Tuliposides H–J and Bioactive Components from the Bulb of Amana edulis
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidations of Isolates 1–3
2.2. Chemical Components Elucidation of TEW
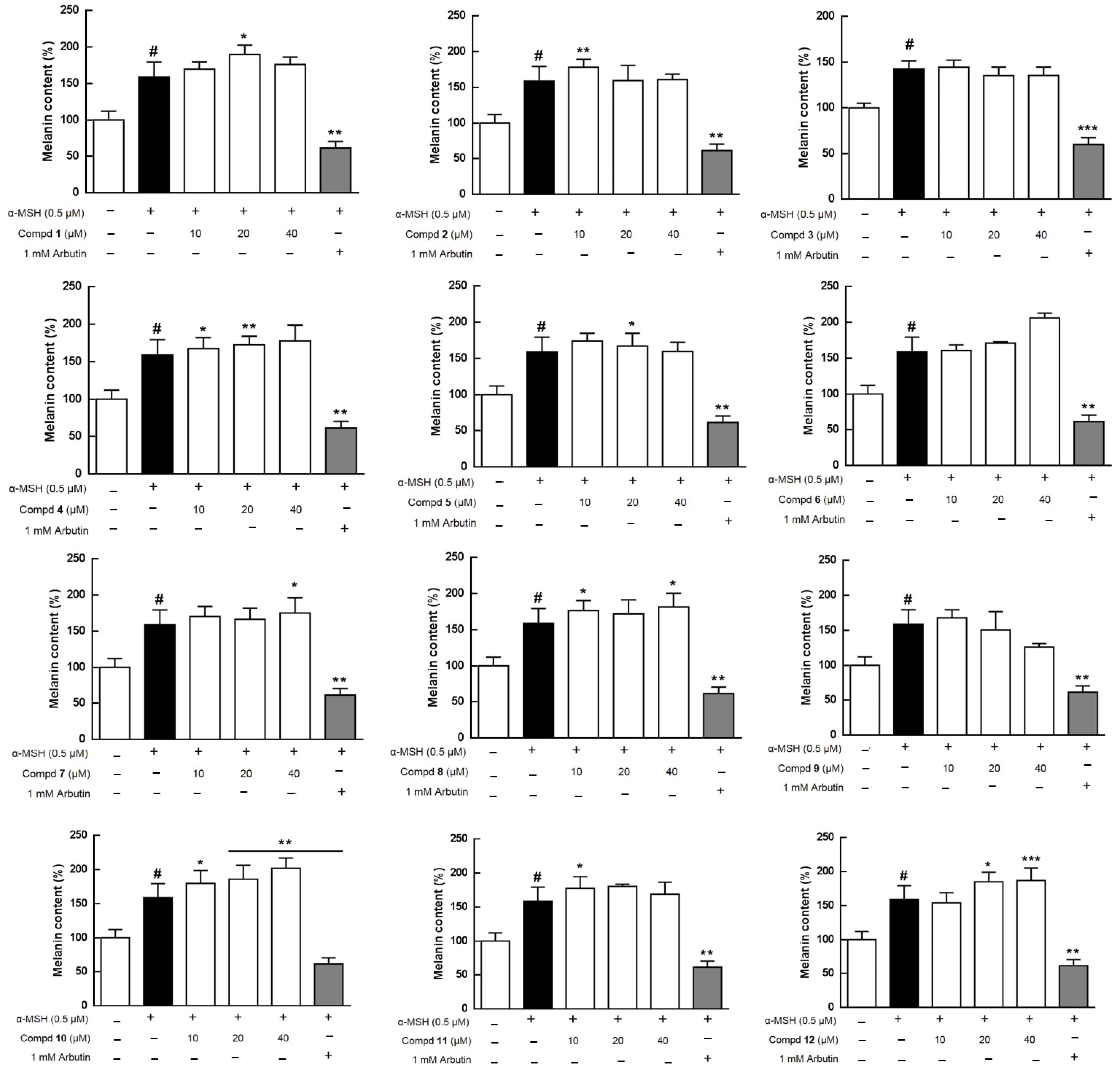
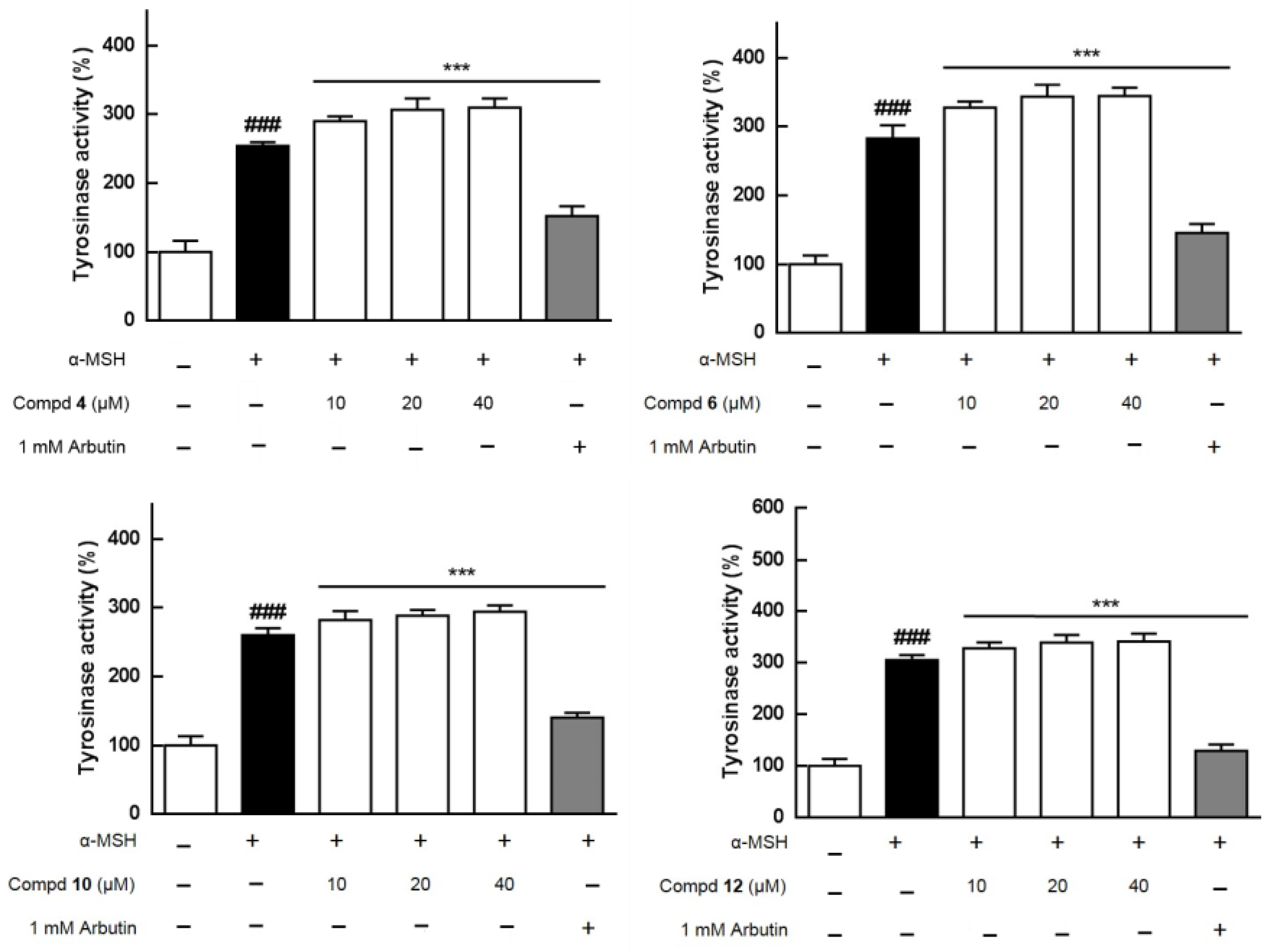
2.3. Effects of Extracts and Isolates on Melanogenesis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Tuliposide H (1)
3.3.2. Tuliposide I (2)
3.3.3. Tuliposide J (3)
3.4. (R)- and (S)-MTPA Derivatives of 1
3.4.1. (S)-MTPA Ester of 1 (1a)
3.4.2. (R)-MTPA Ester of 1 (1b)
3.5. Acid Hydrolysis of Tuliposide I (2)
3.6. Cell Culture
3.7. Measurement of B16 Cell Viability
3.8. Measurement of Melanin Content in B16 Cells
3.9. Intracellular Tyrosinase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lin, R.; Li, Z.; Lin, J.; Ye, J.; Cai, Q.; Chen, L.; Peng, J. Ethanolic extract of Tulipa edulis Bak induces apoptosis in SGC-7901 human gastric carcinoma cells via the mitochondrial signaling pathway. Oncol. Lett. 2015, 10, 2371–2377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, Y.; Hou, X.; Guo, P.; Lv, X.; Zhao, L.; Wang, H.; Zhou, L.; Feng, Y. Extraction of Amana edulis induces liver cancer apoptosis. Evid.-Based Complement. Altern. Med. 2018, 2018, 3927075. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Wei, Z.J. Physicochemical and antioxidant potential of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 131, 453–460. [Google Scholar] [CrossRef]
- Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Wei, Z.J. The rheological properties and emulsifying behavior of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 137, 160–168. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Hu, F.; Zhang, J.G.; Wei, Z.J. Effects of sulfated, phosphorylated and carboxymethylated modifications on the antioxidant activities in-vitro of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2020, 146, 887–896. [Google Scholar] [CrossRef]
- Lai, K.Y.; Hu, H.C.; Chiang, H.M.; Liu, Y.J.; Yang, J.C.; Lin, Y.A.; Chen, C.J.; Chang, Y.S.; Lee, C.L. New diterpenes leojaponins G−L from Leonurus japonicus. Fitoterapia 2018, 130, 125−133. [Google Scholar] [CrossRef]
- Ullah, S.; Chung, Y.C.; Hyun, C.G. Induction of melanogenesis by fosfomycin in B16F10 cells through the upregulation of P-JNK and P-p38 signaling pathways. Antibiotics 2020, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Kristiansen, K. Isolation and quantification of tuliposides and tulipalins in tulips (Tulipa) by high-performance liquid chromatography. Contact Dermat. 1999, 40, 300−309. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Hantupeptins B and C, cytotoxic cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 307−311. [Google Scholar] [CrossRef]
- Qabaja, G.; Wilent, J.E.; Benavides, A.R.; Bullard, G.E.; Petersen, K.S. Facile synthesis of versatile enantioenriched α-substituted hydroxy esters through a Brønsted acid catalyzed kinetic resolution. Org. Lett. 2013, 15, 1266−1269. [Google Scholar] [CrossRef]
- Nomura, T.; Kato, Y. Identification of tuliposide G, a novel glucoside ester-type tuliposide, and its distribution in tulip. Z. Naturforsch. C. J. Biosci. 2020, 75, 75−86. [Google Scholar] [CrossRef]
- Gang, F.L.; Zhu, F.; Li, X.T.; Wei, J.L.; Wu, W.J.; Zhang, J.W. Synthesis and bioactivities evaluation of L-pyroglutamic acid analogues from natural product lead. Bioorg. Med. Chem. 2018, 26, 4644–4649. [Google Scholar] [CrossRef]
- Vereshchagin, A.L.; Anikina, E.V.; Syrchina, A.I.; Lapin, M.F.; Azin, L.A.; Semenov, A.A. Chemical investigation of the bitter substances of the fruit of Lonicera caerulea. Chem. Nat. Compd. 1989, 25, 289−292. [Google Scholar] [CrossRef]
- Jaime, C.; Ortuño, R.M.; Font, J. Di- and trisubstituted γ-lactones. Conformational study by molecular mechanics calculations and coupling constant analysis. J. Org. Chem. 1986, 51, 3946−3951. [Google Scholar] [CrossRef]
- Larchevêque, M.; Henrot, S. Enantiomerically pure β,γ-epoxyesters from β-hydroxylactones: Synthesis of β-hydroxyesters and (−)-GABOB. Tetrahedron 1990, 46, 4277−4282. [Google Scholar] [CrossRef]
- Jaime, C.; Segura, C.; Dinarés, I.; Font, J. Substituted γ-lactones with vicinal hydrogen atoms. Conformation study by MM2 calculations and coupling constant analysis. J. Org. Chem. 1993, 58, 154−158. [Google Scholar] [CrossRef]
- Mori, K.; Fukamatsu, K. A synthesis of (1R,5S)-(+)-frontalin from (S)-(−)-2-hydroxyparaconic acid. Liebigs Ann. Chem. 1992, 11, 1191−1193. [Google Scholar]
- Miyazawa, M.; Anzai, J.; Fujioka, J.; Isikawa, Y. Insecticidal compounds against Drosophila melanogaster from Cornus officinalis Sieb. et Zucc. Nat. Prod. Res. 2003, 17, 337−339. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Wang, C.M.; Hu, H.C.; Yen, H.R.; Song, Y.C.; Yu, S.J.; Chen, C.J.; Li, W.C.; Wu, Y.C. Indole alkaloids indigodoles A–C from aerial parts of Strobilanthes cusia in the traditional Chinese medicine Qing Dai have anti-IL-17 properties. Phytochemistry 2019, 162, 39−46. [Google Scholar] [CrossRef] [PubMed]
- Faizi, S.; Ali, M.; Saleem, R.; Irfanullah; Bibi, S. Complete 1H- and 13C-NMR assignments of stigma-5-en-3-O-β-glucoside and its acetyl derivative. Magn. Reson. Chem. 2001, 39, 399–405. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.C.; Wu, P.Y.; Wu, C.S.; Sung, P.J.; Lin, C.Y.; Chiang, H.M. N-(4-methoxyphenyl) caffeamide-induced melanogenesis inhibition mechanisms. BMC Complement. Altern. Med. 2017, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092−4096. [Google Scholar] [CrossRef]
- Lee, C.L.; Chang, F.R.; Hsieh, P.W.; Chiang, M.Y.; Wu, C.C.; Huang, Z.Y.; Lan, Y.H.; Chen, M.; Lee, K.H.; Yen, H.F.; et al. Cytotoxic ent-abietane diterpenes from Gelonium aequoreum. Phytochemistry 2008, 69, 276−287. [Google Scholar] [CrossRef] [PubMed]
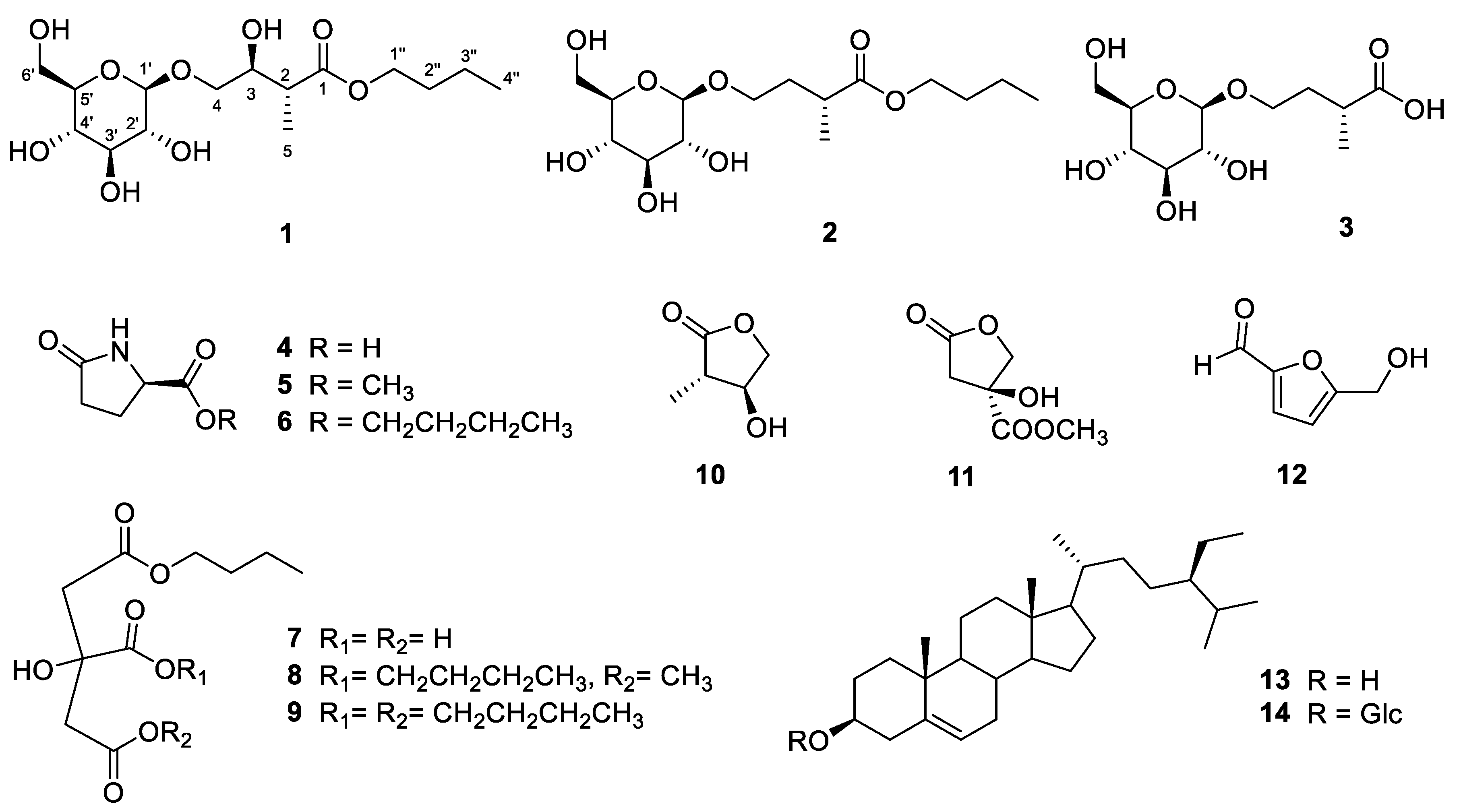
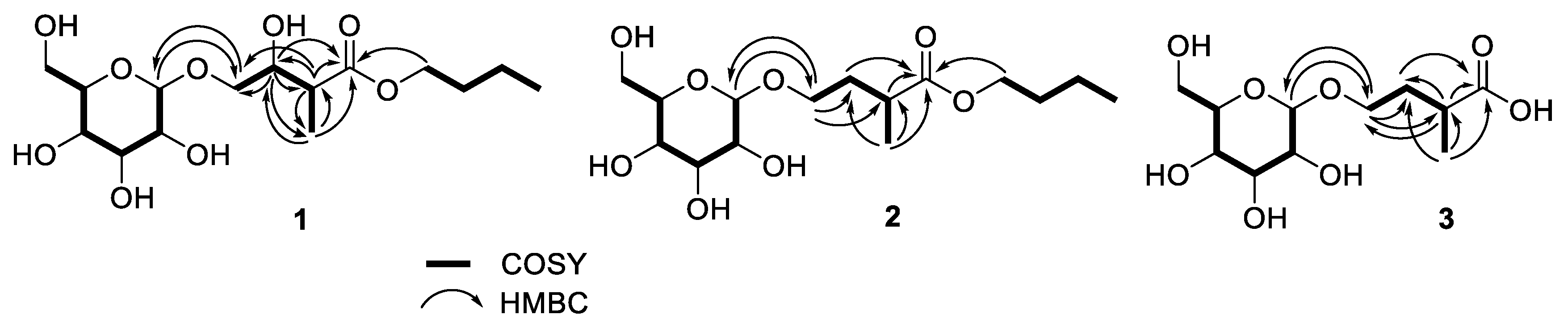

| Tuliposide H (1) | Tuliposide I (2) | Tuliposide J (3) | ||||
|---|---|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC |
| Isoprenoid | ||||||
| 1 | 176.6 | 178.5 | 180.6 | |||
| 2 | 2.67 (quint, 7.0) | 44.6 | 2.63 (sext, 7.0) | 37.8 | 2.63 (sext, 7.0) | 37.6 |
| 3 | 3.89 (td, 7.0, 2.5) | 73.6 | 1.65 (sext, 7.0) 1.96 (sext, 7.0) | 34.6 | 1.68 (sext, 7.0) 2.00 (sext, 7.0) | 34.7 |
| 4 | 3.57 (dd, 10.5, 7.0) 4.01 (dd, 10.5, 2.5) | 73.1 | 3.54 (dt, 10.0, 7.0) 3.87 (dt, 10.0, 7.0) | 68.4 | 3.61 (dt, 10.0, 7.0) 3.92 (dt, 10.0, 7.0) | 68.6 |
| 5 | 1.14 (d, 7.0) | 13.9 | 1.12 (d, 7.0) | 17.5 | 1.17 (d, 7.0) | 17.6 |
| Glc | ||||||
| 1′ | 4.27 (d, 8.0) | 104.9 | 4.19 (d, 8.0) | 104.4 | 4.23 (d, 8.0) | 104.4 |
| 2′ | 3.21 (t, 8.0) | 75.1 | 3.14 (t, 8.0) | 75.6 | 3.16 (t, 8.0) | 75.1 |
| 3′ | 3.35 (t, 8.0) | 77.9 | 3.30 (t, 8.0) | 78.0 | 3.34 (t, 8.0) | 77.9 |
| 4′ | 3.28 (overlap) | 71.6 | 3.22 (overlap) | 71.4 | 3.27 (overlap) | 71.6 |
| 5′ | 3.28 (overlap) | 78.0 | 3.22 (overlap) | 77.8 | 3.27 (overlap) | 78.0 |
| 6′ | 3.66 (dd, 12.0, 5.0) 3.85 (brd, 12.0) | 62.7 | 3.62 (dd, 12.0, 5.5) 3.80 (dd, 12.0, 2.0) | 62.8 | 3.66 (dd, 12.0, 5.5) 3.85 (brd, 12.0) | 62.8 |
| n-butyl | ||||||
| 1″ | 4.10 (2H, t, 7.5) | 65.5 | 4.04 (2H, t, 7.5) | 65.4 | ||
| 2″ | 1.63 (2H, quint, 7.5) | 31.8 | 1.57 (2H, quint, 7.5) | 31.8 | ||
| 3″ | 1.41 (2H, sext, 7.5) | 20.2 | 1.34 (2H, sext, 7.5) | 20.2 | ||
| 4″ | 0.94 (3H, t, 7.5) | 14.0 | 0.90 (3H, t, 7.5) | 14.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Gao, Z.-A.; Jhan, Y.-L.; Chang, Y.-S.; Chen, C.-J. Tuliposides H–J and Bioactive Components from the Bulb of Amana edulis. Molecules 2021, 26, 5907. https://doi.org/10.3390/molecules26195907
Lee C-L, Gao Z-A, Jhan Y-L, Chang Y-S, Chen C-J. Tuliposides H–J and Bioactive Components from the Bulb of Amana edulis. Molecules. 2021; 26(19):5907. https://doi.org/10.3390/molecules26195907
Chicago/Turabian StyleLee, Chia-Lin, Zhi-An Gao, Yun-Lian Jhan, Yuan-Shiun Chang, and Chao-Jung Chen. 2021. "Tuliposides H–J and Bioactive Components from the Bulb of Amana edulis" Molecules 26, no. 19: 5907. https://doi.org/10.3390/molecules26195907
APA StyleLee, C.-L., Gao, Z.-A., Jhan, Y.-L., Chang, Y.-S., & Chen, C.-J. (2021). Tuliposides H–J and Bioactive Components from the Bulb of Amana edulis. Molecules, 26(19), 5907. https://doi.org/10.3390/molecules26195907







