Detection of Circulating Serum microRNA/Protein Complexes in ASD Using Functionalized Chips for an Atomic Force Microscope
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Participants
2.3. Preparation of Serum Samples
2.4. Preparation of AFM Chips
- -
- before the immobilization of oligonucleotides (the height of objects should not exceed 0.5 nm);
- -
- after the immobilization of oligonucleotides (the height of the objects should not exceed 1.5 nm);
- -
- after the incubation of the AFM chip in the analyzed samples and washing procedures (the amount of AFM-visualized objects with heights greater than 1.5 nm should be at least 1000 per 400 μm2).
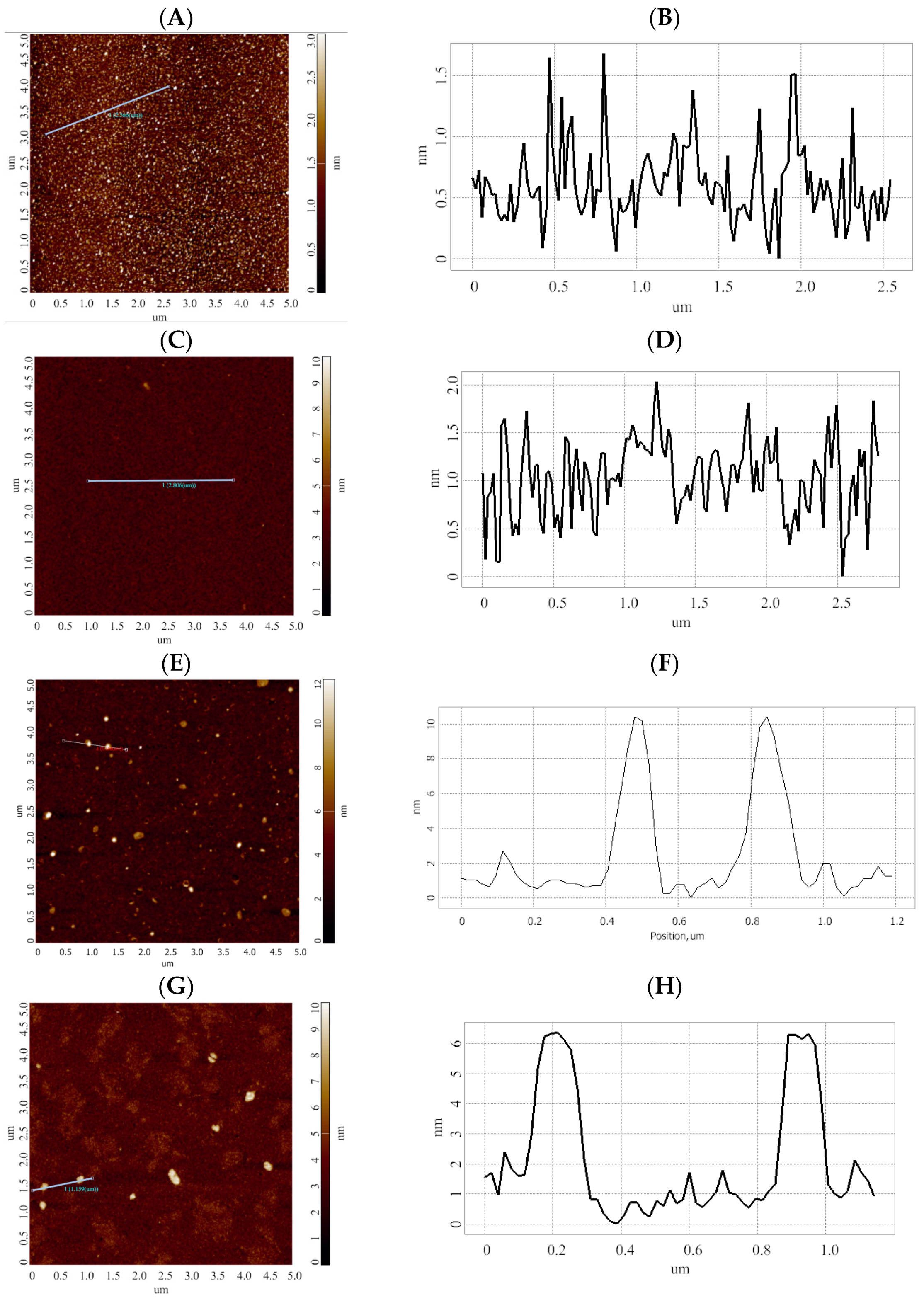
2.5. AFM Measurements
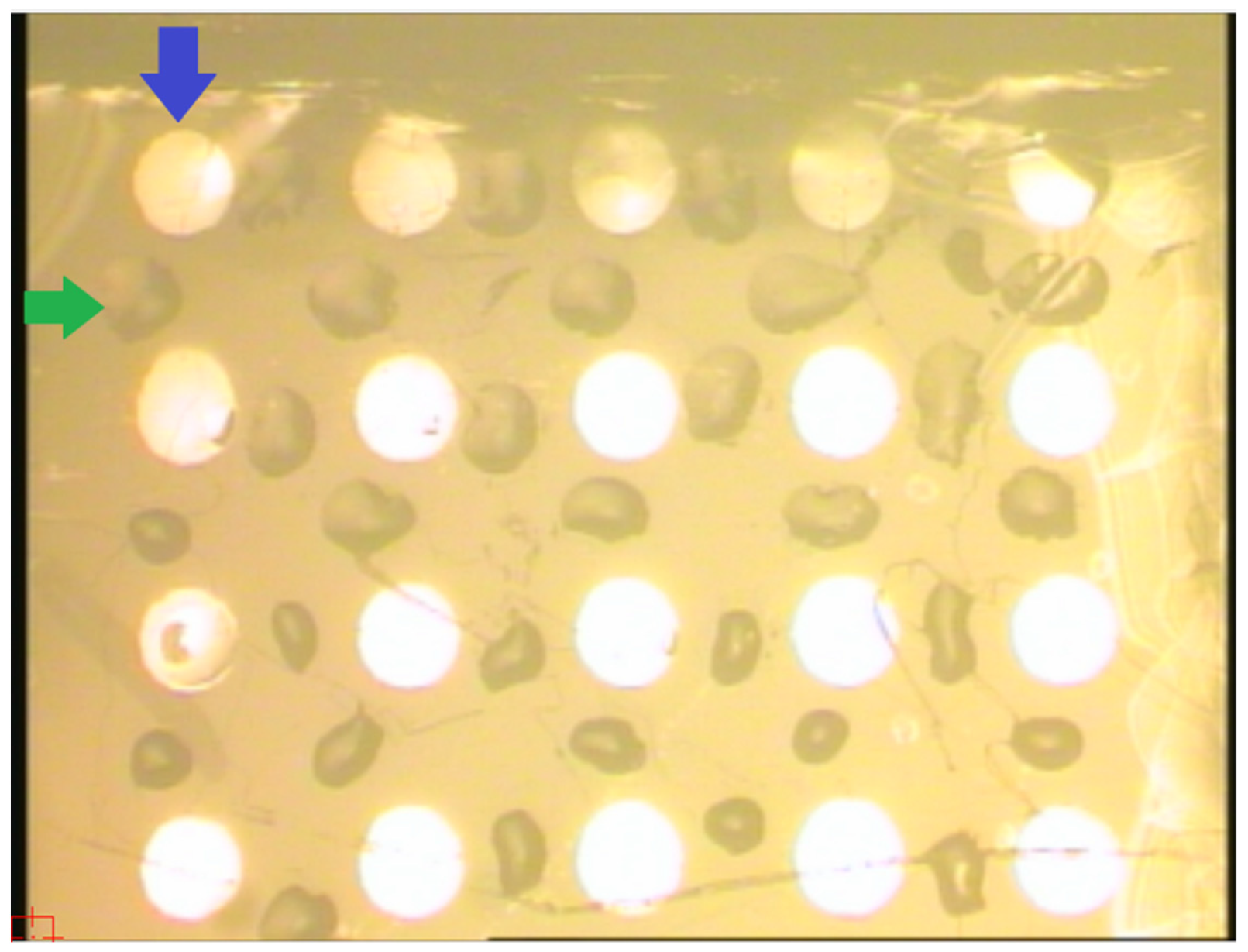
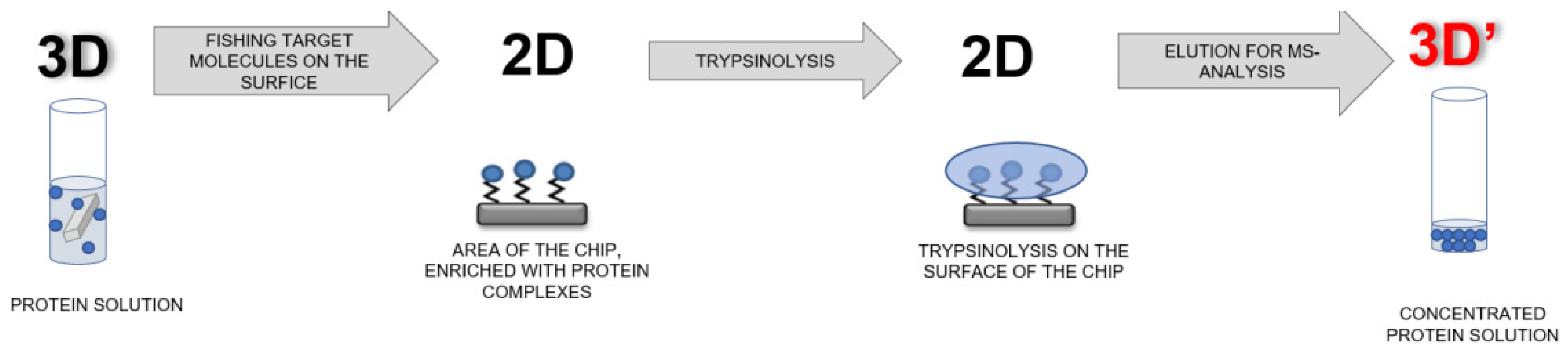
2.6. Preparation of AFM Chips for Mass Spectrometric Analysis
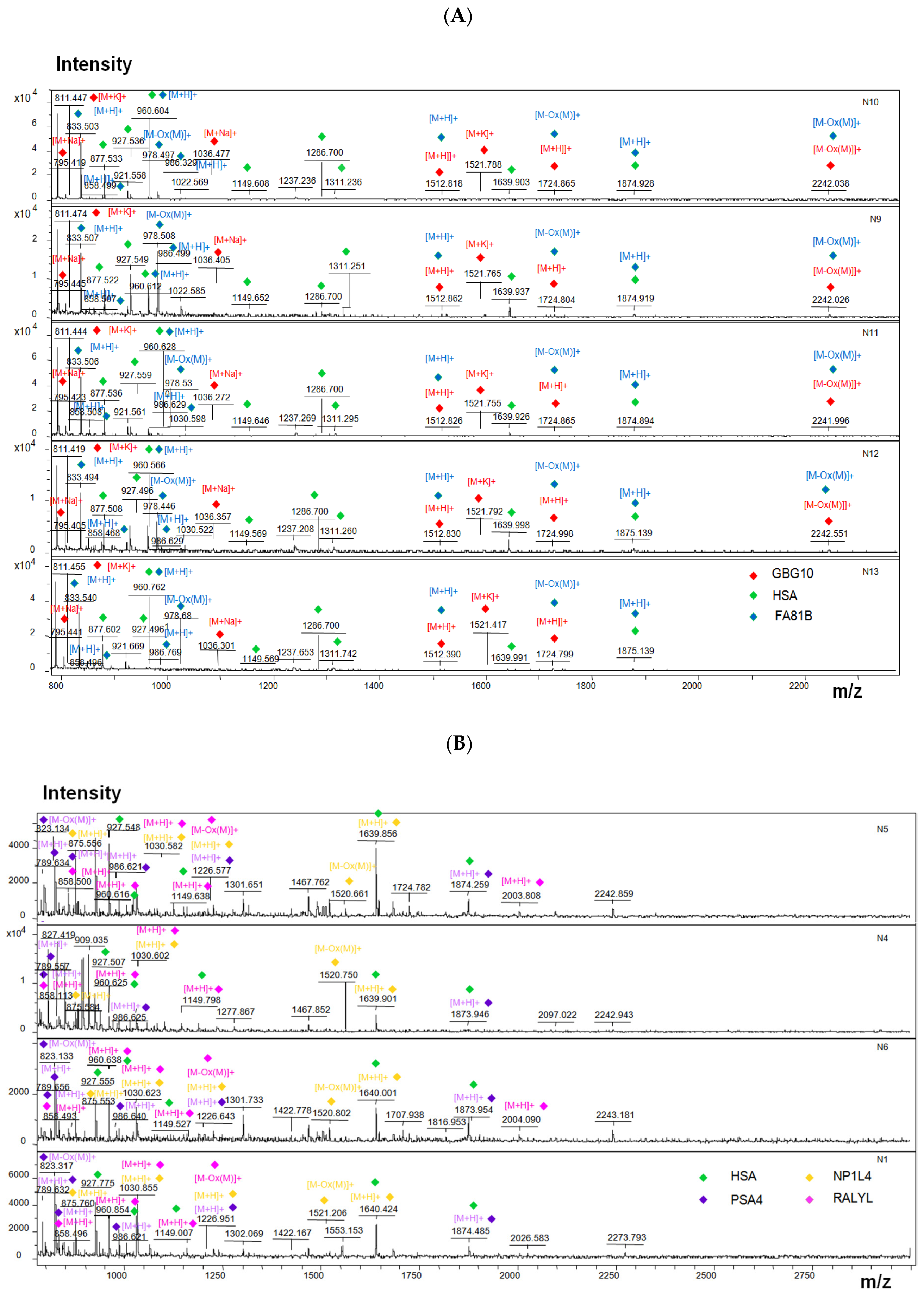
2.7. MALDI-TOF Measurements
2.8. Schematic of the AFM-MS Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Probe No. | Sequence |
|---|---|
| Probe 1 | (NH2)-(T)10 TC AAC ATC AGT CTG ATA AGC TA |
| Probe 2 | (NH2)-(T)10 AC AGC CCA TCG ACT GGT GTT G |
| Probe 3 | (NH2)-(T)10 TC CAA CAC TGT ACT GGA AGA TG |
| Probe 4 | (NH2)-(T)10 CC ATC TTT ACC AGA CAG TGT TA |
| Probe 5 | (NH2)-(T)10 TC CAA TGC TGC CCA GTA AGA TG |
| Probe 6 | (NH2)-(T)10 TC ATC ATT ACC AGG CAG TAT TA |
| Probe 7 | (NH2)-(T)10 CC AAA CAC TGC TGG GTA AGA CG |
| Probe 8 | (NH2)-(T)10 TC CAT CAT TAC CCG GCA GTA TTA |
| Probe 9 | (NH2)-(T)10 CA GAC TCC GGT GGA ATG AAG GA |
| Probe 10 | (NH2)-(T)10 GA ACT TCA CTC CAC TGA AAT C |
References
- El-Ansary, A.; Cannell, J.J.; Bjørklund, G.; Bhat, R.S.; Al Dbass, A.M.; Alfawaz, H.A.; Chirumbolo, S.; Al-Ayadhi, L. In the search for reliable biomarkers for the early diagnosis of autism spectrum disorder: The role of vitamin D. Metab. Brain Dis. 2018, 33, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Khramova, T.V.; Kaysheva, A.L.; Ivanov, Y.D.; Pleshakova, T.O.; Iourov, I.Y.; Vorsanova, S.G.; Yurov, Y.B.; Schetkin, A.A.; Archakov, A.I. Serologic markers of autism spectrum disorder. J. Mol. Neurosci. 2017, 62, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Kaysheva, A.L.; Kopylov, A.T.; Pleshakova, T.O.; Iourov, I.Y.; Vorsanova, S.G.; Yurov, Y.B.; Shchetkin, A.A.; Archakov, A.I.; Ivanov, Y.D. Proteomic analysis of serum proteins of children with autism. Biotecnol. Apl. 2017, 34, 2211–2214. [Google Scholar]
- Abu-Elneel, K.; Liu, T.; Gazzaniga, F.S.; Nishimura, Y.; Wall, D.P.; Geschwind, D.H.; Lao, K.; Kosik, K.S. Heterogeneous dysregulation of micrornas across the autism spectrum. Neurogenetics 2008, 9, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Barger, N.; Stamova, B.; Sharp, F.R.; Schumann, C.M. Atypical MiRNA expression in temporal cortex associated with dysregulation of immune, cell cycle, and other pathways in autism spectrum disorders. Mol. Autism 2015, 6, 37. [Google Scholar] [CrossRef]
- Geekiyanage, H.; Rayatpisheh, S.; Wohlschlegel, J.A.; Brown, R.; Ambros, V. Extracellular MicroRNAs in human circulation are associated with mirisc complexes that are accessible to anti-ago2 antibody and can bind target mimic oligonucleotides. Proc. Natl. Acad. Sci. USA 2020, 117, 24213–24223. [Google Scholar] [CrossRef]
- Koshiol, J.; Wang, E.; Zhao, Y.; Marincola, F.; Landi, M.T. Strengths and limitations of laboratory procedures for MicroRNA Detection: Table 1. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 907–911. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, Z. Detection of MicroRNAs using electrocatalytic nanoparticle tags. Anal. Chem. 2006, 78, 1470–1477. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M. Label-free direct detection of MiRNAs with silicon nanowire biosensors. Biosens. Bioelectron. 2009, 24, 2504–2508. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M.; Buddharaju, K.D.; Balasubramanian, N. Highly sensitive measurements of PNA-DNA hybridization using oxide-etched silicon nanowire biosensors. Biosens. Bioelectron. 2008, 23, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Kappel, A.; Keller, A. MiRNA Assays in the clinical laboratory: Workflow, detection technologies and automation aspects. Clin. Chem. Lab. Med. 2017, 55. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Huang, Y.; Paulicka, P.; Kappel, A.; Katus, H.A.; Keller, A.; Meder, B.; Stähler, C.F.; Gumbrecht, W. Double-Stranded ligation assay for the rapid multiplex quantification of MicroRNAs. Anal. Chem. 2015, 87, 12104–12111. [Google Scholar] [CrossRef] [PubMed]
- Malsagova, K.A.; Popov, V.P.; Kupriyanov, I.N.; Pleshakova, T.O.; Galiullin, R.A.; Kozlov, A.F.; Shumov, I.D.; Larionov, D.I.; Tikhonenko, F.V.; Kapustina, S.I.; et al. Raman spectroscopy-based quality control of “Silicon-On-Insulator” nanowire chips for the detection of brain cancer-associated microrna in plasma. Sensors 2021, 21, 1333. [Google Scholar] [CrossRef]
- Malsagova, K.A.; Pleshakova, T.O.; Galiullin, R.A.; Kozlov, A.F.; Romanova, T.S.; Shumov, I.D.; Popov, V.P.; Tikhonenko, F.V.; Glukhov, A.V.; Smirnov, A.Y.; et al. SOI-Nanowire Biosensor for the Detection of Glioma-Associated MiRNAs in Plasma. Chemosensors 2020, 8, 95. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Malsagova, K.A.; Kozlov, A.F.; Kaysheva, A.L.; Shumov, I.D.; Galiullin, R.A.; Kurbatov, L.K.; Popov, V.P.; Naumova, O.V.; et al. Detection of Marker MiRNAs in Plasma Using SOI-NW Biosensor. Sens. Actuators B Chem. 2018, 261, 566–571. [Google Scholar] [CrossRef]
- Benes, V.; Castoldi, M. Expression profiling of MicroRNA using real-time quantitative pcr, how to use it and what is available. Methods 2010, 50, 244–249. [Google Scholar] [CrossRef]
- Chen, Y.; Gelfond, J.A.; McManus, L.M.; Shireman, P.K. Reproducibility of Quantitative RT-PCR Array in MiRNA Expression Profiling and Comparison with Microarray Analysis. BMC Genom. 2009, 10, 407. [Google Scholar] [CrossRef]
- Baffa, R.; Fassan, M.; Volinia, S.; O’Hara, B.; Liu, C.-G.; Palazzo, J.P.; Gardiman, M.; Rugge, M.; Gomella, L.G.; Croce, C.M.; et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J. Pathol. 2009, 219, 214–221. [Google Scholar] [CrossRef]
- Mees, S.T.; Mardin, W.A.; Wendel, C.; Baeumer, N.; Willscher, E.; Senninger, N.; Schleicher, C.; Colombo-Benkmann, M.; Haier, J. EP300-A MiRNA-Regulated metastasis suppressor gene in ductal adenocarcinomas of the pancreas: Role of EP300 in metastasis of pancreatic cancer. Int. J. Cancer 2010, 126, 114–124. [Google Scholar] [CrossRef]
- Kelly, A.D.; Hill, K.E.; Correll, M.; Hu, L.; Wang, Y.E.; Rubio, R.; Duan, S.; Quackenbush, J.; Spentzos, D. Next-Generation sequencing and microarray-based interrogation of microRNAs from formalin-fixed, paraffin-embedded tissue: Preliminary assessment of cross-platform concordance. Genomics 2013, 102, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ach, R.A.; Wang, H.; Curry, B. Measuring MicroRNAs: Comparisons of microarray and quantitative pcr measurements, and of different total RNA prep methods. BMC Biotechnol. 2008, 8, 69. [Google Scholar] [CrossRef]
- Beuvink, I.; Kolb, F.A.; Budach, W.; Garnier, A.; Lange, J.; Natt, F.; Dengler, U.; Hall, J.; Filipowicz, W.; Weiler, J. A Novel microarray approach reveals new tissue-specific signatures of known and predicted mammalian MicroRNAs. Nucleic Acids Res. 2007, 35, e52. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhao, J.-J.; He, L.; Cheng, J.Q. Strategies for Profiling MicroRNA Expression. J. Cell. Physiol. 2009, 218, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Qavi, A.J.; Kindt, J.T.; Bailey, R.C. Sizing up the Future of MicroRNA Analysis. Anal. Bioanal. Chem. 2010, 398, 2535–2549. [Google Scholar] [CrossRef][Green Version]
- Marín-Romero, A.; Robles-Remacho, A.; Tabraue-Chávez, M.; López-Longarela, B.; Sánchez-Martín, R.M.; Guardia-Monteagudo, J.J.; Fara, M.A.; López-Delgado, F.J.; Pernagallo, S.; Díaz-Mochón, J.J. A PCR-Free technology to detect and quantify MicroRNAs directly from human plasma. Analyst 2018, 143, 5676–5682. [Google Scholar] [CrossRef]
- Ouyang, T.; Liu, Z.; Han, Z.; Ge, Q. MicroRNA detection specificity: Recent advances and future perspective. Anal. Chem. 2019, 91, 3179–3186. [Google Scholar] [CrossRef]
- Kang, K.; Peng, X.; Luo, J.; Gou, D. Identification of circulating MiRNA biomarkers based on global quantitative real-time PCR profiling. J. Anim. Sci. Biotechnol. 2012, 3, 4. [Google Scholar] [CrossRef]
- Moldovan, L.; Batte, K.E.; Trgovcich, J.; Wisler, J.; Marsh, C.B.; Piper, M. Methodological challenges in utilizing MiRNAs as circulating biomarkers. J. Cell. Mol. Med. 2014, 18, 371–390. [Google Scholar] [CrossRef]
- Archakov, A.; Ivanov, Y.; Lisitsa, A.; Zgoda, V. Biospecific Irreversible Fishing Coupled with Atomic Force Microscopy for Detection of Extremely Low-Abundant Proteins. Proteomics 2009, 9, 1326–1343. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef]
- Kaysheva, A.L.; Stepanov, A.A.; Kopylov, A.T.; Butkova, T.V.; Pleshakova, T.; Ryabtsev, V.V.; Iourov, I.Y.; Vorsanova, S.G.; Ivanov, Y.D. Pilot data of serum proteins from children with autism spectrum disorders. Data Brief 2019, 27, 104558. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Ivanov, Y.D.; Bykov, V.A.; Saunin, S.A.; Fedorov, I.A.; Lemeshko, S.V.; Bon Hoa, H.; Archakov, A.I. Atomic force microscopy detection of molecular complexes in multiprotein P450cam containing monooxygenase system. Proteomics 2002, 2, 1699–1705. [Google Scholar] [CrossRef]
- Shumov, I.D.; Kanashenko, S.L.; Ziborov, V.S.; Ivanov, Y.D.; Archakov, A.I.; Pleshakova, T.O. Formation of sensor array on the AFM chip surface by magnetron sputtering. J Phys. Conf. Ser. 2017, 789, 012053. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Danichev, V.V.; Pleshakova, T.O.; Shumov, I.D.; Ziborov, V.S.; Krokhin, N.V.; Zagumenniy, M.N.; Ustinov, V.S.; Smirnov, L.P.; Shironin, A.V.; et al. Irreversible chemical AFM-based fishing for detection of low-copied proteins. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2013, 46–61. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef] [PubMed]
- Kaysheva, A.L.; Pleshakova, T.O.; Stepanov, A.A.; Ziborov, V.S.; Saravanabhavan, S.S.; Natesan, B.; Archakov, A.I.; Ivanov, Y.D. Immuno-MALDI MS dataset for improved detection of HCVcoreAg in sera. Data Brief 2019, 25, 104240. [Google Scholar] [CrossRef] [PubMed]
- Pleshakova, T.O.; Kaysheva, A.L.; Bayzyanova, J.M.; Anashkina, A.S.; Uchaikin, V.F.; Shumov, I.D.; Ziborov, V.S.; Konev, V.A.; Archakov, A.I.; Ivanov, Y.D. Advantages of aptamers as ligands upon protein detection by AFM-based fishing. Anal. Methods 2017, 9, 6049–6060. [Google Scholar] [CrossRef]
- Bukharina, N.S.; Ivanov, Y.D.; Pleshakova, T.O.; Frantsuzov, P.A.; Andreeva, E.Y.; Kaysheva, A.L.; Izotov, A.A.; Pavlova, T.I.; Ziborov, V.S.; Radko, S.P.; et al. Atomic force microscopy fishing of GP120 on immobilized aptamers and its mass spectrometry identification. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2014, 8, 115–124. [Google Scholar] [CrossRef]
- Hansma, H.G.; Oroudjev, E.; Baudrey, S.; Jaeger, L. TectoRNA and “kissing-Loop” RNA: Atomic force microscopy of self-assembling RNA structures. J. Microsc. 2003, 212, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hansma, H.G.; Revenko, I.; Kim, K.; Laney, D.E. Atomic force microscopy of long and short double-stranded, single-stranded and triple-stranded nucleic acids. Nucleic Acids Res. 1996, 24, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, M.G. Microscale sample preparation. Nat. Biotechnol. 2000, 18, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Joyner, J.C.; Keuper, K.D.; Cowan, J.A. Analysis of RNA cleavage by MALDI-TOF mass spectrometry. Nucleic Acids Res. 2013, 41, e2. [Google Scholar] [CrossRef] [PubMed]
- Zagorevskii, D.V.; Aldersley, M.F.; Ferris, J.P. MALDI analysis of oligonucleotides directly from montmorillonite. J. Am. Soc. Mass Spectrom. 2006, 17, 1265–1270. [Google Scholar] [CrossRef]
- Nordhoff, E.; Kirpekar, F.; Roepstorff, P. Mass Spectrometry of Nucleic Acids. Mass Spectrom. Rev. 1996, 15, 67–138. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Bukharina, N.S.; Pleshakova, T.O.; Frantsuzov, P.A.; Andreeva, E.Y.; Kaysheva, A.L.; Zgoda, V.G.; Izotov, A.A.; Pavlova, T.I.; Ziborov, V.S.; et al. Atomic force microscopy fishing and mass spectrometry identification of Gp120 on immobilized aptamers. Int. J. Nanomed. 2014, 9, 4659–4670. [Google Scholar] [CrossRef][Green Version]
- Ivanov, Y.D.; Kaysheva, A.L.; Frantsuzov, P.A.; Pleshakova, T.O.; Krohin, N.V.; Izotov, A.A.; Shumov, I.D.; Uchaikin, V.F.; Konev, V.A.; Ziborov, V.S.; et al. Detection of hepatitis C Virus core protein in serum by atomic force microscopy combined with mass spectrometry. Int. J. Nanomed. 2015, 10, 1597–1608. [Google Scholar] [CrossRef][Green Version]
- Hicks, S.D.; Middleton, F.A. A Comparative review of MicroRNA expression patterns in autism spectrum disorder. Front. Psychiatry 2016, 7. [Google Scholar] [CrossRef]
- Zamil, B.M.; Ali-Labib, R.; Youssef, W.Y.; Khairy, E. Evaluation of MiR-106a and ADARB1 in Autistic Children. Gene Rep. 2020, 18, 100586. [Google Scholar] [CrossRef]
- Zhi, F.; Shao, N.; Wang, R.; Deng, D.; Xue, L.; Wang, Q.; Zhang, Y.; Shi, Y.; Xia, X.; Wang, S.; et al. Identification of 9 serum MicroRNAs as potential noninvasive biomarkers of human astrocytoma. Neuro-Oncol. 2015, 17, 383–391. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Geng, L.; Streck, D.L.; Dermody, J.J.; Toruner, G.A. MicroRNA expression changes in association with changes in interleukin-1ß/interleukin10 ratios produced by monocytes in autism spectrum disorders: Their association with neuropsychiatric symptoms and comorbid conditions (observational study). J. Neuroinflamm. 2017, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Mundalil Vasu, M.; Anitha, A.; Thanseem, I.; Suzuki, K.; Yamada, K.; Takahashi, T.; Wakuda, T.; Iwata, K.; Tsujii, M.; Sugiyama, T.; et al. Serum MicroRNA profiles in children with autism. Mol. Autism 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Nardone, S.; Sams, D.S.; Elliott, E. Hypomethylation of MiR-142 Promoter and upregulation of MicroRNAs that target the oxytocin receptor gene in the autism prefrontal cortex. Mol. Autism 2015, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Long, Z.; Chen, Z.; Li, J.; Hu, Z.; Qiu, R.; Zhuang, W.; Tang, B.; Xia, K.; Jiang, H. Investigation of gene regulatory networks associated with autism spectrum disorder based on MiRNA expression in china. PLoS ONE 2015, 10, e0129052. [Google Scholar] [CrossRef] [PubMed]

| Probes for microRNAs | Secondary Structure | Sequence of Probes |
|---|---|---|
| miR-106a-5p |  | NH2-(T)10 CT ACC TGC ACT GTA AGC ACT TTT |
| miR-106b-5p | 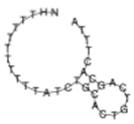 | NH2-(T)10 AT CTG CAC TGT CAG CAC TTT A |
| miR-19b-3p |  | NH2-(T)10TC AGT TTT GCA TGG ATT TGC ACA |
| miR-494-5p | 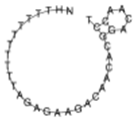 | NH2-(T)10AG AGA AGA CAA CAC GGA CAA CCT |
| miR-494-3p |  | NH2-(T)10GA GGT TTC CCG TGT ATG TTT CA |
| miR-15b-5p |  | NH2-(T)10TG TAA ACC ATG ATG TGC TGC TA |
| miR-320a | 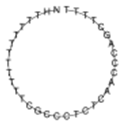 | NH2-(T)10TC GCC CTC TCA ACC CAG CTT TT |
| Sample No. | Age | Sex | Additional Information |
|---|---|---|---|
| Family 1 | |||
| 7 | 8 | M | F84.1 Son |
| 6 | 38 | F | Healthy, Mother |
| 8 | 6 | M | Healthy, Son |
| Family 2 | |||
| 5 | 7 | M | F84.1 Son |
| 4 | 34 | F | Healthy, Mother |
| Participants | |||
| 11 | 7 | M | F84.1 Son |
| 12 | 11 | M | F84.1 Son |
| 13 | 11 | F | F84.1 Daughter |
| 1 | 48 | F | Healthy, Mother |
| 9 | 8 | M | F84.1 Son |
| 10 | 11 | M | F84.1 Son |
| Protein | Frequency of Occurrence in Samples | Function (According to UniProt) | Mw, Da | pI | Aliphatic Index |
|---|---|---|---|---|---|
| GBG10 | 5 | Guanine nucleotide-binding proteins (G proteins) are involved into various transmembrane signaling systems as modulators or transducers | 7201 | 7.7 | 95 |
| FA81B | 5 | Protein FAM81B | 51,990 | 9.2 | 82 |
| CDN2C | 10 | Inhibits cell growth and proliferation | 18,116 | 7.7 | 95 |
| RT24 | 5 | 28S ribosomal protein | 19,003 | 9.5 | 90 |
| KCNV2 | 10 | Potassium voltage-gated channel subfamily | 62,419 | 6.1 | 84 |
| CCL27 | 5 | Chemotactic factor, which attracts skin-associated memory T-lymphocytes | 12,610 | 9 | 100 |
| THA11 | 10 | Transcriptional repressor, playing a central role in embryogenesis and pluripotency of embryonic stem cells | 34,433 | 9.2 | 71 |
| PSA4 | 5 | Proteasome subunit alpha type-4 | 29,465 | 7.6 | 87 |
| NP1L4 | 5 | Nucleosome assembly protein 1-like 4 | 42,797 | 4.6 | 65 |
| KCNE5 | 10 | Potassium voltage-gated channel subfamily | 14,984 | 5.9 | 91 |
| RALYL | 5 | RNA-binding Raly-like protein | 32,311 | 7.7 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaysheva, A.L.; Isaeva, A.I.; Pleshakova, T.O.; Shumov, I.D.; Valueva, A.A.; Ershova, M.O.; Ivanova, I.A.; Ziborov, V.S.; Iourov, I.Y.; Vorsanova, S.G.; et al. Detection of Circulating Serum microRNA/Protein Complexes in ASD Using Functionalized Chips for an Atomic Force Microscope. Molecules 2021, 26, 5979. https://doi.org/10.3390/molecules26195979
Kaysheva AL, Isaeva AI, Pleshakova TO, Shumov ID, Valueva AA, Ershova MO, Ivanova IA, Ziborov VS, Iourov IY, Vorsanova SG, et al. Detection of Circulating Serum microRNA/Protein Complexes in ASD Using Functionalized Chips for an Atomic Force Microscope. Molecules. 2021; 26(19):5979. https://doi.org/10.3390/molecules26195979
Chicago/Turabian StyleKaysheva, Anna L., Arina I. Isaeva, Tatyana O. Pleshakova, Ivan D. Shumov, Anastasia A. Valueva, Maria O. Ershova, Irina A. Ivanova, Vadim S. Ziborov, Ivan Y. Iourov, Svetlana G. Vorsanova, and et al. 2021. "Detection of Circulating Serum microRNA/Protein Complexes in ASD Using Functionalized Chips for an Atomic Force Microscope" Molecules 26, no. 19: 5979. https://doi.org/10.3390/molecules26195979
APA StyleKaysheva, A. L., Isaeva, A. I., Pleshakova, T. O., Shumov, I. D., Valueva, A. A., Ershova, M. O., Ivanova, I. A., Ziborov, V. S., Iourov, I. Y., Vorsanova, S. G., Ryabtsev, S. V., Archakov, A. I., & Ivanov, Y. D. (2021). Detection of Circulating Serum microRNA/Protein Complexes in ASD Using Functionalized Chips for an Atomic Force Microscope. Molecules, 26(19), 5979. https://doi.org/10.3390/molecules26195979







