Clerodane Diterpenoids from an Edible Plant Justicia insularis: Discovery, Cytotoxicity, and Apoptosis Induction in Human Ovarian Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Samples
2.3. Extraction Procedure for Justicia insularis
2.4. Solvent Partition of Plant Extracts
2.5. Bioassay-Guided Purification of Bioactive Fraction of Justicia insularis
2.6. Isolation of Compound 1 and 2 Using High Performance Liquid Chromatography (HPLC)
2.7. Quantification of Compound 1 in the Extracts and Plant Materials
2.8. Gas Chromatography Mass Spectrometry Analysis
2.9. Liquid Chromatography Mass Spectrometry (LC-MS) Analysis
2.10. NMR Spectroscopy
2.11. Cell Culture
2.12. Sulforhodamine B Cell Growth Inhibitory Assay
2.13. Apoptosis Detection Using Caspase-Glo 3/7, 8 and 9 Activity Assay
2.14. Evaluation of Early and Late Apoptosis Using Flow Cytometry
2.15. Bioinformatic Analysis
2.16. Statistical Analysis
3. Results
3.1. Bioassay-Guided Isolation of Diterpenoids from J. insularis
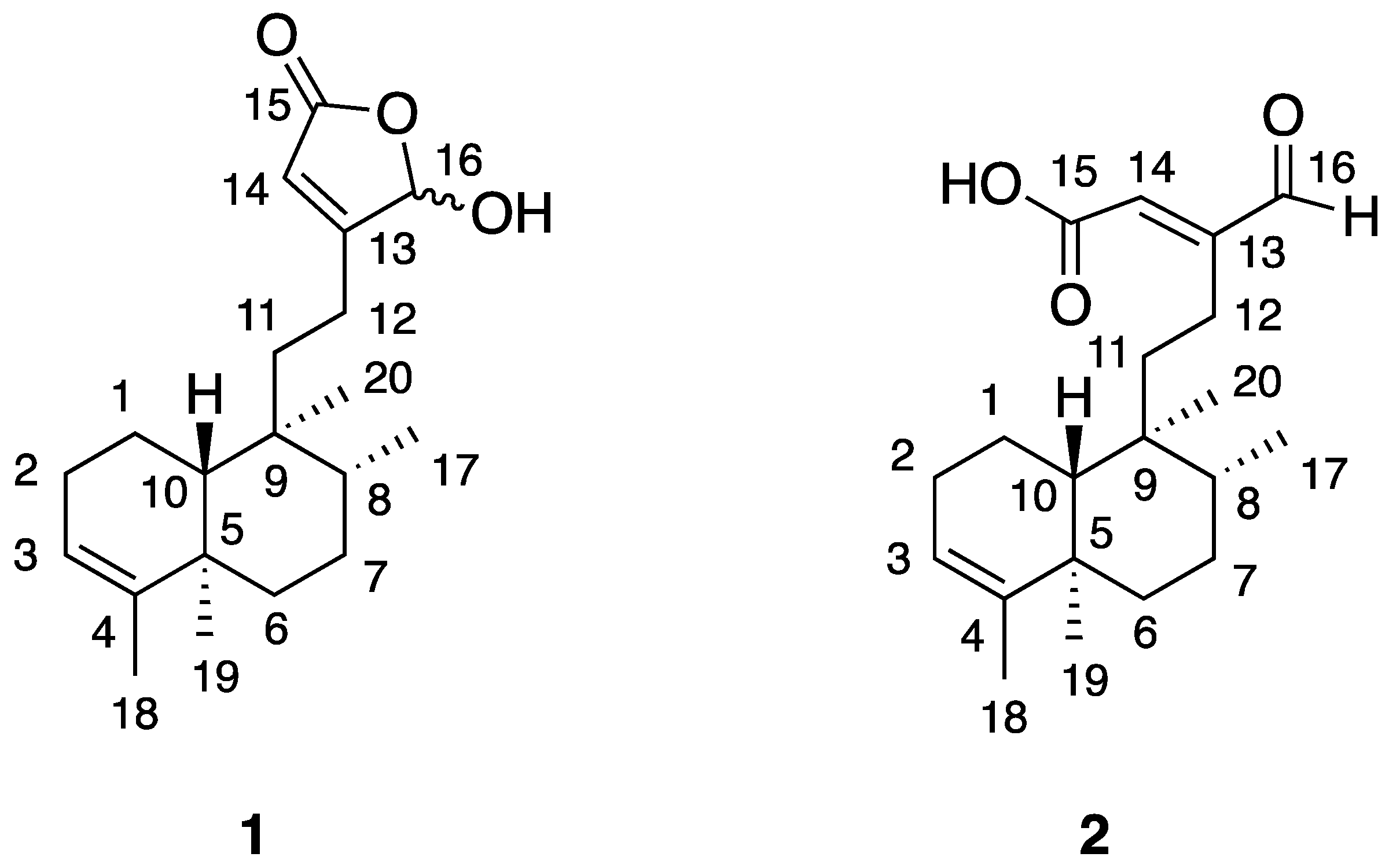
3.2. Chemical Identification of the Isolated Bioactive Compounds of J. insularis
3.3. In Vitro Cytotoxicity of Compounds 1 and 2
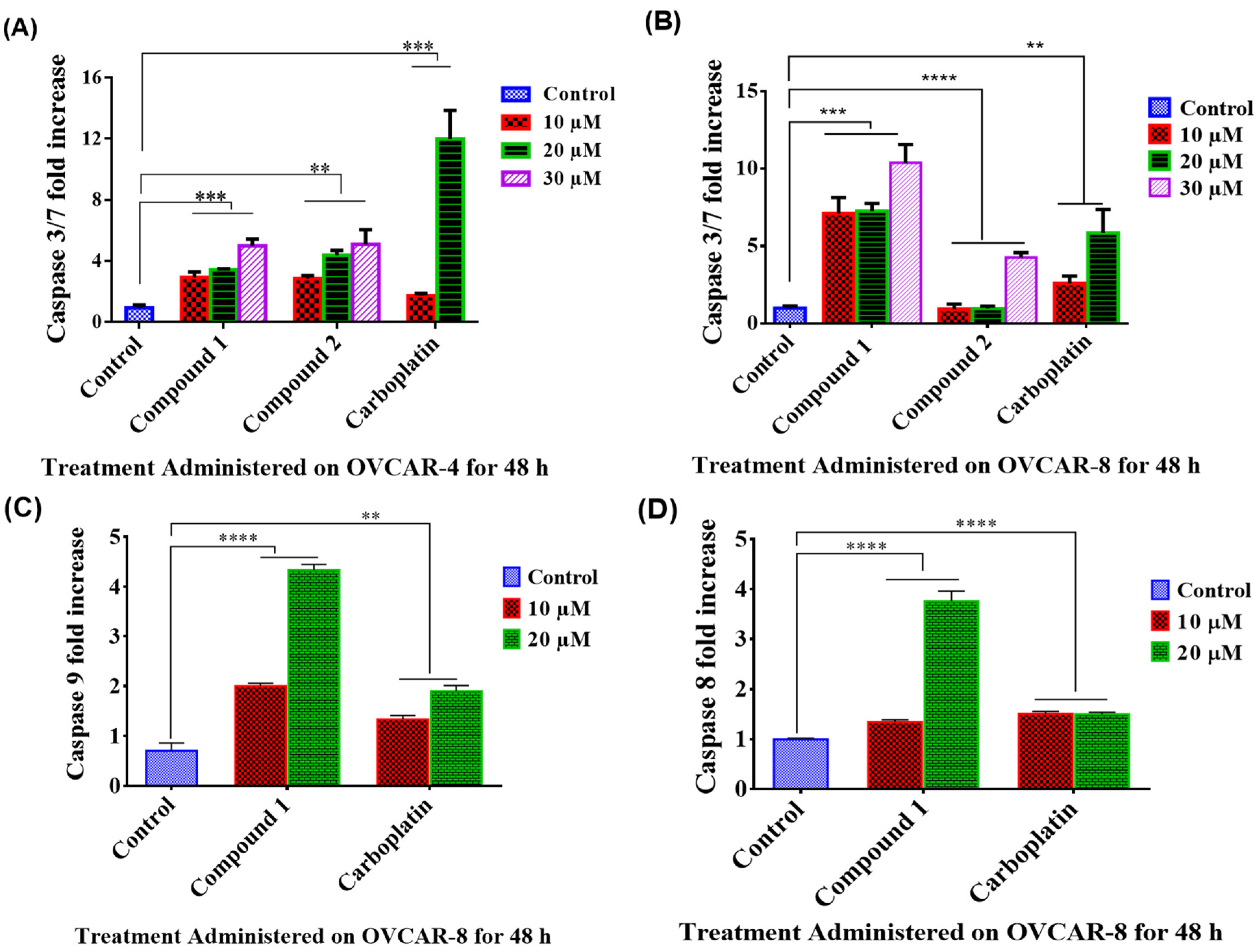
3.4. Apoptosis Study
3.5. Bioinformatic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Johnson-Ajinwo, O.R.; Uche, F.I. Advances of Plant-Derived Natural Products in Ovarian Cancer Therapy. Int. J. Cancer Res. Prev. 2016, 9, 81–135. [Google Scholar]
- Kuroki, L.; Guntupalli, S.R. Treatment of epithelial ovarian cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandrich, L.; Caputo, E. Brassicaceae-Derived Anticancer Agents: Towards a Green Approach to Beat Cancer. Nutrients 2020, 12, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madunic, J.; Madunic, I.V.; Gajski, G.; Popic, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Khor, T.O.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, B.V.; Rammohan, A.; Babu, T.M.; Zheng, G.Y.; Chen, W.; Rajendra, W.; Zyryanov, G.V.; Gu, W. Molecular insight into isoform specific inhibition of PI3K-α and PKC-η with dietary agents through an ensemble pharmacophore and docking studies. Sci Rep. 2021, 11, 12150. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Cytotoxic effects of stem bark extracts and pure compounds from Margaritaria discoidea on human ovarian cancer cell lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Uche, F.I.; Drijfhout, F.P.; McCullagh, J.; Richardson, A.; Li, W.W. Cytotoxicity Effects and Apoptosis Induction by Bisbenzylisoquinoline Alkaloids from Triclisia subcordata. Phytother. Res. 2016, 30, 1533–1539. [Google Scholar] [CrossRef]
- Uche, F.I.; Abed, M.N.; Abdullah, M.I.; Drijfhout, F.P.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.W. Isolation, identification and anti-cancer activity of minor alkaloids from Triclisia subcordata Oliv. Biochem Pharm. 2017, 139, 112. [Google Scholar] [CrossRef]
- Uche, F.I.; Abed, M.N.; Abdullah, M.I.; Drijfhout, F.P.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.W. Isochondodendrine and 2′-norcocsuline: Additional alkaloids from Triclisia subcordata induce cytotoxicity and apoptosis in ovarian cancer cell lines. Rsc Adv. 2017, 7, 44154–44161. [Google Scholar] [CrossRef] [Green Version]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Identification and evaluation of anticancer compounds from three Nigerian plants used in traditional medicines. Biochem. Pharm. 2017, 139, 128. [Google Scholar] [CrossRef]
- Uche, F.I.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.W. Synthesis of (aminoalkyl)cycleanine analogues: Cytotoxicity, cellular uptake, and apoptosis induction in ovarian cancer cells. Bioorg. Med. Chem. Lett. 2018, 28, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Palmatine from Unexplored Rutidea parviflora Showed Cytotoxicity and Induction of Apoptosis in Human Ovarian Cancer Cells. Toxins 2019, 11, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uche, F.I.; Guo, X.; Okokon, J.; Ullah, I.; Horrocks, P.; Boateng, J.; Huang, C.; Li, W.W. In Vivo Efficacy and Metabolism of the Antimalarial Cycleanine and Improved In Vitro Antiplasmodial Activity of Semisynthetic Analogues. Antimicrob. Agents Chemother. 2021, 65, e01995-20. [Google Scholar] [CrossRef] [PubMed]
- Telefo, P.B.; Moundipa, P.F.; Tchouanguep, F.M. Inductive effect of the leaf mixture extract of Aloe buettneri, Justicia insularis, Dicliptera verticillata and Hibiscus macranthus on in vitro production of estradiol. J. Ethnopharmacol. 2004, 91, 225–230. [Google Scholar] [CrossRef]
- Ajibesin, K.K.; Ekpo, B.A.; Bala, D.N.; Essien, E.E.; Adesanya, S.A. Ethnobotanical survey of Akwa Ibom State of Nigeria. J. Ethnopharmacol. 2008, 115, 387–408. [Google Scholar] [CrossRef]
- Telefo, P.B.; Tagne, S.R.; Koona, O.E.; Yemele, D.M.; Tchouanguep, F.M. Effect of the aqueous extract of Justicia insularis T. Anders (Acanthaceae) on ovarian folliculogenesis and fertility of female rats. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeyemi, O.T.; Babatunde, O. Chemical composition and anti-oxidant capacity of the leaf extract of Justicia insularis. Intern. J. Phys. Sci. 2014, 9, 454–458. [Google Scholar]
- Correa, G.M.; Alcantara, A.F.D. Chemical constituents and biological activities of species of Justicia—A review. Rev. Bras. Farm. 2012, 22, 220–238. [Google Scholar] [CrossRef]
- Wood, J.; Yasmin-Karim, S.; Moreau, M.; Kumar, R.; Akwanwi, J.; Derek, A.; Atoneche, F.; Kress, J.; Ngwa, A.W. Characterization of Isolated Extracts from Justicia Plant Leaves used as Remedy for Anemia. Molecules 2020, 25, 534. [Google Scholar] [CrossRef] [Green Version]
- Logue, S.E.; Elgendy, M.; Martin, S.J. Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat. Protoc. 2009, 4, 1383–1395. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.swissadme.ch/index.php (accessed on 23 September 2021).
- Available online: http://www.swisstargetprediction.ch/ (accessed on 23 September 2021).
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Phadnis, A.P.; Patwardhan, S.A.; Dhaneshwar, N.N.; Tavale, S.S.; Row, T.N.G. Clerodane Diterpenoids from Polyalthia-longifolia. Phytochemistry 1988, 27, 2899–2901. [Google Scholar] [CrossRef]
- Hara, N.; Asaki, H.; Fujimoto, Y.; Gupta, Y.K.; Singh, A.K.; Sahai, M. Clerodane and Ent-Halimane Diterpenes from Polyalthia-longifolia. Phytochemistry 1995, 38, 189–194. [Google Scholar] [CrossRef]
- Muller, D.S.; Untiedt, N.L.; Dieskau, A.P.; Lackner, G.L.; Overman, L.E. Constructing Quaternary Stereogenic Centers Using Tertiary Organocuprates and Tertiary Radicals. Total Synthesis of trans-Clerodane Natural Products. J. Am. Chem. Soc. 2015, 137, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Aranjani, J.M.; Pai, K.S.; Srinivasan, K.K. Promising anticancer activities of Justicia simplex D. Don. in cellular and animal models. J. Ethnopharmacol. 2017, 199, 231–239. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Zhang, P.; Dong, X.Z.; Yang, M.H.; Chen, S.L.; Bi, M.G. JR6, a new compound isolated from Justicia procumbens, induces apoptosis in human bladder cancer EJ cells through caspase-dependent pathway. J. Ethnopharmacol 2012, 144, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.P.; Yang, S.; Dong, J.X.; Jin, H. New cyclopeptide alkaloids from the whole plant of Justicia procumbens L. Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Lee, I.S.; Chai, H.B.; Zaw, K.; Farnsworth, N.R.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic clerodane diterpenes from Polyalthia barnesii. Phytochemistry 1994, 37, 1659–1662. [Google Scholar] [CrossRef]
- Duan, X.Y.; Guo, K.Y.; Lv, D.J.; Mei, R.Q.; Zhang, M.D. Terpenes isolated from Polyalthia simiarum and their cytotoxic activities. Fitoterapia 2020, 147, 104734. [Google Scholar] [CrossRef]
- Fukamiya, N.; Lee, K.H. Antitumor agents, 81. Justicidin-A and diphyllin, two cytotoxic principles from Justicia procumbens. J. Nat. Prod. 1986, 49, 348–350. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, C.H.; Su, C.L.; Huang, C.W.; Liu, H.S.; Lin, C.N.; Won, S.J. Justicidin A decreases the level of cytosolic Ku70 leading to apoptosis in human colorectal cancer cells. Carcinogenesis 2005, 26, 1716–1730. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagaron, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.P.; Ninomiya, M.; Efdi, M.; Santoni, A.; Ibrahim, S.; Tanaka, K.; Koketsu, M. Clerodane diterpenes isolated from Polyalthia longifolia induce apoptosis in human leukemia HL-60 cells. J. Oleo Sci. 2013, 62, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.H.; Lee, C.C.; Chang, F.R.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-hydroxycleroda-3,13-dien-15,16-olide regulates the expression of histone-modifying enzymes PRC2 complex and induces apoptosis in CML K562 cells. Life Sci. 2011, 89, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lee, C.C.; Chan, W.L.; Chang, W.H.; Wu, Y.C.; Chang, J.G. 16-Hydroxycleroda-3,13-dien-15,16-olide deregulates PI3K and Aurora B activities that involve in cancer cell apoptosis. Toxicology 2011, 285, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.F.; Lin, S.R.; Tseng, F.J.; Huang, Y.C.; Tsai, M.J.; Fu, Y.S.; Weng, C.F. The autophagic inhibition oral squamous cell carcinoma cancer growth of 16-hydroxy-cleroda-3,14-dine-15,16-olide. Oncotarget 2017, 8, 78379–78396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Lee, W.C.; Huang, B.M.; Chia, Y.C.; Chen, Y.C.; Chen, Y.C. 16-Hydroxycleroda-3, 13-dien-15, 16-olide inhibits the proliferation and induces mitochondrial-dependent apoptosis through Akt, mTOR, and MEK-ERK pathways in human renal carcinoma cells. Phytomedicine 2017, 36, 95–107. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, B.M.; Lee, W.C.; Chen, Y.C. 16-Hydroxycleroda-3,13-dien-15,16-olide induces anoikis in human renal cell carcinoma cells: Involvement of focal adhesion disassembly and signaling. OncoTargets Ther. 2018, 11, 7679–7690. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Wang, P.Y.; Huang, B.M.; Chen, Y.J.; Lee, W.C.; Chen, Y.C. 16-Hydroxycleroda-3,13-dien-15,16-olide Induces Apoptosis in Human Bladder Cancer Cells through Cell Cycle Arrest, Mitochondria ROS Overproduction, and Inactivation of EGFR-Related Signalling Pathways. Molecules 2020, 25, 3958. [Google Scholar] [CrossRef]
- Velmurugan, B.K.; Wang, P.C.; Weng, C.F. 16-Hydroxycleroda-3,13-dien-15,16-olide and N-Methyl-Actinodaphine Potentiate Tamoxifen-Induced Cell Death in Breast Cancer. Molecules 2018, 23, 1966. [Google Scholar] [CrossRef] [Green Version]
- Misra, P.; Sashidhara, K.V.; Singh, S.P.; Kumar, A.; Gupta, R.; Chaudhaery, S.S.; Gupta, S.S.; Majumder, H.K.; Saxena, A.K.; Dube, A. 16alpha-Hydroxycleroda-3,13 (14)Z-dien-15,16-olide from Polyalthia longifolia: A safe and orally active antileishmanial agent. Br. J. Pharm. 2010, 159, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Sashidhara, K.V.; Singh, S.P.; Srivastava, A.; Puri, A.; Chhonker, Y.S.; Bhatta, R.S.; Shah, P.; Siddiqi, M.I. Discovery of a new class of HMG-CoA reductase inhibitor from Polyalthia longifolia as potential lipid lowering agent. Eur. J. Med. Chem. 2011, 46, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Vu, T.Y.; Chandi, V.; Polimati, H.; Tatipamula, V.B. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020, 10, 15965. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Cheng, Y.Y.; Chen, C.J.; Ng, L.T.; Chou, L.C.; Huang, L.J.; Chen, Y.H.; Kuo, S.C.; El-Shazly, M.; Wu, Y.C.; et al. Three new clerodane diterpenes from Polyalthia longifolia var. pendula. Molecules 2014, 19, 2049–2060. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.K.; Ahmed, A.; Hussain, M.; Khan, I.A.; Ali, S.A.; Farooq, A.D.; Faizi, S. Antibiofilm potential of 16-oxo-cleroda-3, 13(14) E-diene-15 oic acid and its five new gamma-amino gamma-lactone derivatives against methicillin resistant Staphylococcus aureus and Streptococcus mutans. Eur. J. Med. Chem. 2017, 138, 480–490. [Google Scholar] [CrossRef]
- Islam, M.T. Diterpenes and Their Derivatives as Potential Anticancer Agents. Phytother. Res. 2017, 31, 691–712. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. Biomed. Res. Int. 2015, 2015, 413076. [Google Scholar] [CrossRef]
- Huang, D.M.; Shen, Y.C.; Wu, C.; Huang, Y.T.; Kung, F.L.; Teng, C.M.; Guh, J.H. Investigation of extrinsic and intrinsic apoptosis pathways of new clerodane diterpenoids in human prostate cancer PC-3 cells. Eur. J. Pharm. 2004, 503, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.J.; Sun, J.Y.; Ren, Y.; Liu, K.; Shen, L. Bioactive ent-clerodane diterpenoids from Scutellaria barbata. Planta Med. 2007, 73, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, Q.; Yang, X.; Li, Y.; Zhang, X.; Li, Y.; Du, Q.; Jin, D.Q.; Cui, J.; Lall, N.; et al. Diterpenoids from the leaves of Casearia kurzii showing cytotoxic activities. Bioorg. Chem. 2020, 98, 103741. [Google Scholar] [CrossRef] [PubMed]
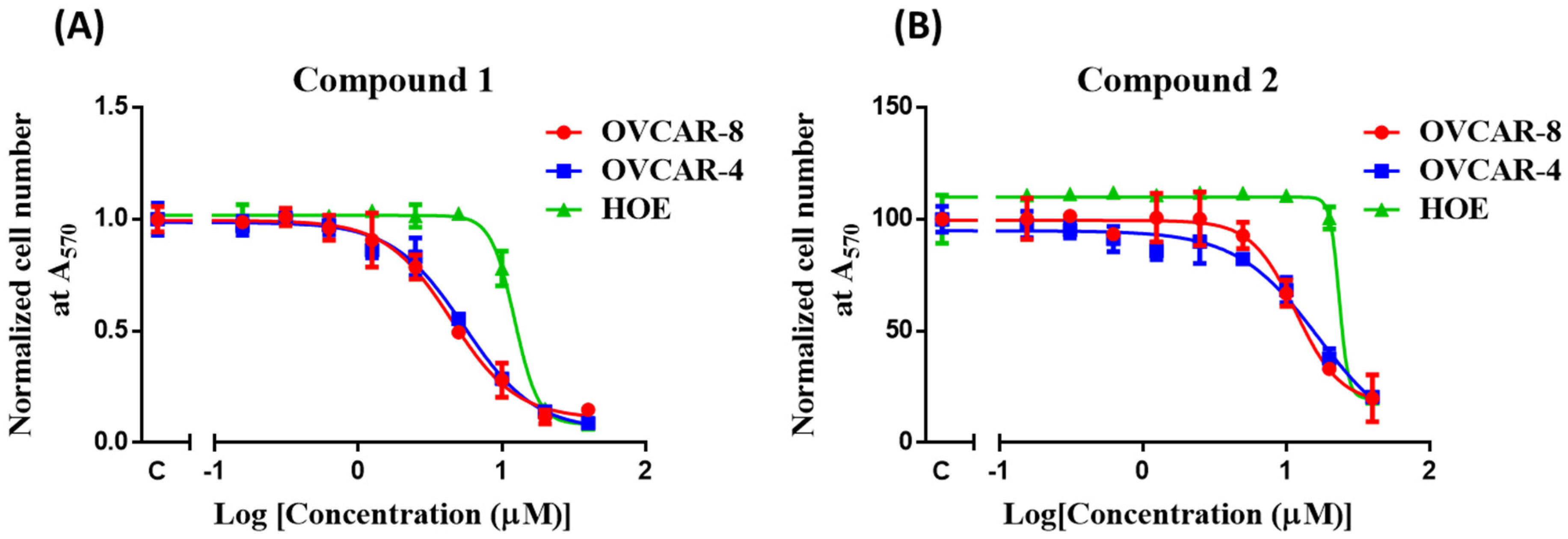

| Compounds | OVCAR-4 (μM) | OVCAR-8 (μM) | HOE (μM) | SI against OVCAR-8 |
|---|---|---|---|---|
| 1 | 5.7 ± 0.3(1.8 μg/mL) | 4.4 ± 0.2 (1.4 μg/mL) | 12.1 ± 0.1 (3.9 μg/mL) | 3 |
| 2 | 16.6 ± 2.8 (5.3 μg/mL) | 11.8 ± 0.5 (3.8 μg/mL) | 22.8 ± 0.7 (7.3 μg/mL) | 2 |
| Carboplatin | 17.6 ± 4.6 | 8.2 ± 2.2 | 13.0 ± 3.7 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadayomi, I.E.; Johnson-Ajinwo, O.R.; Pires, E.; McCullagh, J.; Claridge, T.D.W.; Forsyth, N.R.; Li, W.-W. Clerodane Diterpenoids from an Edible Plant Justicia insularis: Discovery, Cytotoxicity, and Apoptosis Induction in Human Ovarian Cancer Cells. Molecules 2021, 26, 5933. https://doi.org/10.3390/molecules26195933
Fadayomi IE, Johnson-Ajinwo OR, Pires E, McCullagh J, Claridge TDW, Forsyth NR, Li W-W. Clerodane Diterpenoids from an Edible Plant Justicia insularis: Discovery, Cytotoxicity, and Apoptosis Induction in Human Ovarian Cancer Cells. Molecules. 2021; 26(19):5933. https://doi.org/10.3390/molecules26195933
Chicago/Turabian StyleFadayomi, Idowu E., Okiemute R. Johnson-Ajinwo, Elisabete Pires, James McCullagh, Tim D.W. Claridge, Nicholas R. Forsyth, and Wen-Wu Li. 2021. "Clerodane Diterpenoids from an Edible Plant Justicia insularis: Discovery, Cytotoxicity, and Apoptosis Induction in Human Ovarian Cancer Cells" Molecules 26, no. 19: 5933. https://doi.org/10.3390/molecules26195933
APA StyleFadayomi, I. E., Johnson-Ajinwo, O. R., Pires, E., McCullagh, J., Claridge, T. D. W., Forsyth, N. R., & Li, W.-W. (2021). Clerodane Diterpenoids from an Edible Plant Justicia insularis: Discovery, Cytotoxicity, and Apoptosis Induction in Human Ovarian Cancer Cells. Molecules, 26(19), 5933. https://doi.org/10.3390/molecules26195933








