Properties of Mosquito Repellent-Plasticized Poly(lactic acid) Strands
Abstract
1. Introduction
2. Modelling Diffusion-Controlled Repellent Release from Round Polymer Strands
3. Results and Discussion
3.1. Thermogravimetric Analysis
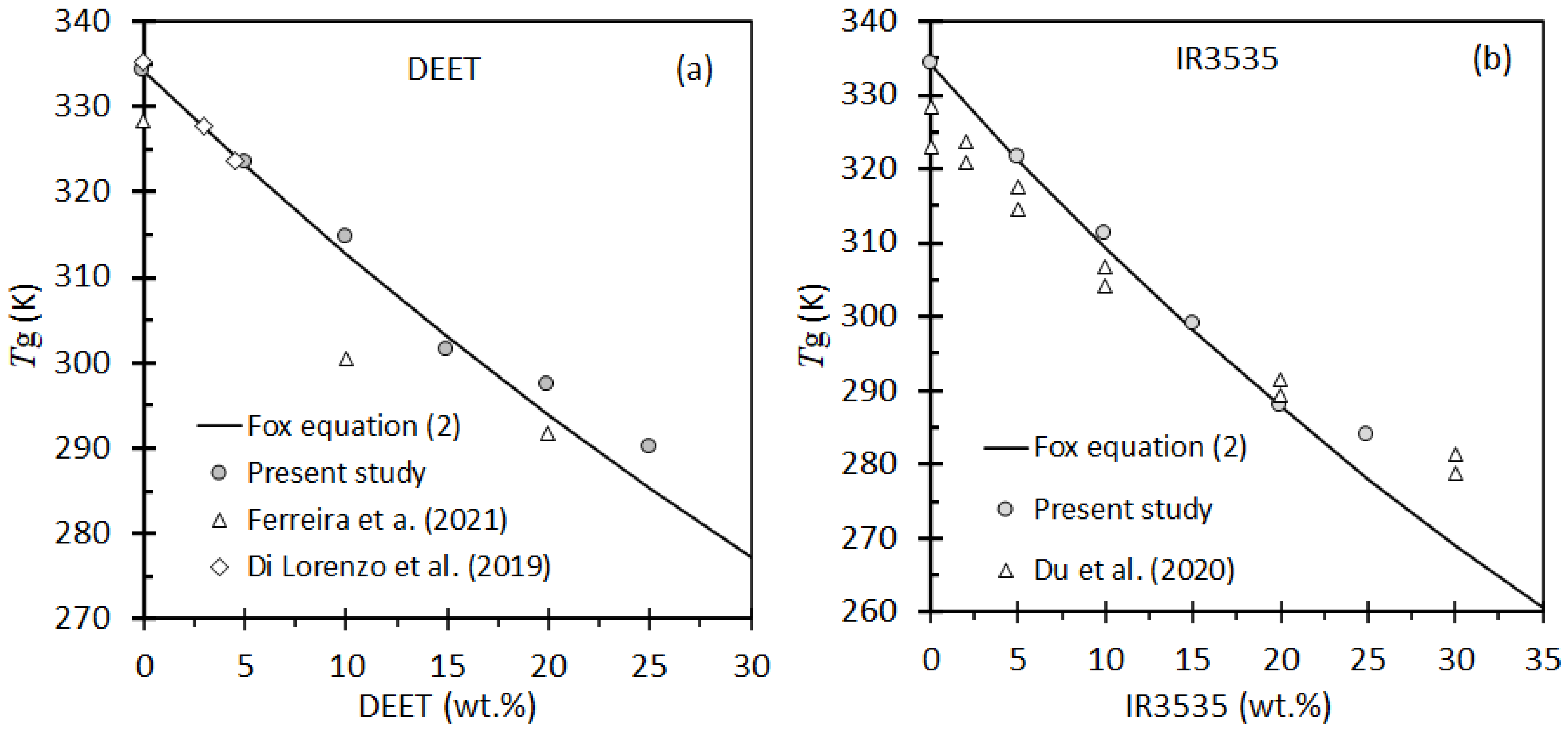
3.2. Differential Scanning Calorimetry
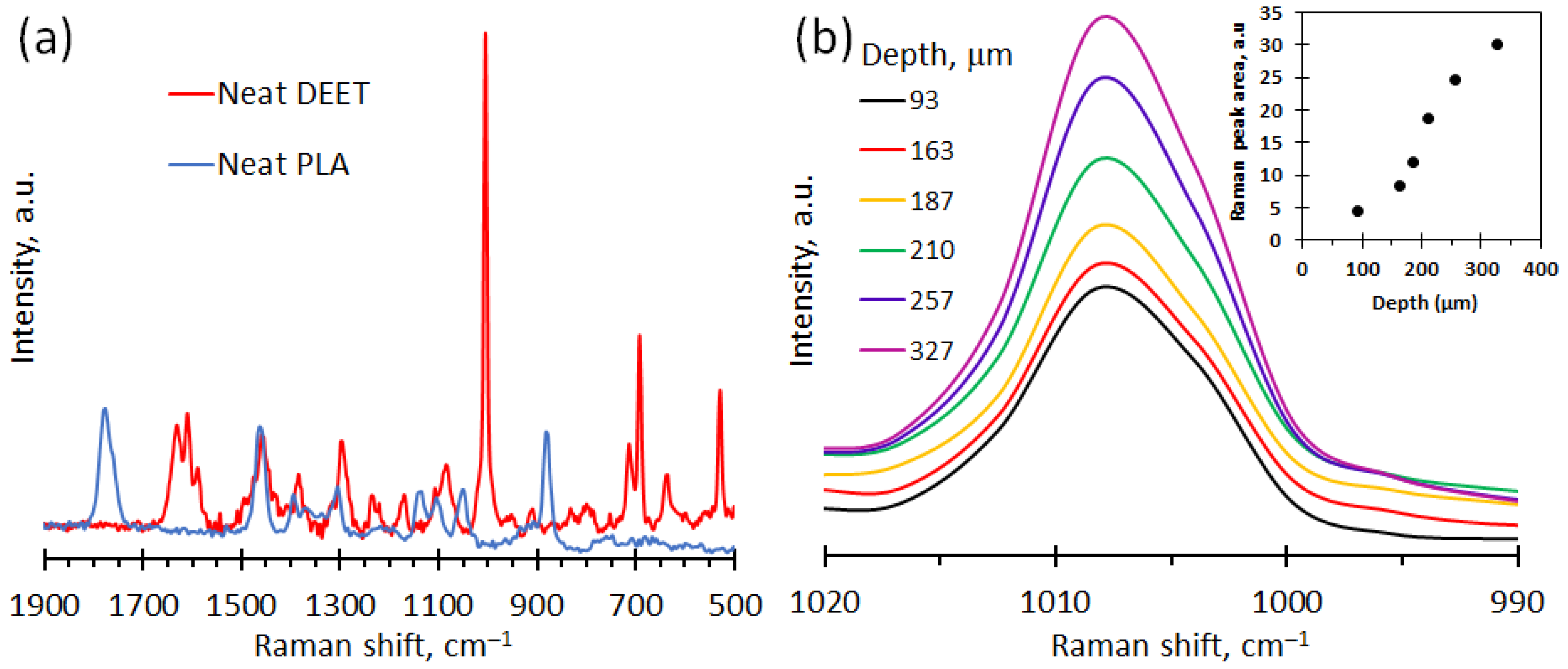
3.3. Raman Spectroscopy
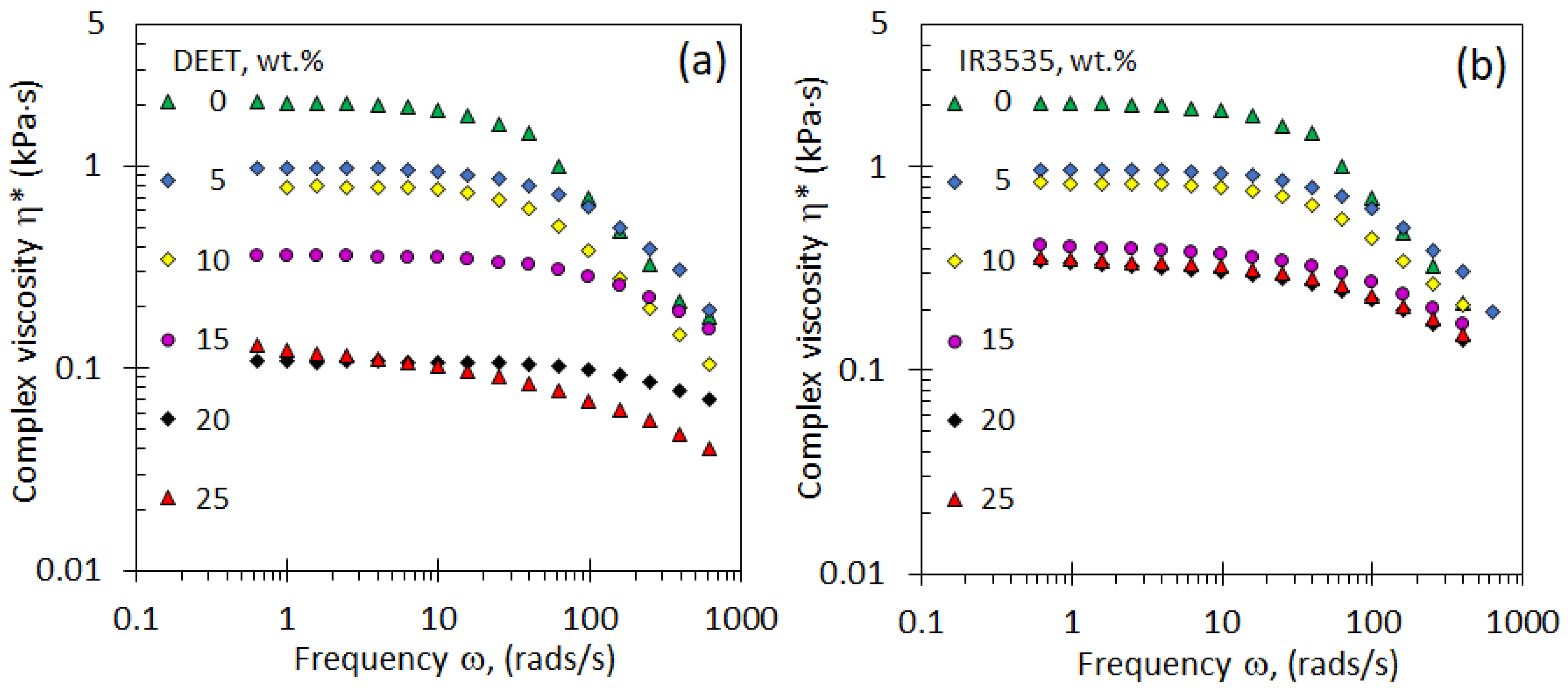
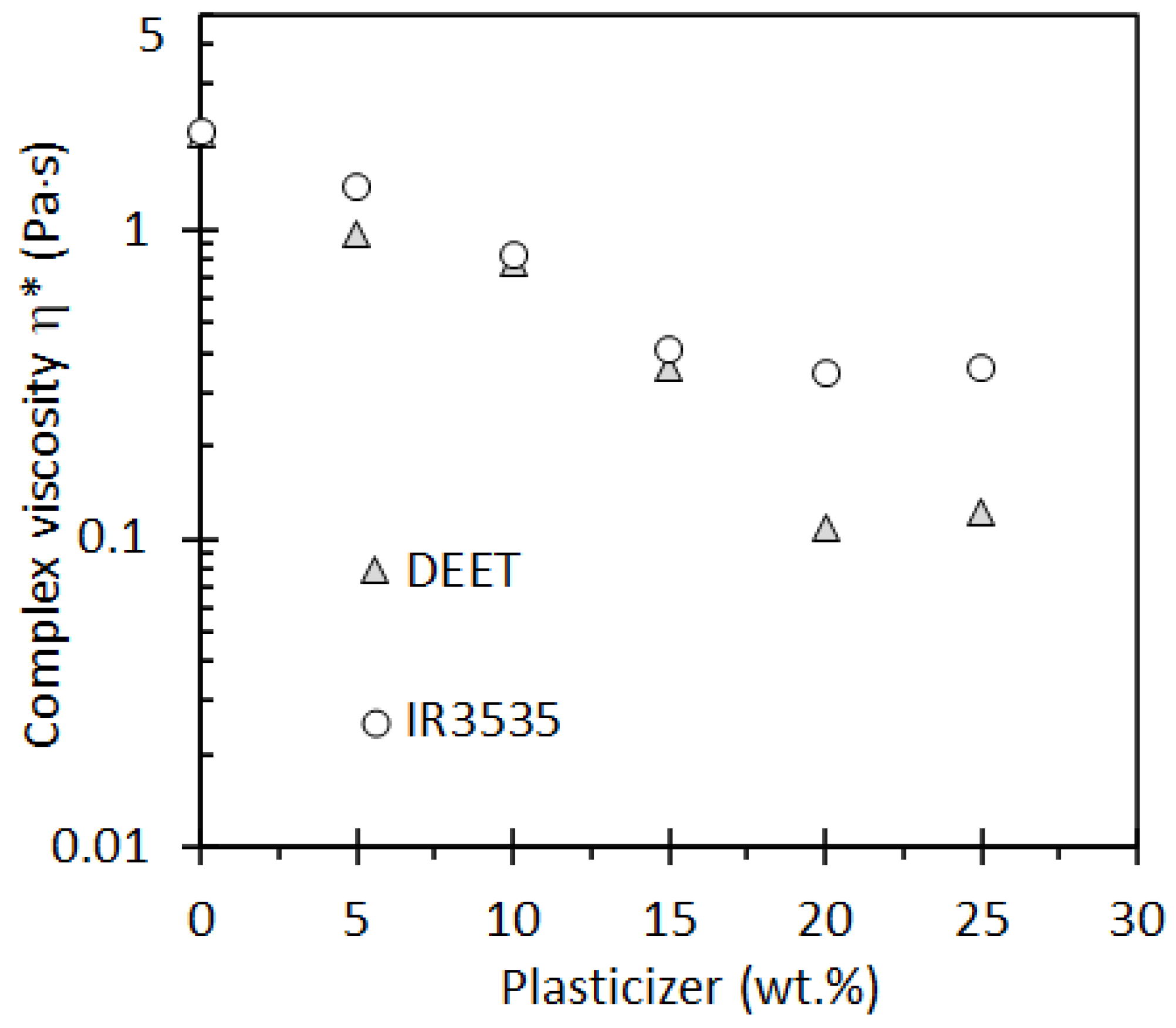
3.4. Rheological Properties
3.5. Repellents Release from PLA Strands
4. Materials and Methods
4.1. Materials
4.2. Extrusion-Compounding
4.3. Thermogravimetric Analysis (TGA)
4.4. Differential Scanning Calorimetry (DSC)
4.5. High-Resolution Confocal Raman Imaging
4.6. Rheological Tests
4.7. Mosquito Repellent Release Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. World Malaria Report 2020; World Health Organization: Geneva, Switzerland, 2020; pp. 1–299. [Google Scholar]
- Mapossa, A.B.; Focke, W.W.; Tewo, R.K.; Androsch, R.; Kruger, T. Mosquito-repellent controlled-release formulations for fighting infectious diseases. Malar. J. 2021, 20, 1–33. [Google Scholar] [CrossRef]
- Sibanda, M.; Focke, W.; Braack, L.; Leuteritz, A.; Brünig, H.; Tran, N.H.A.; Wieczorek, F.; Trümper, W. Bicomponent fibres for controlled release of volatile mosquito repellents. Mater. Sci. Eng. C 2018, 91, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Mapossa, A.B.; Sibanda, M.M.; Sitoe, A.; Focke, W.W.; Braack, L.; Ndonyane, C.; Mouatcho, J.; Smart, J.; Muaimbo, H.; Androsch, R. Microporous polyolefin strands as controlled-release devices for mosquito repellents. Chem. Eng. J. 2019, 360, 435–444. [Google Scholar] [CrossRef]
- Godfrey, L.; Ahmed, M.T.; Gebremedhin, K.G.; Katima, J.H.; Oelofse, S.; Osibanjo, O.; Richter, U.H.; Yonli, A.H. Solid Waste Management in Africa: Governance Failure or Development Opportunity? In Regional Development in Africa; Books on Demand GmbH: Norderstedt, Germany, 2019; p. 235. [Google Scholar] [CrossRef]
- Ferreira, I.; Brünig, H.; Focke, W.; Boldt, R.; Androsch, R.; Leuteritz, A. Melt-Spun Poly(d,l-lactic acid) Monofilaments Containing N, N-Diethyl-3-Methylbenzamide as Mosquito Repellent. Materials 2021, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Sungkapreecha, C.; Focke, W.W.; Androsch, R. Competition between liquid-liquid de-mixing, crystallization, and glass transition in solutions of PLA of different stereochemistry and DEET. Chin. J. Polym. Sci. 2020, 38, 174–178. [Google Scholar] [CrossRef]
- Du, F.; Schick, C.; Androsch, R. Full-composition-range glass transition behavior of the polymer/solvent system poly(lactic acid)/ethyl butylacetylaminopropionate (PLA/IR3535®). Polymer 2020, 209, 123058. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Iqbal, N.; Focke, W.W.; Androsch, R. Crystallization of poly(l-lactic acid) in solution with the mosquito-repellent N, N-diethyl-3-methylbenzamide. Polym. Cryst. 2019, 2, e10029. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Beily, M.J.; Kressler, J.; Focke, W.W.; Androsch, R. Phase behavior of the polymer/drug system PLA/DEET: Effect of PLA molar mass on subambient liquid-liquid phase separation. Thermochim. Acta 2018, 660, 77–81. [Google Scholar] [CrossRef]
- Sungkapreecha, C.; Iqbal, N.; Gohn, A.M.; Focke, W.W.; Androsch, R. Phase behavior of the polymer/drug system PLA/DEET. Polymer 2017, 126, 116–125. [Google Scholar] [CrossRef]
- Luo, J.; Meng, X.; Gong, W.; Jiang, Z.; Xin, Z. Improving the stability and ductility of polylactic acid via phosphite functional polysilsesquioxane. RSC Adv. 2019, 9, 25151–25157. [Google Scholar] [CrossRef]
- Zhang, C. Biodegradable Polyesters: Synthesis, Properties, Applications. Biodegradable Polyester; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C. Crystal nucleation in random L/D-lactide copolymers. Eur. Polym. J. 2016, 75, 474–485. [Google Scholar] [CrossRef]
- Enumo, A., Jr.; Gross, I.P.; Saatkamp, R.H.; Pires, A.T.; Parize, A.L. Evaluation of mechanical, thermal and morphological properties of PLA films plasticized with maleic acid and its propyl ester derivatives. Polym. Test. 2020, 88, 106552. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpă, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Penczek, S. Ring-Opening Polymerization and Special Polymerization Processes; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Di Lorenzo, M.L.; Longo, A. N,N-Diethyl-3-methylbenzamide (DEET): A mosquito repellent as functional plasticizer for poly(l-lactic acid). Thermochim. Acta 2019, 677, 180–185. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized jatropha oil as a sustainable plasticizer to poly(lactic acid). Polymers 2017, 9, 204. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Dos Santos, D.C.; Motta, J.F.G.; Dos Santos, R.R.; Chávez, D.W.H.; De Melo, N.R. Structure and functional properties of cellulose acetate films incorporated with glycerol. Carbohydr. Polym. 2019, 209, 190–197. [Google Scholar] [CrossRef]
- Mapossa, A.B.; Sitoe, A.; Focke, W.W.; Izadi, H.; Du Toit, E.L.; Androsch, R.; Sungkapreecha, C.; Van Der Merwe, E.M. Mosquito repellent thermal stability, permeability and air volatility. Pest Manag. Sci. 2020, 76, 1112–1120. [Google Scholar] [CrossRef]
- Weeks, J.; Guiney, P.; Nikiforov, A. Assessment of the environmental fate and ecotoxicity of N,N-diethyl-m-toluamide (DEET). Integr. Environ. Assess. Manag. 2012, 8, 120–134. [Google Scholar] [CrossRef]
- Tavares, M.; Da Silva, M.R.M.; De Siqueira, L.B.D.O.; Rodrigues, R.A.S.; Bodjolle-D’almeida, L.; Dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef]
- Sitoe, A.; Mapossa, A.B.; Focke, W.W.; Muiambo, H.; Androsch, R.; Wesley-Smith, J. Development, characterization and modeling of mosquito repellent release from microporous devices. SPE Polym. 2020, 1, 90–100. [Google Scholar] [CrossRef]
- Mphateng, T.N.; Mapossa, A.B.; Focke, W.W.; Ramjee, S. Cellulose diacetate/organoclay nanocomposites as controlled-release matrices for pest control applications. ACS Appl. Mater. Interfaces 2021. Submitted. [Google Scholar]
- Mapossa, A.B.; Focke, W.W.; Sitoe, A.; Androsch, R. Mosquito repellent microporous polyolefin strands. AIP Conf. Proc. 2020, 2289, 020062. [Google Scholar]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Marabi, A.; Livings, S.; Jacobson, M.; Saguy, I.S. Normalized Weibull distribution for modeling rehydration of food particulates. Eur. Food Res. Technol. 2003, 217, 311–318. [Google Scholar] [CrossRef]
- Kosmidis, K.; Argyrakis, P.; Macheras, P. A Reappraisal of Drug Release Laws Using Monte Carlo Simulations: The Prevalence of the Weibull Function. Pharm. Res. 2003, 20, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Ignacio, M.; Chubynsky, M.V.; Slater, G.W. Interpreting the Weibull fitting parameters for diffusion-controlled release data. Phys. A Stat. Mech. Appl. 2017, 486, 486–496. [Google Scholar] [CrossRef]
- Frisch, H.L. Sorption and transport in glassy polymers—A review. Polym. Eng. Sci. 1980, 20, 2–13. [Google Scholar] [CrossRef]
- Edwards, D.A. Skinning during desorption of polymers: An asymptotic analysis. SIAM J. Appl. Math. 1998, 59, 1134–1155. [Google Scholar] [CrossRef][Green Version]
- Joshi, S.; Astarita, G. Mathematical model of the desorption of swelling solvents from swollen polymer films. Polymer 1979, 20, 1217–1220. [Google Scholar] [CrossRef]
- Bruschi, M.L. 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L., Ed.; Woodhead Publishing: Sawsto, UK, 2015. [Google Scholar]
- Alfrey, T., Jr.; Gurnee, E.F.; Lloyd, W.G. Diffusion in glassy polymers. J. Polym. Sci. Part C Polym. Symp. 1966, 12, 249–261. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Sarasua, J.-R.; Prud’homme, R.E.; Wisniewski, M.; Le Borgne, A.; Spassky, N. Crystallization and melting behavior of polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Díaz-Díaz, A.M.; López-Beceiro, J.; Li, Y.; Cheng, Y.; Artiaga, R. Crystallization kinetics of a commercial poly(lactic acid) based on characteristic crystallization time and optimal crystallization temperature. J. Therm. Anal. Calorim. 2021, 145, 3125–3132. [Google Scholar] [CrossRef]
- Gracia-Fernández, C.A.; Gómez-Barreiro, S.; López-Beceiro, J.; Naya, S.; Artiaga, R. New approach to the double melting peak of poly(l-lactic acid) observed by DSC. J. Mater. Res. 2012, 27, 1379–1382. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α′- and α-crystals of poly(l-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Ge, H.; Yang, F.; Hao, Y.; Wu, G.; Zhang, H.; Dong, L. Thermal, mechanical, and rheological properties of plasticized poly(l-lactic acid). J. Appl. Polym. Sci. 2013, 127, 2832–2839. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Polymer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Edwards, D.A. A spatially nonlocal model for polymer desorption. J. Eng. Math. 2005, 53, 221–238. [Google Scholar] [CrossRef][Green Version]
- Yener, H.E.; Hillrichs, G.; Androsch, R. Phase behavior of solvent-rich compositions of the polymer/drug system poly(butylene succinate) and N,N-diethyl-3-methylbenzamide (DEET). Colloid Polym. Sci. 2021, 299, 873–881. [Google Scholar] [CrossRef]
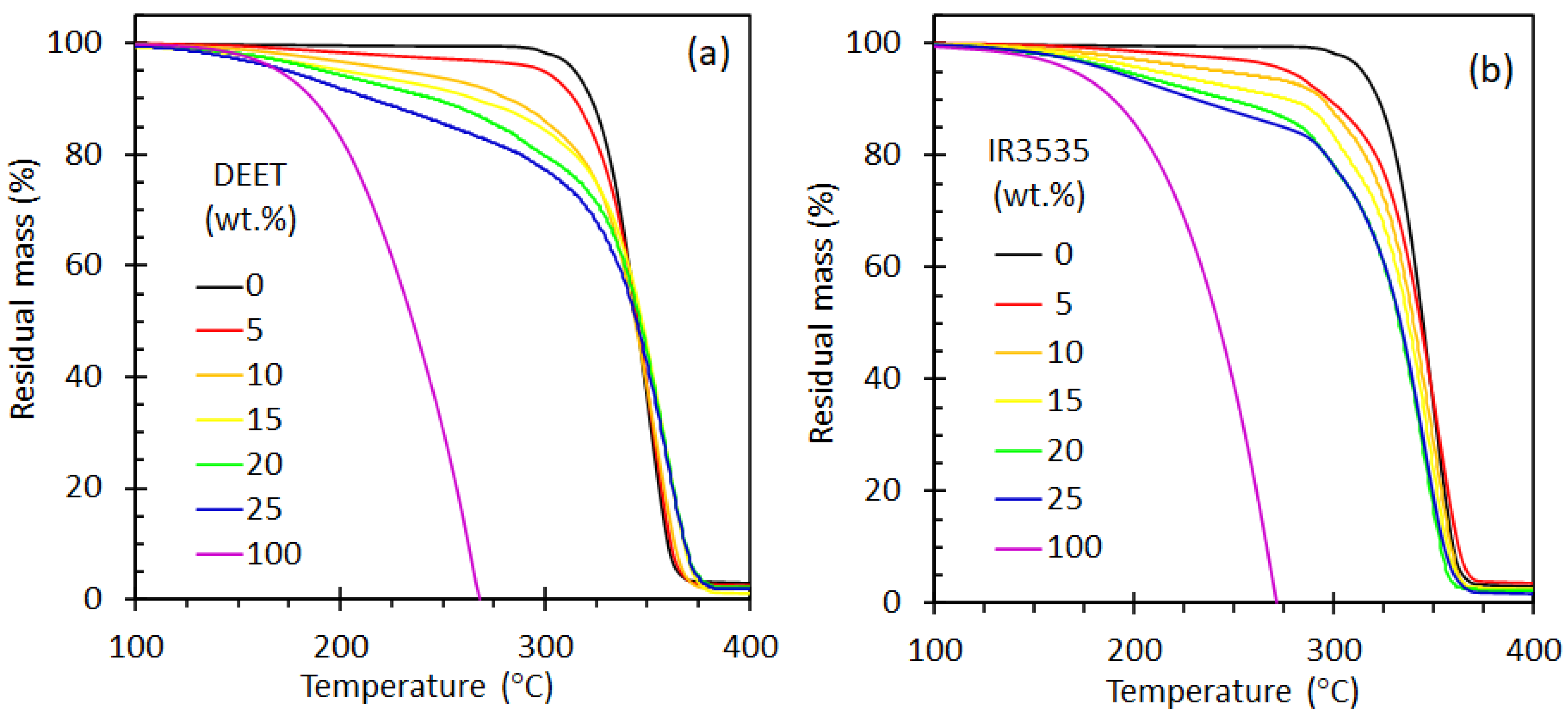
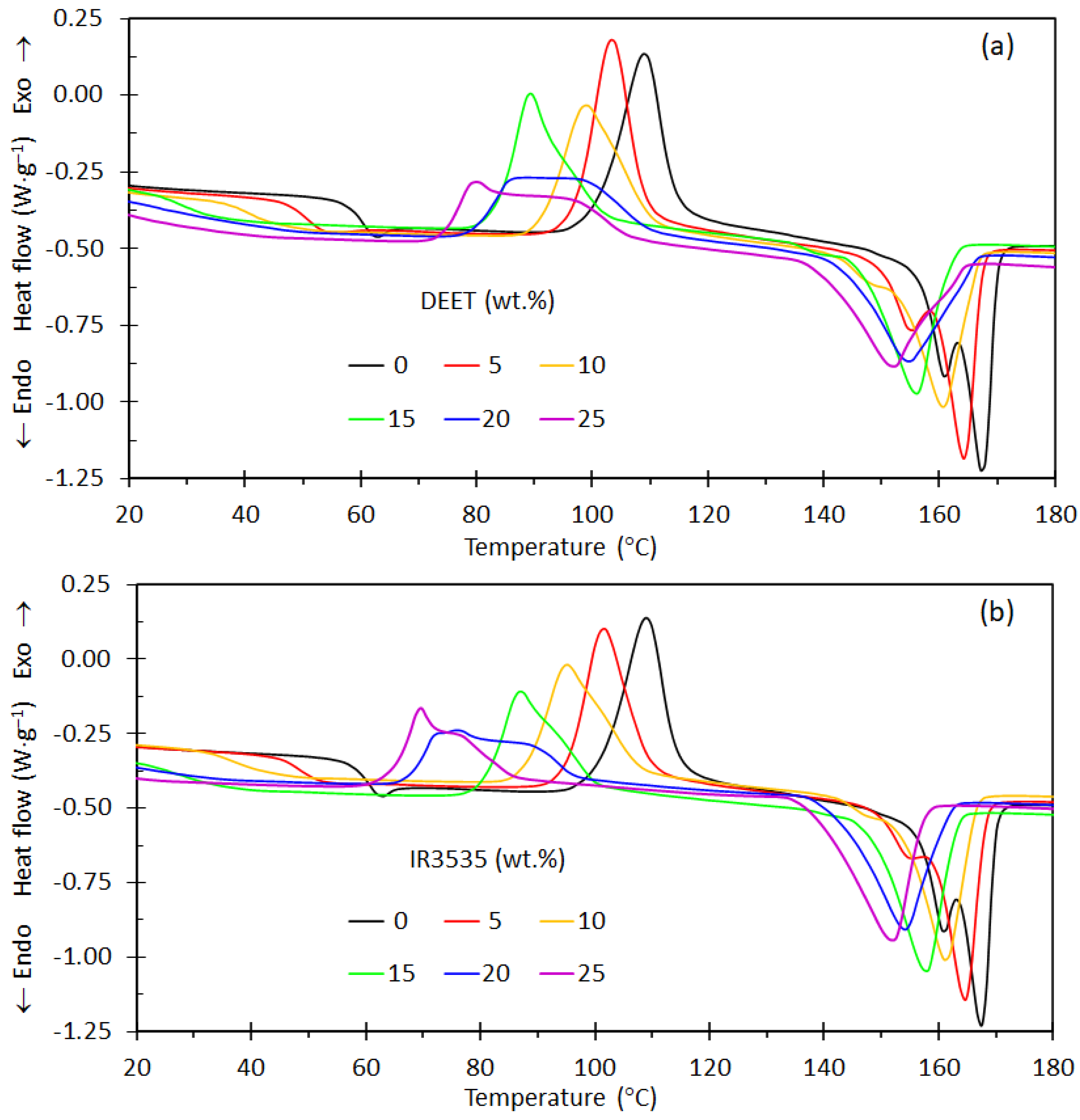

| Repellent | (wt.%) | Tg (°C) | Toc (°C) | Tpc (°C) | Tom(1) (°C) | Tpm(1) (°C) | Tom(2) (°C) | Tpm(2) (°C) | ΔHcc (J⋅g−1) | ΔHm (J⋅g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| None | 0 | 61.0 | 100.9 | 109.0 | 156.4 | 160.8 | 162.2 | 167.5 | 31.2 | 34.9 |
| DEET | 5 | 50.2 | 97.0 | 103.3 | 150.2 | 155.1 | 158.3 | 164.3 | 29.5 | 33.7 |
| 10 | 41.5 | 91.3 | 98.8 | 142.6 | 150.8 | 151.7 | 160.6 | 28.9 | 31.8 | |
| 15 | 28.3 | 83.7 | 89.2 | 134.6 | 140.9 | 145.8 | 155.9 | 21.8 | 30.8 | |
| 20 | 24.3 | 79.8 | 88.7 | - | - | 143.3 | 154.5 | 26.8 | 30.0 | |
| 25 | 17.0 | 74.5 | 79.9 | - | - | 139.8 | 152.0 | 25.3 | 29.2 | |
| IR3535 | 5 | 48.5 | 95.2 | 101.6 | 148.9 | 155.9 | 158.4 | 164.6 | 26.9 | 33.3 |
| 10 | 38.0 | 88.1 | 95.0 | 142.7 | 148.0 | 153.2 | 161.2 | 27.3 | 31.9 | |
| 15 | 25.9 | 80.9 | 86.8 | 136.9 | 142.1 | 148.4 | 157.6 | 22.9 | 29.9 | |
| 20 | 14.9 | 67.9 | 76.0 | - | - | 142.4 | 154.1 | 20.9 | 30.1 | |
| 25 | 10.7 | 64.7 | 69.5 | - | - | 138.7 | 151.5 | 17.8 | 30.7 |
| Strand Type | Initial Diameter (mm) | Final Diameter (mm) | Difference (%) |
|---|---|---|---|
| Neat PLA | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.0 |
| IR3535 (20 wt.%) | 4.3 ± 0.5 | 3.9 ± 0.4 | 9.3 |
| IR3535 (25 wt.%) | 4.6 ± 0.3 | 4.0 ± 0.4 | 13.0 |
| DEET (20 wt.%) | 3.1 ± 0.1 | 2.7 ± 0.2 | 12.9 |
| DEET (25 wt.%) | 3.5 ± 0.1 | 2.9 ± 0.1 | 17.1 |
| Strand Type | X1 (-) | τ1 (Days) | τ2 (Days) | R |
|---|---|---|---|---|
| IR3535 (20 wt.%) | 27.6 | 7.66 | 607 | 0.9982 |
| IR3535 (25 wt.%) | 32.1 | 7.66 | 607 | 0.9986 |
| DEET (20 wt.%) | 9.96 | 12.0 | 1397 | 0.9962 |
| DEET (25 wt.%) | 23.1 | 12.0 | 1397 | 0.9993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapossa, A.B.; López-Beceiro, J.; Díaz-Díaz, A.M.; Artiaga, R.; Moyo, D.S.; Mphateng, T.N.; Focke, W.W. Properties of Mosquito Repellent-Plasticized Poly(lactic acid) Strands. Molecules 2021, 26, 5890. https://doi.org/10.3390/molecules26195890
Mapossa AB, López-Beceiro J, Díaz-Díaz AM, Artiaga R, Moyo DS, Mphateng TN, Focke WW. Properties of Mosquito Repellent-Plasticized Poly(lactic acid) Strands. Molecules. 2021; 26(19):5890. https://doi.org/10.3390/molecules26195890
Chicago/Turabian StyleMapossa, António B., Jorge López-Beceiro, Ana María Díaz-Díaz, Ramón Artiaga, Dennis S. Moyo, Thabang N. Mphateng, and Walter W. Focke. 2021. "Properties of Mosquito Repellent-Plasticized Poly(lactic acid) Strands" Molecules 26, no. 19: 5890. https://doi.org/10.3390/molecules26195890
APA StyleMapossa, A. B., López-Beceiro, J., Díaz-Díaz, A. M., Artiaga, R., Moyo, D. S., Mphateng, T. N., & Focke, W. W. (2021). Properties of Mosquito Repellent-Plasticized Poly(lactic acid) Strands. Molecules, 26(19), 5890. https://doi.org/10.3390/molecules26195890






