1. Introduction
Modified biopolymers offer a broad spectrum of hydrogel forming materials that, in recent years, have become increasingly important in the fields of drug delivery, tissue engineering, and regenerative medicine [
1,
2,
3]. As materials from natural origin, they are found in many different mammalian extracellular matrices (ECM), such as HA or collagen, which are derived due to processing slightly degraded gelatin [
4,
5]. Especially the biotechnologically produced HA and its derivatives are already in clinical use for many specific applications due to their excellent biocompatibility and availability in high purity [
6]. For a chemical modification, HA offers several functional groups available for different modification schemes, additionally the polymer is also known to bind to CD44 receptors [
7,
8,
9] and to enhance the chondrogenic differentiation of mesenchymal stem cells in general [
10,
11].
In the field of tissue engineering, the dissolution stability and mechanical strength of applied biopolymers-based hydrogels is especially crucial for culture and for tissue development under physiological conditions. Without a stable crosslinking, water-soluble polymers will dissolve slowly and accordingly disappear from the application site [
12]. Natural HA hydrogels possess only very low mechanical properties as physically crosslinked hydrogels. and therefore are limited in their applications for tissue engineering approaches [
13]. To achieve a proper dissolution and mechanical stability, HA needs to be functionalized and, subsequently, chemically crosslinked with other nondetrimental molecules to form long term stable hydrogel networks [
14]. For these applications the carboxyl group of HA can be modified, for example, with thiol, tyramine, or dihydrazide, whereas the primary 6-hydroxy groups can be alternatively functionalized with methacrylate and bromoacetate for various chemical or enzymatic crosslinking reactions such as thiol-ene, peroxidase, or photo-crosslinking reactions. These applied modifications of HA have been previously summarized in a review article by Burdick et al. [
9]. In addition to the frequently applied chemical networks, there are alternative more stable physical, chemically reversible or irreversible crosslinking mechanisms used for hydrogel formation. For example, physically crosslinked HA hydrogels via a more stable guest–host interaction were introduced by Highley et al., by esterification of the primary alcohol with adamantane acetic acid as guest and amidation of the carboxylate with β-cyclodextrin as host. For the later application in cell culture, they still needed a secondary and irreversible chemical crosslinking by applying radical polymerization of methacrylate, which they attached to non-modified primary alcohols for enhanced mechanical stability [
15]. Wang et al., in a similar fashion, introduced HA hydrogels containing adipic acid dihydrazide modified HA as well as periodate oxidized HA (oxHA). This dynamic covalent system, based on reversible Schiff base chemistry, additionally needed chemical fixation by a thiol-ene crosslinked secondary network with norbornene modified HA and a tetrathiol crosslinker to achieve a long-term tissue culture [
16].
Many of the physical or dynamically crosslinked hydrogels can also be successfully applied for three-dimensional bioprinting approaches due to their excellent shear thinning properties, but as stated above, those systems require a secondary crosslinking to achieve sufficient mechanical strength and long-term stability for subsequent cell culture. Therefore, other common crosslinking mechanisms, which can also lead to hydrogels without any pre-crosslinking systems, were used to generate irreversible covalent bonds via UV crosslinking, for example, via thiol-ene reaction [
17,
18] or radical polymerization [
19]. However, the UV light used here can harm cells by direct DNA damage, and therefore should be avoided or at least applied with the necessary care [
20,
21]. In general, therefore, it is desirable to apply mechanisms without any UV crosslinking to provide good cell viability during covalent hydrogel formation. One alternative crosslinking reaction previously described for HA hydrogels is based on Schiff base chemistry, which has been previously reported for pre crosslinking [
16,
22,
23], including different reactive and stable amine derivatives, such as imines, hydrazones, and oximes [
24].
For the necessary aldehyde functionalization, one commonly applied method is the oxidation of HA using sodium periodate to generate a dialdehyde in a ring opening reaction of the glucuronic acid unit, which results in a massive alteration of the HA backbone structure. Linkers containing amine, dihydrazide, or aminooxy groups can, subsequently, be used as crosslinkers [
22,
23,
24,
25]. Weis et al., showed that hydrogels formed using these materials are not stable for longer than six days in PBS and even less stable in cell culture medium containing free amino acids. At the same time, the degree of oxidation (DO) varies even with same molar stoichiometry and is usually quite moderate, most likely due to numerous side reactions of the oxidation agent periodate [
16,
22,
23]. Accordingly, hydrogels based on Schiff base chemistry using low molecular weight crosslinkers always need a secondary networking, for example, UV crosslinking, to achieve sufficient mechanical and long-term stability [
16].
A promising alternative to modify HA with aldehyde functionalities is the selective oxidation of the primary alcohol of the
N-acetylglucosamine unit with (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO)/trichloroisocyanuric acid (TCC), as described for the selective oxidation of glycosides. To enable this reaction of HA in DMF, the solubility of HA needs to be enhanced by exchanging the sodium ion of hyaluronan for a tetrabutylammonium ion (
Scheme 1). Angelin et al., proved the selectivity of the oxidation to primary alcohols with unprotected monoglycosides with high yields achieved after at least 7 h [
26]. For this approach, anhydrous DMF was used as the solvent to avoid overoxidation of the primary alcohol to the carboxylic acid, and sodium bicarbonate was shown to be most efficient to generate the required alkaline conditions. Buffa et al., performed this oxidation in a mixture of water (H
2O) and dimethylformamide (DMF) to generate degrees of oxidation between 5 and 15%. Additionally, they also tested various secondary oxidation reagents, such as sodium hypochlorite (NaClO) or sodium hypobromite (NaBrO). However, a maximum DO of only 18% was obtained in their studies [
27].
In this study, we aimed to oxidize the primary alcohol of HA selectively by using TEMPO/TCC oxidation in anhydrous DMF. We expected preservation of the HA backbone without ring-opening and possibly higher DO. We also assumed that, due to the intact polymer backbone and higher DO, more stable hydrogels could be prepared even in cell culture medium without any secondary crosslinking systems to achieve higher mechanical and potentially long-term stability. Additionally, the reversible chemical crosslinks should allow an erosion of the hydrogels without a chain cleavage catalyzed by hyaluronidase. In these hydrogels, we exemplarily analyzed the chondrogenic differentiation potential of human mesenchymal stromal cells (hMSCs) within these hydrogels. Accordingly, the prepared HA-based hydrogels have already been widely used as favorable matrices in tissue engineering approaches to study the chondrogenic differentiation of encapsulated cells [
9,
17,
28,
29]. In our approach, hMSCs were embedded in in situ forming hydrogels and, subsequently, analyzed regarding cell viability and their chondrogenic differentiation potential in vitro. Since it is known that modifications of the HA backbone can affect the binding affinity to CD44 receptors [
28], we additionally studied the binding properties of our proxHA and compared this to oxHA and native HA using surface plasmon resonance (SPR) affinity measurements.
3. Discussion
According to this study, we established a new synthesis path of a primary oxidized HA for the formation of dynamic chemically crosslinked hydrogels with high mechanical strength and long-term stability for cell culture. Additionally, we optimized the determination method for the aldehyde content of oxidized HA and other comparable biopolymers. In studies in the literature,
tert-butyl carbazate, a BOC-protected hydrazine, is often used for the quantitative analysis [
23], but we found out that the BOC protection group can already be cleaved under the mild acidic conditions during the modification [
30]. Since the reductive amination is performed in acetate buffer with pH 5.2, the use of
tert-butyl carbazate can deceive the obtained DO. With
tert-butyl hydrazine, the cleavage of the
tert-butyl group used for quantification is avoided and reliable results are obtained.
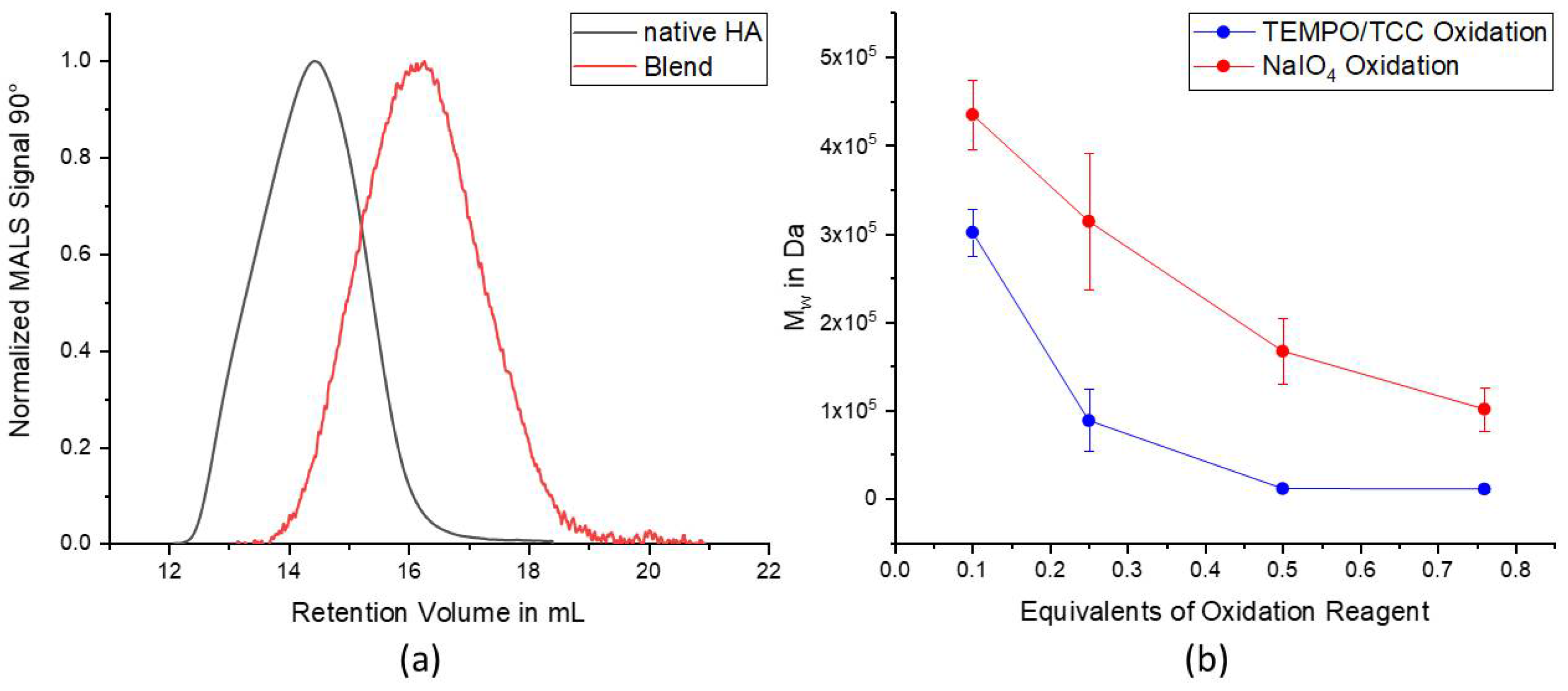
During the characterization of proxHA using TBH, we observed that both higher TCC equivalents of 0.76 and 0.5 led to a similar DO over 70%. The oxidation of the primary alcohol of HA is performed by TEMPO which is acting as catalyst and needs activation and regeneration by a secondary oxidation reagent. For this, TCC is used both to activate and regenerate TEMPO in a two-step oxidation [
26]. Since all three chlorine atoms are active, TCC can function three times as oxidation reagent and finally convert to cyanuric acid with an aromatic ring, which is the driving force for the reaction [
31] (
Scheme 2). Additionally, the reaction must be performed under strictly anhydrous conditions to avoid subsequent oxidation to carboxylic acid functions [
26]. For this reason, 0.5 and 0.76 equivalents TCC should be able to convert nearly all primary alcohol groups of N-acetyl-
D-glucosamine during the reaction. Still, no complete conversion of all HA dimer units was achieved, therefore, we assume that the oxidation limit could capped at a certain degree of oxidation due to steric effects, since the obtained degrees of oxidation for higher amounts of TCC were quite similar (
Figure 1b). Taking the three active chlorine species into account, it was expected to achieve a DO of over 70% already with 0.25 eq. of TCC, which occurred only once with DO 73.7% due to excellent reaction conditions. Since HA is a very sensitive and eventually also hygroscopic biomacromolecule, reproducibility of the reactions with HA is often difficult to accomplish. Under best conditions, a DO of around 60% was achieved on average and this is, nonetheless, 2.4 times higher than the equivalents of added TCC. A similar effect was observed for the reactions with only 0.1 equivalents of TCC, indicating the multiple step oxidation of the secondary oxidation reagent. As compared with the oxidation with NaIO
4, Dos that were at least 3–12 times higher were obtained depending on the used equivalents of TCC. Unfortunately, the GPC measurements, nevertheless, also showed a massive degradation of the HA chains after TEMPO/TCC oxidation. However, it is well known that degradation of HA chains can occur under oxidative stress (e.g., caused by free radicals) or under non physiological pH conditions both during chemical reactions as well as in biological systems [
32,
33,
34,
35].
When using the polymers for the formulation of hydrogel, we observed very fast gelation with adipic acid dihydrazide, within 10 s or faster, especially with higher polymer contents and oxidation degrees. Due to the immediate gelation, the obtained hydrogels were quite stiff and brittle, therefore, we assume that these hydrogel systems cannot be used for extrusion-based 3D bioprinting. Since a higher DO led to more crosslinking and, consequently, higher network density, it is also expected that a higher DO would result in a higher Young’s modulus value. Upon incubation in PBS, we observed increased Young’s modulus values of almost all hydrogels, except the hydrogels with DO 49.5%, which even dissolved completely between Days 14 and 21, due to the lowest DO and resulting low crosslinking efficiency. One possible explanation for this increase in the value of Young’s modulus is the dynamic reversible bond of the Schiff base chemistry, which can be rearranged due to the reversibility until the most stable thermodynamic state and, consequently, highest mechanical stability is reached. Additionally, we assume that the shrinkage of the hydrogels that occurs after storage in PBS is also related to this rearrangement of the network. The higher hydrogel network density caused by syneresis again explains the occurring increase in the mechanical strength at the later timepoints. A similar effect of higher network density can also be achieved by increasing the content of the hydrogel forming polymer solutions, which is demonstrated with the blend hydrogels formed by different polymer contents (
Figure 3b). As compared to the individual polymer batches, the blend hydrogels showed a reduced mechanical strength and rather moderate swelling behavior, which can be attributed to the overall mixed behavior of the differently oxidized polymer batches. Accordingly, the behavior of the hydrogels can be tuned by the oxidation degree of the used polymers. In general, the formed hydrogels with an average oxidation degree above 50%, proved to be stable for extended periods of time even without cells (over 3 weeks), which was never been achieved with hydrogels based on NaIO
4 oxidized HA and the low molecular weight crosslinker ADH [
22]. As compared with other HA hydrogels crosslinked only via reversible Schiff base, for example, the hydrazine modified HA crosslinked with NaIO
4 oxidized HA [
16], our proxHA hydrogels are also mechanically much stiffer. We presume this is mainly induced by the high degree of oxidation and eventually also the preservation of the HA backbone structure.
Since we only modified the primary alcohol of HA without changing the backbone structure excessively, we were interested in the receptor binding affinity of our HA derivative as compared with the commonly performed HA oxidation using periodate. Bhattacharya et al., summarized the interactions between the functional groups of HA and the CD44 receptor, and showed that hydrogen bonds between the charged glucuronic acid and the amino acids predominate the hydrogen bonds and hydrophobic interaction of the N-acetylglucosamine [
28,
36]. Oxidation with NaIO
4 obviously leads to an opening of saccharide rings, which causes an extreme interference with the retention of HA backbone structure. We assumed that this interference by ring opening, and oxidation has a much higher influence on the CD44 receptor binding properties of HA than the oxidation of the primary alcohol group to an aldehyde function. However, both binding affinities of the differently oxidized HA derivatives were significantly lower than the native HA, which demonstrates the specificity of CD44 for the preserved structure of HA. Despite the fact that NaIO
4 oxidized HA only had an almost six-fold lower DO and a ten-fold higher molecular weight than proxHA, the
KD values of both oxidized HA were in a similar range. This demonstrates that even with preservation of the backbone the high DO negatively affects the binding affinity of the HA derivative (
Table 2). However, given the fact that the CD44 receptor needs at least four intact HA repeating units for binding [
8], which is achievable by oxHA with a DO of 11.7% (every 10th repeating unit is oxidized), it is impossible to find as much unchanged repeating units in the proxHA, where six out of ten repeating units are already oxidized. Accordingly, we suppose that the NaIO
4 oxidation massively destroys the backbone structure, and thereby interferes with the receptor interaction leading to the weak binding affinity even with a lower oxidation degree. The exchange of the primary alcohol to an aldehyde function, accordingly, seems to have a minor effect on the binding ability (
Figure 5) in this study.
Further striking differences were observed during the in vitro cell culture in the formed hydrogels. The hydrogels were only allowed to crosslink for 30 min, since the supply of cells with nutrients should not be disrupted for too long. Without storage in any aqueous solutions, there is no possibility for the hydrogels to absorb additional water for enhancement of the dynamic Schiff base formation. Here, it was observed that the hydrogels formed out of 3 and 4 wt% polymer dissolved completely during in vitro culture in proliferation medium, which was most likely caused by the reversible dynamic Schiff base system where the formed hydrazones undergo hydrolysis [
37]. Additionally, the dissolution of the hydrogels was possibly enhanced by the presence of amino acids in cell culture medium, which can obviously replace ADH as a bifunctional crosslinker. In strong contrast, all hydrogels which underwent differentiation retained their stability in cell culture, which we attributed to the newly formed ECM by the hMSCs, which was most likely integrated in the reversible hydrogel network. Nevertheless, a higher DO could still be employed to improve stability due to more crosslinking possibilities for ADH, and thereby also allow a longer culture for undifferentiated cells (
Figure S2). In general, all hMSCs (undifferentiated and differentiated) within the hydrogels initially showed an excellent cell viability with over 85% after encapsulation. The viability of cells decreased after seven days, especially for the higher polymer containing 5 wt% hydrogels (
Figure 6b), which was also observed in other HA-based hydrogels and could probably be caused by limitations of nutrient and waste diffusion due to the denser polymer network [
38,
39,
40]. The toxicity of the also present low molecular weight crosslinker was only minute as demonstrated in cell culture studies (data not shown).
Furthermore, the deposition of GAG and collagen throughout the hydrogel matrix proved the formation of cartilage specific ECM [
41]. The significant reduction in the GAG/DNA ratio in 5 wt% hydrogels as compared with lower concentrated hydrogels, i.e., 3 wt% (
Figure 7a), was in line with reports for other HA hydrogels which showed that higher polymer content can reduce GAG production of encapsulated cells [
42,
43,
44]. In addition to this, the total collagen content did not indicate significant differences between the different contents (
Figure 7b). The considerable shrinkage of hydrogels cultured under chondrogenic conditions after 21 days, especially for high concentrated gels with less ECM production (
Figure S2), could also be explained with the dynamic bonds of the Schiff base chemistry, which allow a steady replacement of the ADH crosslinker by the ECM produced by the cells, and thereby a further contraction or condensation of the hydrogel. Here, the reversible Schiff base chemistry also enabled a continuous remodeling of the hydrogels without any necessary cleavable crosslinkers. Additional histological and IHC staining, accordingly, showed an aggregation of cells and homogeneous distribution of cartilaginous matrix components such as GAG, aggrecan, and collagen II, especially throughout the lower concentrated hydrogels, providing more space for the formed ECM components (
Figure 7c). The deposition of cellular ECM in these reversibly crosslinked hydrogels appeared to be more even as compared with other HA hydrogel systems [
17,
42,
43]. Taken together, in this study, the presented hydrogel system based on highly oxidized HA seems to be well suited for the culture of ECM forming cells, especially hMSCs undergoing chondrogenesis in this pilot experiment.
4. Materials and Methods
4.1. Materials
HA (1.0–2.0 MDa, 80–100 kDa), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS) were purchased from Carbosynth Limited (Berkshire, UK). Adipic acid dihydrazide (ADH), anhydrous dimethylformamide (DMF), dimethylhydrazine, DOWEX® 50WX8-400 ion exchanger, Dulbecco’s phosphate buffered saline (DPBS), ethidium homodimer-I (EthD-I), sodium azide, sodium chloride, sodium cyanoborohydride, sodium periodate, tert-butyl hydrazine hydrochloride (TBH), tetrabutylammonium (TBA) hydroxide solution (~40% in water), and trichloroisocyanuric acid (TCC) were purchased from Sigma-Aldrich, St. Louis, MO, USA. 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) was acquired from Alfa Aesar (Karlsruhe, Germany). Human CD44 Fc Chimera Recombinant Protein was obtained from R&D Systems (Minneapolis, MN, USA). The acetic acid, ethylene glycol, potassium chloride, and sodium nitrate were purchased from Merck KGaA (Darmstadt, Germany). Proteinase K was purchased from Merck Millipore (Burlington, MA, USA). Calcein-acetoxymethyl ester (calcein-AM), Dulbecco’s modified Eagle’s medium (DMEM), 0.25% trypsin-EDTA, HEPES 1M, penicillin-streptomycin, Quant-iT™ PicoGreen® dsDNA Reagent and Kit, and sodium hydrogencarbonate were acquired from Thermo Fisher Scientific (Waltham, MA, USA). ROTI®Histofix 4.5% and ZelluTrans/Roth dialysis tubing (regenerated cellulose, MWCO 3500 Da) were purchased from Carl Roth GmbH and Co. KG, Karlsruhe, Germany.
4.2. Syntheses of Tetrabutylammonium Hyaluronate (TBA-HA)
The transformation of sodium hyaluronate into tetrabutylammonium hyaluronate (TBA-HA) was performed, according to Oudshoorn et al., with slight modifications [
45]. The exchange of the sodium counterions was performed with 50 g DOWEX
® 50WX8-400. The resin was washed three times with 1 L Millipore H
2O and, afterwards, suspended in 98 mL TBA hydroxide solution, stirred for two hours at room temperature (RT), and then separated from the solution by filtration. For the preparation of TBA-HA, 5 g HA was dissolved in 600 mL Millipore water overnight at RT, and then suspended with 50 g resin. The suspension was shaken for 3 h and the resin was removed by centrifugation. TBA-HA was obtained by lyophilization (Epsilon 2–4 LSCplus, Martin Christ Gefriertrocknungsanlage GmbH, Osterode, Germany).
4.3. Syntheses of Primary Oxidized Hyaluronic Acid (proxHA)
All subsequently used equivalents were calculated corresponding to one repeating unit of the TBA-HA (M = 621.79 g/mol). TBA-HA was dissolved overnight at RT in anhydrous DMF (0.5% w/v) under inert gas. TEMPO (0.0513 eq., catalytical amount) and excess of solid NaHCO3 (30 eq.) were added. Then, the suspension was cooled to 4 °C and different amounts of TCC (0.76, 0.5, 0.25, and 0.1 eq.) were added. The reaction was subsequently stirred for 4 h, and then slowly warmed to RT. Afterwards, an excess of ethylene glycol (30 mL for 750 mg TBA-HA and 0.25 eq. of TCC) was added to quench the reaction and remaining oxidative potential. Then, the obtained reaction mixture was dialyzed against 150 mM NaCl for 2 d, and then against Millipore H2O for another 2 d (MWCO 3500 Da). The final modified polymer was obtained after lyophilization as a white solid foam. For cell studies and mechanical testing, several batches obtained with 0.25 eq. of TCC were consolidated to receive one larger blend batch to achieve sufficient reproducibility of subsequent experiments and to investigate the influence of batch-to-batch variations.
4.4. Syntheses of Hyaluronic Acid Dialdehyde (oxHA)
The synthesis was performed according to Jia et al., with slight modifications [
23]. HA was dissolved in Millipore H
2O overnight. A 0.4
M sodium periodate solution was added dropwise under exclusion of light with the following equivalents: 0.76, 0.5, 0.25, and 0.1. The reaction was stirred for 2 h at RT and similarly quenched by adding 30 mL ethylene glycol. The oxHA was obtained after dialysis (MWCO 3500 Da) against ddH
2O and lyophilization as a white solid foam.
4.5. Determination of the Degree of Oxidation (DO)
Aldehyde containing HA was dissolved in acetate buffer (pH 5.2) overnight at RT. Stock solutions of
tert-butyl hydrazine hydrochloride and sodium cyanoborohydride (0.5
m) were added. The mixtures were shaken at RT for 24 h at 850 rpm. Then, the solutions were dialyzed against Millipore H
2O (MWCO 3500) and lyophilized.
1H-NMR spectra of the products were measured with 300 MHz NMR (Bruker NMR Fourier 300) in D
2O. The acetyl group of HA (2.05 ppm) was calibrated as 3 protons. The
tert-butyl signal (1.40 ppm) of each sample was accordingly divided by 9 protons to estimate the achieved degree of oxidation (DO) as follows:
4.6. Gel Permeation Chromatography (GPC)
Weight and number average molecular weights (Mw and Mn) of proxHA and oxHA were determined by using a GPC system from Malvern (Herrenberg, Germany). The system consisted of a Viscotek GPCmax (in-line degasser, 2-piston pump and autosampler), a column oven (35 °C) refractive index (RI) detector (Viscotek VE3580), multiple angle light scattering detector (Viscotek SEC-MALS 20, laser wavelength 660 nm), and two Viscotek A-columns (A6000M, length = 300 mm, width = 8 mm, porous poly(methyl methacrylate), particle size 13 µm). An aqueous solution of Millipore H2O with 8.5 g/L NaNO3 and 0.2 g/L NaN3 was used as eluent and solvent for the polymers. The samples were dissolved with a concentration of 0.5 mg/mL overnight at RT and filtered with a 0.45 µm regenerated cellulose membrane. The measurements were performed with 100 µL injection volume with an elution rate of 0.7 mL/min. The molecular weights of the HA derivatives were calculated using a MALS calibration performed with a narrowly distributed PEG standard of Mw = 45,000.
4.7. SPR Measurements for Binding Affinity of HA Derivatives
Interactions between the differently modified HA and the corresponding HA receptor CD44 (R&D Systems, Inc., Minneapolis, MN, USA) were measured by SPR using a Reichert
® 4SPR system (Reichert Technologies, Unterschleissheim, Germany). For immobilization of the CD44 receptor on the sensor surface, the carboxylate groups of a carboxymethyldextran hydrogel coated sensor chip (XanTec bioanalytics GmbH, Düsseldorf, Germany) were activated, first, by perfusing a 1:1 mixture of NHS and EDC (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide), according to the manufacturer’s recommendation. Then, CD44 (10 µg/mL in 10 mM NaOAc, pH 4.5) was injected onto the activated sensor for 8 min at a flow rate of 10 µL/min to cause CD44 coating to a final density of 2000 resonance units. Unreacted activated carboxylate groups were quenched by injecting 1
M ethanolamine (pH 8.5) for 300 s. Afterwards, measurements were performed at 25 °C using HBS150T (10 mM HEPES (pH 7.4), 150 mM NaCl, 3.4 mM EDTA, 0.005% (
v/
v) Tween 20) as running buffer. Native HA or oxidized HA derivatives were used as analyte at four different concentrations, ranging from 32.7 to 2.7 µM. The flowrate for the acquisition of interaction data was set to 25 µL/min in all experiments. Association was measured for 120 s, then, dissociation was initiated by perfusing running buffer, and the dissociation phase was monitored for 300 s. Regeneration of the chip surface was performed by injecting of two 60 s pulses of 100 mM glycine (pH 2.5) at a flow rate of 100 µL/min. Interaction data were analyzed using the software TraceDrawer version 1.8.1 (Ridgeview Instruments AB, Uppsala, Sweden), applying a simple Langmuir-type 1:1 interaction model and using global fitting for the rate constants. Association rate constant
kon values and dissociation rates constant
koff values were obtained by fitting data of individual experiments. Equilibrium binding
KD values were deduced using the equation:
All SPR experiments were performed in at least three independent experiments.
4.8. Hydrogel Preparation
Hydrogels were generally prepared in PBS with final polymer contents of 2, 3, 4 and 5% (
w/
w) for proxHA (
Table S1). The polymers were dissolved for 3 h at 37 °C and the 1 or 2% (
w/
w) ADH in PBS was also prepared fresh for every experiment. For all hydrogels, equimolar ratios of aldehyde to hydrazide were used. The ADH solution was added to the polymer solution, thoroughly mixed with the pipette and then the solution was immediately transferred to appropriate cylindrical molds. The gels were allowed to cross-link in petri dishes with added wet tissues to avoid drying out, the 3, 4 and 5 wt% hydrogels for 1 h and the 2% hydrogels for 2 h to achieve sufficient crosslinking before adding them into the incubation media.
4.9. Mechanical Testing
For mechanical testing, as well as the swelling/degradation behavior, hydrogels were prepared in PBS as described in
Section 4.8 using cylindrical molds (4 mm diameter, 4 mm height). Mechanical strength was measured from the resulting hydrogels directly after preparation and after storage in 1 mL PBS for 1 h, and 24 h, 7 days, 14 days, and 21 days. The linear compression tests were performed with a mechanical test instrument (ElectroForce 5500, TA Instruments, Eden Prairie, MN, USA) with a load cell of 250 g and a rate of 0.005 mm/s. The height and diameter of each sample were measured with a sliding caliper before each mechanical testing. The strain
γ was calculated by dividing displacement (disp) of the measurement by the height
h0, as shown Equation (3):
True stress was calculated as follows:
By plotting strain versus true stress and using the linear fit of the strain ranges showing, Young’s modulus was obtained from the slope of the linear fit. On the basis of the incubation period of the hydrogels, the compression depth and the strain ranges for the calculation of Young’s modulus were adjusted, due to the contraction of the hydrogels (0 and 1 h: l = 1 mm, strain 10 to 20%; all other time points: l = 0.5 mm, strain 2.5 to 12.5%).
4.10. Swelling and Degradation Behavior
To observe the swelling or degradation behavior of the hydrogels after incubation in PBS, the hydrogels were taken out of the solution after 1 h, 24 h, 7 d, 14 d, and 21 d; blotted dry using analytical tissues; weighed; and compared to initial hydrogels at time 0 h. The ratios of the weight of incubated hydrogels (
ws) and weight of non-incubated hydrogels (
w) were calculated using the following equation:
4.11. Cell Culture
Human bone marrow-derived mesenchymal stromal cells (hMSC) were used for biological evaluation of the obtained hydrogels. The hMSC were isolated from the cancellous bone of patients undergoing hip replacement (as approved by the Local Ethics Committee of the University of Wuerzburg (186/18) with the written informed consent of each donor patient.) The hMSC were extracted by extensively washing of the bone fragments and bone marrow with PBS. Following this, the cell containing suspension was centrifuged and the obtained cell pellet was resuspended in proliferation medium (DMEM/F12, supplemented with 10% FCS, 1% PS, 50 μg/mL L-ascorbic acid, 2-phosphate sesquimagnesium salt hydrate, and 5 ng/mL bFGF (BioLegend, London, UK)) and seeded into T175 cm2 flasks (Greiner Bio-One, Frickenhausen, Germany). After several days the nonadherent cells were removed by washing carefully with PBS and adherent cells were cultured to a sub-confluent level at 37 °C and 5% CO2 in proliferation medium. Finally, MSCs were detached with 0.25% trypsin-EDTA and seeded at a density of 3–5×104 cells mL−1 into T175 cm2 flasks.
4.12. MSC Encapsulation in Blend Hydrogels
Blend hydrogels were prepared as described in
Section 4.8 for encapsulation of MSCs. Briefly, proxHA was sterilized using germicidal UV light at 254 nm for 20 min (UVL hand lamp with filter, A. Hartenstein, Wuerzburg, Germany). Afterwards, the proxHA was dissolved in PBS to achieve final polymer contents of 3, 4, and 5% (
Table S2). The ADH solution was sterile filtered through a 0.2 µm syringe filter.
Initially, MSCs at Passage 4 were resuspended in the corresponding blend proxHA solutions. Then, the cell-laden proxHA solution was mixed with the ADH solution separately for each individual hydrogel and immediately transferred into a silicon mold with 6 mm diameter and 2 mm height (60 µL). The hydrogel precursor solutions were allowed to gel for 30 min at 37 °C and 5% CO2 in humidified environment. Then, the hydrogels were transferred into the cell culture medium using a spatula and were cultivated in vitro for 21 days either in proliferation medium or in chondrogenic medium (Dulbecco’s modified Eagle’s medium high glucose 4.5 g/L (DMEM) supplemented with 1% ITS + Premix (Corning, NY, USA), 40 μg/mL L-proline, 50 μg/mL L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate, 0.1 μM dexamethasone, 1 mM sodium pyruvate, 1% PS, and 10 ng/mL transforming growth factor-β1 (TGF-β1, BioLegend, London, UK)).
4.13. Cell Viability Assay
The viability of encapsulated MSCs cultivated in proliferation medium was analyzed on days 1, 7, and 21 using calcein acetoxymethyl ester (calcein-AM) to detect viable cells and ethidium homodimer-I (EthD-I) for the detection of dead cells. Therefore, the cell containing hydrogels were washed two times with PBS and were subsequently incubated for 45 min at RT in the staining solution (1 µM EthD-I, 2 µM calcein-AM). Then, the constructs were washed with PBS and top view images were taken using a fluorescence microscope (Axio Observer Z1, equipped with epifluorescence optics and an MRm camera, Carl Zeiss, Jena, Germany). The ratio of viable and dead cells was determined for three samples per condition, counting at least at eight different locations within each sample using “Find Maxima” (settings for “live” and “dead” channel: prominence = 75, strict, exclude edge maxima, output = count) with Fiji [
46].
4.14. Histology and Immunohistochemistry
The hydrogel constructs undergoing chondrogenic differentiation were harvested on day 21 and fixed in ROTI®Histofix 4% for 60 min, washed afterwards in PBS, and incubated in Tissue Tek® O.C.T. (Sakura Finetek, Torrance, CA, USA) overnight at 4 °C. The next day, the constructs were transferred into fresh O.C.T., shock frozen in liquid nitrogen, and stored at −20 °C until the cryosection procedure. Longitudinal sections of 8 μm thickness were prepared and collected on Super Frost® plus glass slides (R. Langenbrinck, Emmendingen, Germany).
For histology, the samples were stained with Weigert’s hematoxylin, fast green, and safranin O to analyze the distribution of GAG [
47], and with Weigert’s hematoxylin and picrosirius red to stain for collagen distribution [
48].
For immunohistochemistry, the sections were washed in ddH2O, and antigen retrieval was performed using proteinase K for 5 min at RT. The blocking was performed with 1% bovine serum albumin (BSA) in PBS for 30 min and primary antibodies were diluted in 1% BSA in PBS and incubated overnight in a humidified chamber at RT. Antibodies against collagen type II (II-4C11, 1:100, Sigma-Aldrich, St. Louis, MO, USA) and aggrecan (969D4D11, 1:300, Thermo Fisher Scientific, Waltham, MA, USA) were used. Sections were washed three times in PBS, and secondary antibodies were diluted in 1% BSA in PBS and applied in the dark for 1 h. A goat anti-mouse (Alexa Fluor 488, 1:100, (115-545-068, Jackson ImmunoResearch, Dianova, Hamburg, Germany)) secondary antibody was used. Finally, the slides were washed three times in PBS and mounted with DAPI mounting medium ImmunoSelect®. Images were taken with a fluorescence microscope (Axio Observer Z1, equipped with epifluorescence optics, an MRm camera, and an Apotome, Carl Zeiss, Jena, Germany).
4.15. Biochemical Analyses
For analyses of DNA, GAG, and collagen content the constructs were digested in 0.5 mL of a papain solution (3 U/mL) for 16 h at 60 °C. Prior to digestion, the constructs were homogenized at 25 Hz for 5 min using a TissueLyser LT (Qiagen, Hilden, Germany).
The DNA content of the hydrogels was determined employing a Quant-iT™PicoGreen
® dsDNA Reagent and Kit (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The DNA quantification was carried out fluorometrically at λ
ex = 485 nm and λ
em = 538 nm, using a microplate reader (Spark 20M, Tecan, Maennedorf, Switzerland) and a Lambda DNA standard. The amount of produced GAG was measured using the DMMB assay. The GAG amount was determined with a spectrophotometer at 525 nm, using bovine chondroitin sulfate as standard [
49]. The content of hydroxyproline was measured, after acid hydrolysis and reaction with DAB and chloramine T. The quantification was carried out with a spectrophotometer at 570 nm, using L-hydroxyproline as standard [
50,
51].
4.16. Statistics
Sigma Plot 12.5 (Systat Software GmbH, Erkrath, Germany) was used for statistical analysis and Origin 2018b (OriginLab Corp., Northampton, MA, USA) for graphical plots. Data are reported as the mean and standard deviation from at least three replicates, if not stated otherwise. For GAG and collagen production, multiple groups were compared using one-way ANOVA with Tukey’s post hoc test. Two-way ANOVA with Tukey’s post hoc test was used with time (days 1 and 7) and blend hydrogel contents (3%, 4%, and 5%) for cell viability. Significant differences are marked as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.