| 4b | no | 52.8 |
| 4c | 54.5 | 22.2 |
| 4d | 75.7 | 11.5 |
| 4e | 52.3 | 43.1 |
| 4f | 62.5 | 45.4 |
| 4h | 33.7 | 12.6 |
| 4i | 21.7 | 6.48 |
| 4j | 33.5 | 11.9 |
| 4k | 66.5 | no |
| Silibinin | 78.3 | 84.4 |
| pH 7.40 | ||||||
|---|---|---|---|---|---|---|
| Compound | MW 1 | logP 2 | logkw | F*+ 3 | F*− 4 | %HOA |
| 3a | 328.7 | 4.42 | 3.58 | 0.000 | 0.000 | 100 |
| 3b | 328.8 | 3.86 | 2.88 | 0.000 | 0.000 | 99.8 |
| 3c | 328.7 | 4.42 | 3.05 | 0.000 | 0.000 | 99.9 |
| 3d | 312.3 | 3.34 | 2.56 | 0.000 | 0.000 | 99.7 |
| 3e | 312.3 | 3.34 | 2.47 | 0.000 | 0.000 | 99.7 |
| 3f | 312.3 | 3.96 | 2.52 | 0.000 | 0.000 | 99.7 |
| 3g | 373.2 | 4.08 | 4.77 | 0.000 | 0.000 | 100.0 |
| 3h | 294.3 | 3.43 | 2.48 | 0.000 | 0.000 | 99.7 |
| 3i | 373.2 | 4.59 | 3.28 | 0.000 | 0.000 | 99.8 |
| 3j | 328.7 | 4.42 | 3.06 | 0.000 | 0.000 | 99.9 |
| 3k | 452.1 | 5.36 | 3.91 | 0.000 | 0.000 | 99.9 |
| 3l | 350.4 | 2.97 | 2.43 | 0.000 | 0.000 | 99.4 |
| 3m | 431.2 | 4.20 | 3.21 | 0.000 | 0.000 | 99.6 |
| 3n | 386.8 | 4.03 | 3.04 | 0.000 | 0.000 | 99.7 |
| 3o | 510.1 | 4.96 | 3.79 | 0.000 | 0.000 | 99.6 |
| 4a | 286.7 | 3.79 | 4.09 | 0.000 | 0.102 | 100 |
| 4b | 286.7 | 3.79 | 3.73 | 0.000 | 0.102 | 99.9 |
| 4c | 270.3 | 3.27 | 4.53 | 0.000 | 0.102 | 100 |
| 4d | 270.3 | 3.27 | 4.69 | 0.000 | 0.102 | 100 |
| 4e | 270.3 | 4.05 | 3.23 | 0.000 | 0.102 | 99.9 |
| 4f | 252.3 | 3.07 | 2.62 | 0.000 | 0.007 | 99.7 |
| 4h | 331.2 | 4.68 | 3.34 | 0.000 | 0.007 | 99.8 |
| 4i | 286.7 | 4.51 | 3.23 | 0.000 | 0.007 | 99.9 |
| 4j | 410.1 | 5.45 | 3.41 | 0.000 | 0.007 | 99.5 |
| pH 7.00 | ||
|---|---|---|
| Compound | logk10 | %PPB |
| 3a | 0.57 | 79.8 |
| 3b | 0.80 | 87.2 |
| 3c | 0.96 | 90.9 |
| 3d | 0.73 | 85.1 |
| 3e | 0.73 | 85.1 |
| 3f | 0.57 | 79.4 |
| 3g | 0.83 | 88.0 |
| 3h | 0.74 | 85.5 |
| 3i | 1.14 | 94.1 |
| 3j | 0.97 | 91.2 |
| 3k | 1.63 | 98.7 |
| 3l | 0.16 | 59.9 |
| 3m | 0.89 | 89.5 |
| 3n | 0.74 | 85.5 |
| 3o | 0.58 | 79.9 |
| 4a | 0.79 | 87.0 |
| 4b | 0.58 | 79.9 |
| 4c | 0.72 | 84.8 |
| 4d | 0.72 | 84.8 |
| 4e | 1.34 | 96.6 |
| 4f | 0.68 | 83.7 |
| 4h | 1.38 | 97.0 |
| 4i | 1.26 | 95.7 |
| 4j | 0.59 | 80.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katopodi, A.; Tsotsou, E.; Iliou, T.; Deligiannidou, G.-E.; Pontiki, E.; Kontogiorgis, C.; Tsopelas, F.; Detsi, A. Synthesis, Bioactivity, Pharmacokinetic and Biomimetic Properties of Multi-Substituted Coumarin Derivatives. Molecules 2021, 26, 5999. https://doi.org/10.3390/molecules26195999
Katopodi A, Tsotsou E, Iliou T, Deligiannidou G-E, Pontiki E, Kontogiorgis C, Tsopelas F, Detsi A. Synthesis, Bioactivity, Pharmacokinetic and Biomimetic Properties of Multi-Substituted Coumarin Derivatives. Molecules. 2021; 26(19):5999. https://doi.org/10.3390/molecules26195999
Chicago/Turabian StyleKatopodi, Annita, Evangelia Tsotsou, Triantafylia Iliou, Georgia-Eirini Deligiannidou, Eleni Pontiki, Christos Kontogiorgis, Fotios Tsopelas, and Anastasia Detsi. 2021. "Synthesis, Bioactivity, Pharmacokinetic and Biomimetic Properties of Multi-Substituted Coumarin Derivatives" Molecules 26, no. 19: 5999. https://doi.org/10.3390/molecules26195999
APA StyleKatopodi, A., Tsotsou, E., Iliou, T., Deligiannidou, G.-E., Pontiki, E., Kontogiorgis, C., Tsopelas, F., & Detsi, A. (2021). Synthesis, Bioactivity, Pharmacokinetic and Biomimetic Properties of Multi-Substituted Coumarin Derivatives. Molecules, 26(19), 5999. https://doi.org/10.3390/molecules26195999









