Ultrasonic-Assisted Enzymolysis Extraction and Protective Effect on Injured Cardiomyocytes in Mice of Flavonoids from Prunus mume Blossom
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Test Results
2.2. Orthogonal Test Results
2.3. Identification of Main Components in FPMB
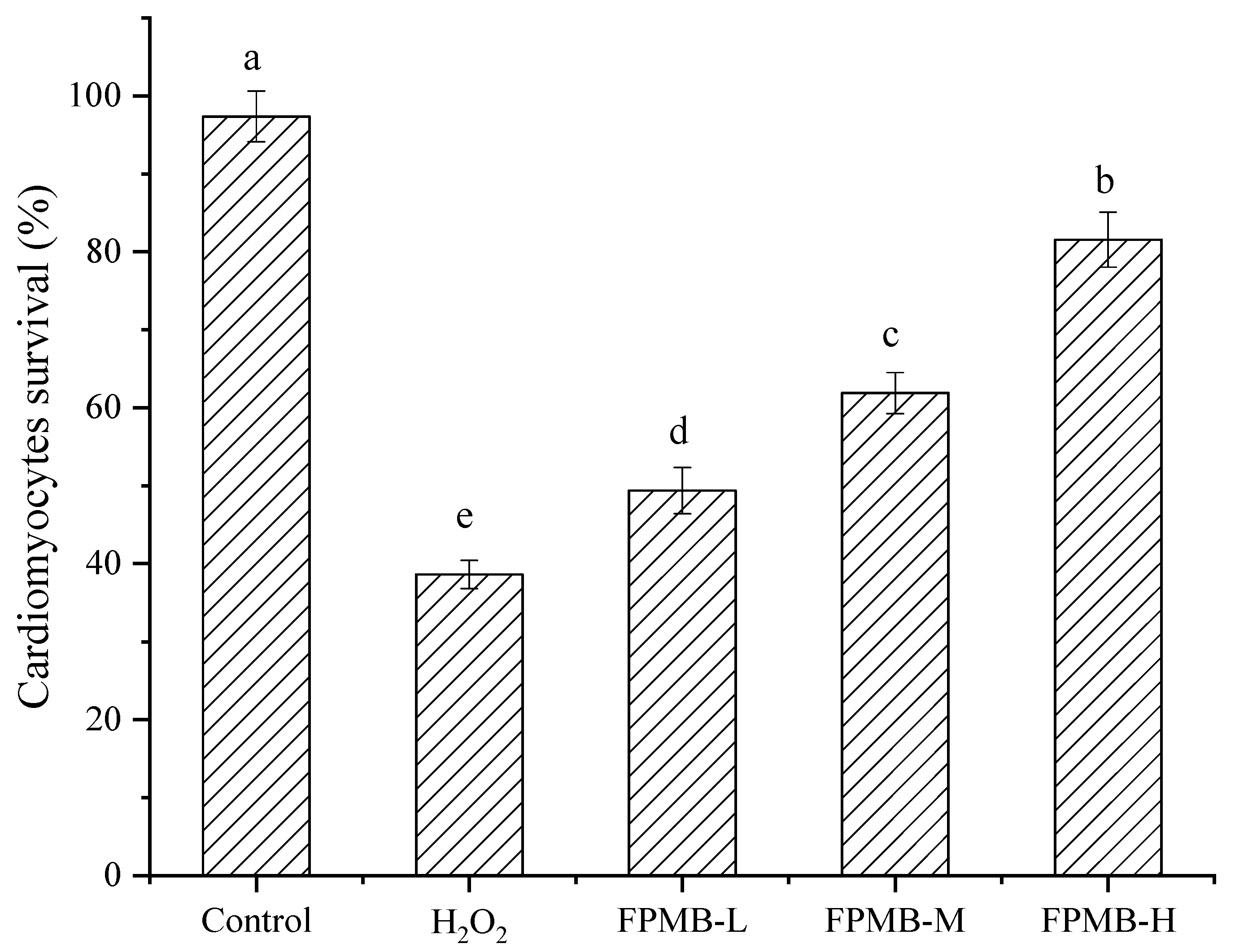
2.4. Effect of FPMB on the Cardiomyocytes’ Survival Rate
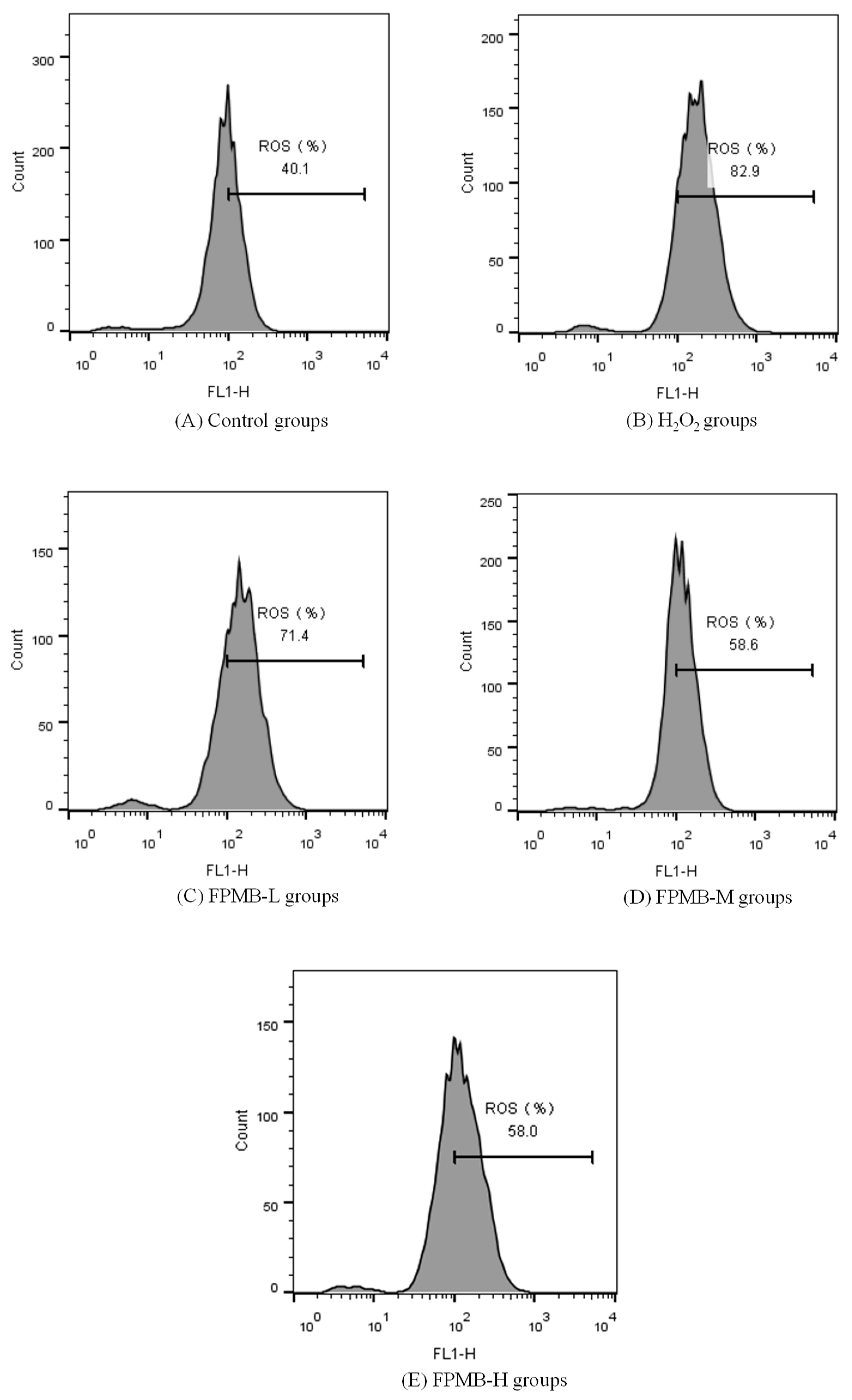
2.5. Effect of FPMB on the ROS Contents
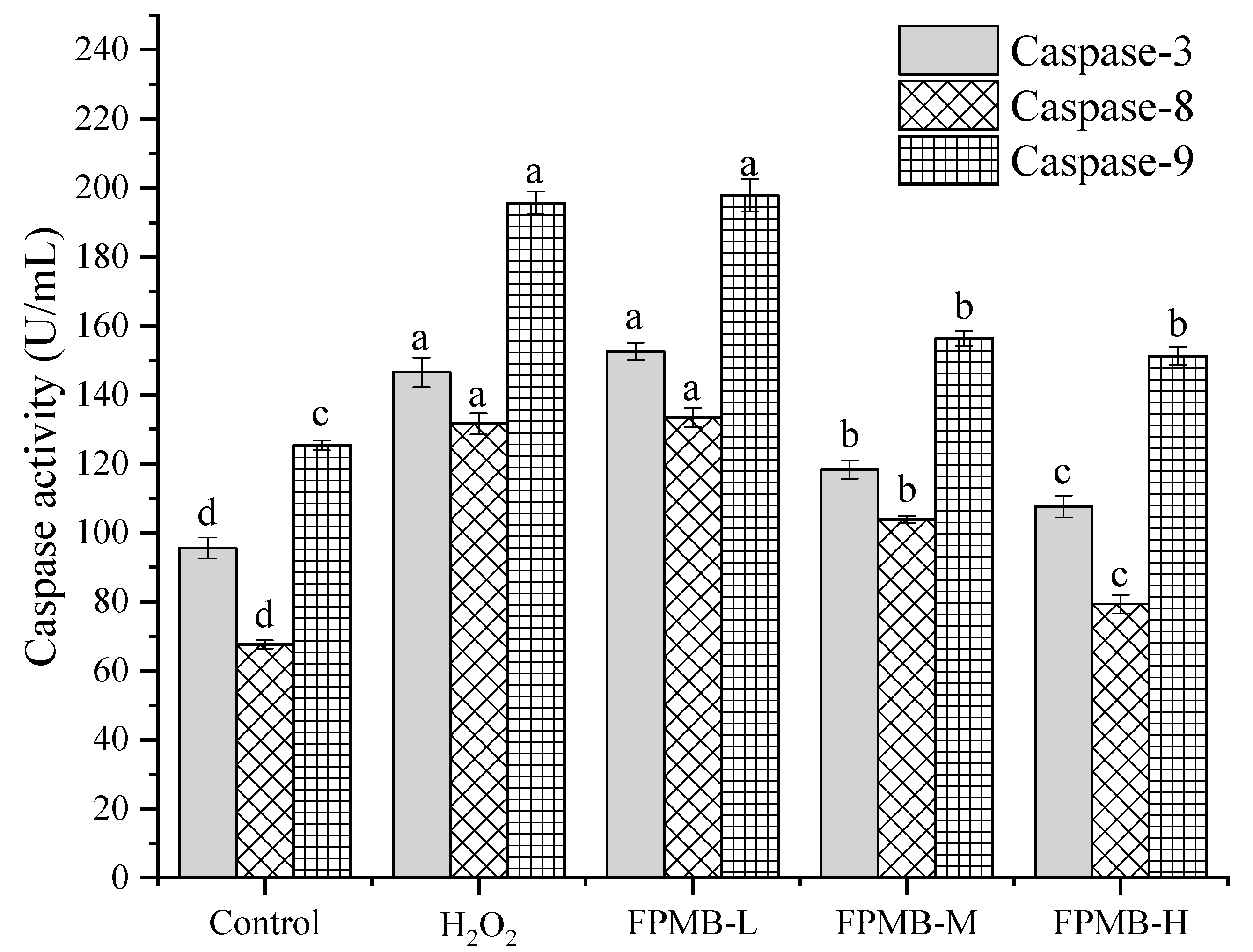
2.6. Effect of FPMB on the Activities of Caspase-3, Caspase-8, and Caspase-9
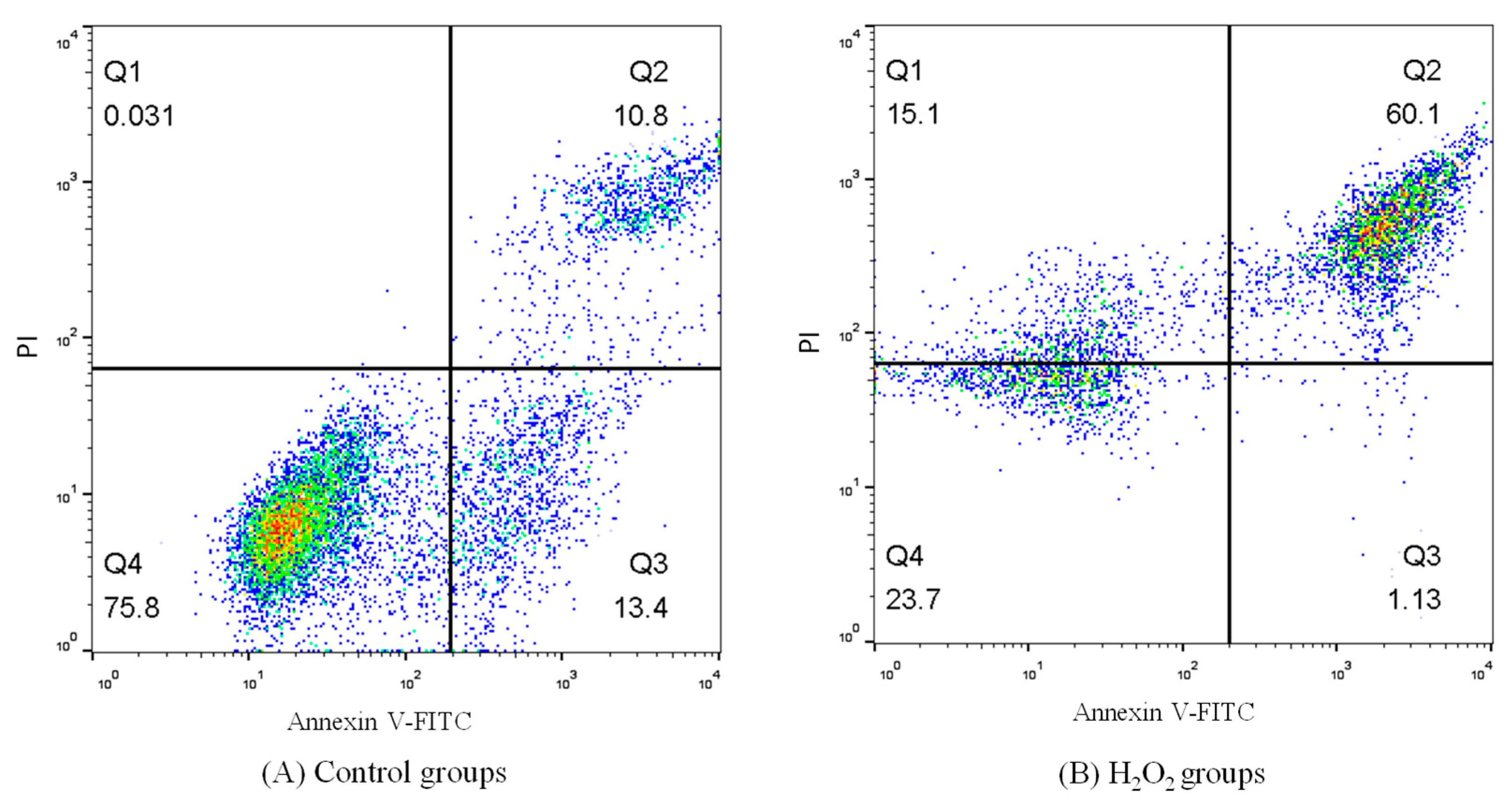
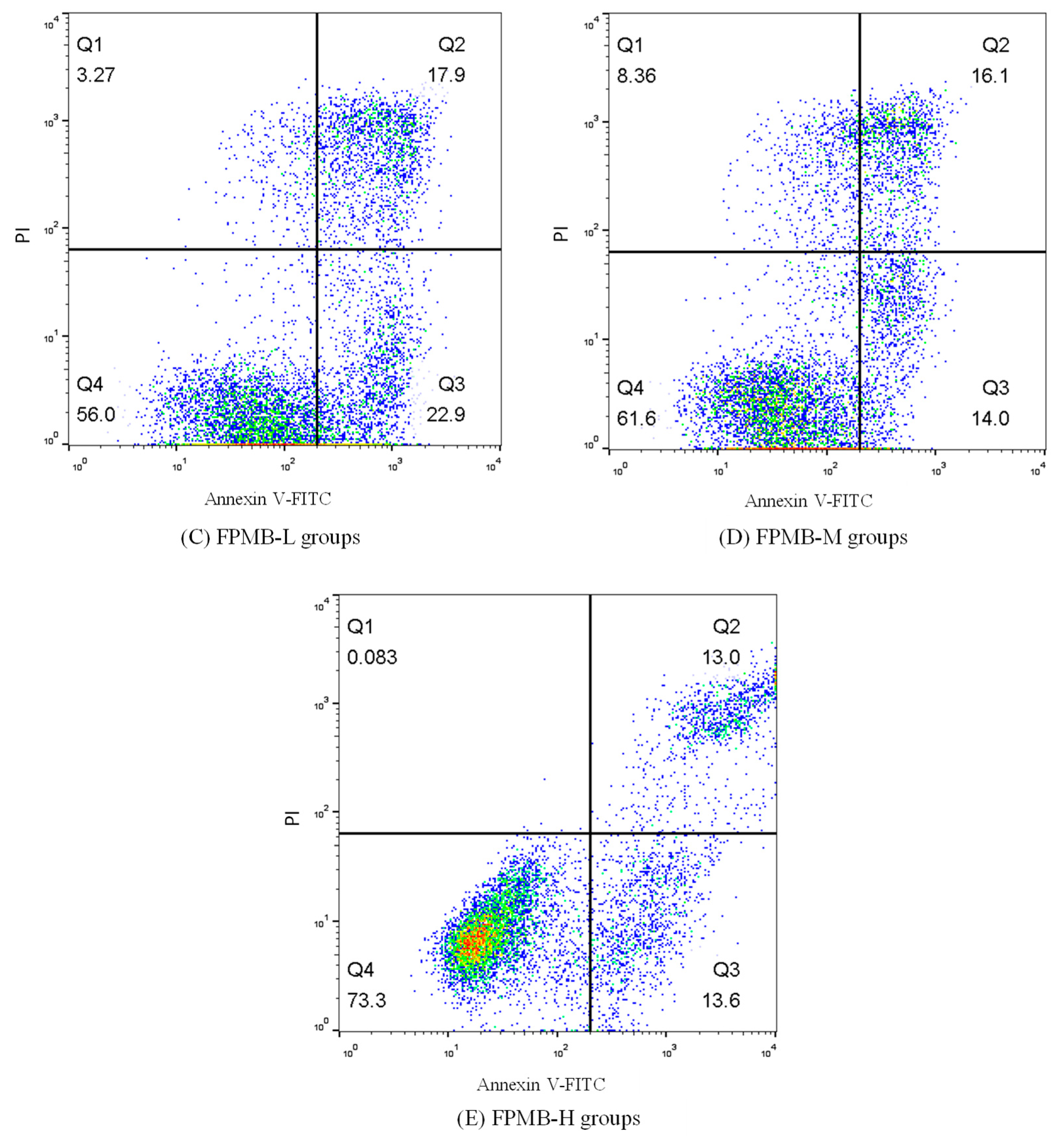
2.7. Effect of on Cardiomyocytes’ Apoptosis
3. Materials and Methods
3.1. Raw Materials
3.2. Extraction of FPMB
3.3. Measurements and Analyses
3.3.1. Determination of Flavonoids Content
Drawing of a Standard Curve
Flavonoids Content Measurement
3.3.2. Single-Factor Test Design
3.3.3. Orthogonal Experimental Design
3.3.4. Identification of FPMB by HPLC
3.3.5. Isolation of Cardiomyocytes
3.3.6. Design of Protective Effect Test on Cardiomyocytes
3.3.7. Measurements of Cardiomyocytes’ Activity
3.3.8. Measurements of ROS Activity
3.3.9. Measurements of Caspase-3, Caspase-8, and Caspase-9 Activity in Cardiomyocytes
3.3.10. Measurements of Cardiomyocytes’ Apoptosis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nakamura, S.; Fujimoto, K.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Structures of acylated sucroses and an acylated flavonol glycoside and inhibitory effects of constituents on aldose reductase from the flower buds of Prunus mume. J. Nat. Med. 2013, 67, 799–806. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crop. Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- Mai, X.; Liu, Y.; Tang, X.; Wang, L.; Lin, Y.; Zeng, H.; Luo, L.; Fan, H.; Li, P. Sequential extraction and enrichment of flavonoids from Euonymus alatus by ultrasonic-assisted polyethylene glycol-based extraction coupled to temperature-induced cloud point extraction. Ultrason. Sonochem. 2020, 66, 105073. [Google Scholar] [CrossRef]
- Qiao, Z.; Han, L.; Liu, X.; Dai, H.; Liu, C.; Yan, M.; Li, W.; Han, W.; Li, X.; Huang, S.; et al. Extraction, radical scavenging activities, and chemical composition identification of flavonoids from sunflower (Helianthus annuus L.) receptacles. Molecules 2021, 26, 403. [Google Scholar] [CrossRef]
- Chaiyana, W.; Chansakaow, S.; Intasai, N.; Kiattisin, K.; Lee, K.H.; Lin, W.C.; Lue, S.C.; Leelapornpisid, P. Chemical constituents, antioxidant, anti-mmps, and anti-hyaluronidase activities of Thunbergia laurifolia lindl. leaf extracts for skin aging and skin damage prevention. Molecules 2020, 25, 1923. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Zhuang, S.; Wen, Y.; Cheng, W.; Zeng, Z.; Jiang, T.; Tang, C. Effect of Anoectochilus roxburghii flavonoids extract on H2O2—Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J. Ethnopharmacol. 2020, 254, 112670. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, L.; Tan, Y.; Liang, Q.; Bai, F.; Mai, W.; Huang, Q.; Ye, Y. Hepatoprotective effect of total flavonoids of Mallotus apelta (Lour.) Muell.Arg. leaf against carbon tetrachloride-induced liver fibrosis in rats via modulation of TGF-beta1/Smad and NF-kappaB signaling pathways. J. Ethnopharmacol. 2020, 254, 112714. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Nakamura, S.; Matsumoto, T.; Ohta, T.; Ogawa, K.; Tamura, H.; Matsuda, H.; Yoshikawa, M. Medicinal flowers. XXXVIII. structures of acylated sucroses and inhibitory effects of constituents on aldose reducatase from the flower buds of Prunus mume. Chem. Pharm. Bull. 2013, 61, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant. Sci. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Lin, C.H.; Huang, T.H.; Chuang, S.Y. In vivo rodent models of type 2 diabetes and their usefulness for evaluating flavonoid bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Tešić, Ž.; Stupar, A.; Bulut, G.; Sinan, K.I.; Uysal, S.; Picot-Allain, M.C.N.; Mahomoodally, M.F. A comparative exploration of the phytochemical profiles and bio-pharmaceutical potential of Helichrysum stoechas subsp. barrelieri extracts obtained via five extraction techniques. Process. Biochem. 2020, 91, 113–125. [Google Scholar] [CrossRef]
- Sekhon, J.K.; Rosentrater, K.A.; Jung, S.; Wang, T. Effect of co-products of enzyme-assisted aqueous extraction of soybeans, enzymes, and surfactant on oil recovery from integrated corn-soy fermentation. Ind. Crop. Prod. 2018, 121, 441–451. [Google Scholar] [CrossRef]
- Sousa, A.D.; Maia, A.I.V.; Rodrigues, T.H.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Cassia Alves Pereira, R.; Vieira, R.F.; de Brito, E.S. Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF. Ind. Crop. Prod. 2016, 79, 91–103. [Google Scholar] [CrossRef]
- Xu, B.; Yuan, J.; Wang, L.; Lu, F.; Wei, B.; Azam, R.S.M.; Ren, X.; Zhou, C.; Ma, H.; Bhandari, B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020, 63, 104930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, C.; Liu, C.; Wang, Y.; Zhang, W.; Xing, Y. Trimetazidine and lcarnitine prevent heart aging and cardiac metabolic impairment in rats via regulating cardiac metabolic substrates. Exp. Gerontol. 2019, 119, 120–127. [Google Scholar] [CrossRef]
- Bode, D.; Lindner, D.; Schwarzl, M.; Westermann, D.; Deissler, P.; Primessnig, U.; Hegemann, N.; Blatter, L.A.; van Linthout, S.; Tschope, C.; et al. The role of fibroblast—Cardiomyocyte interaction for atrial dysfunction in HFpEF and hypertensive heart disease. J. Mol. Cell. Cardiol. 2019, 131, 53–65. [Google Scholar] [CrossRef]
- Cao, J.W.; Duan, S.Y.; Zhang, H.X.; Chen, Y.; Guo, M. Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol. Trace Elem. Res. 2020, 196, 145–152. [Google Scholar] [CrossRef]
- Tang, M.; Huang, Z.; Luo, X.; Liu, M.; Wang, L.; Qi, Z.; Huang, S.; Zhong, J.; Chen, J.X.; Li, L.; et al. Ferritinophagy activation and sideroflexin1-dependent mitochondria iron overload is involved in apelin-13-induced cardiomyocytes hypertrophy. Free. Radic. Biol. Med. 2019, 134, 445–457. [Google Scholar] [CrossRef]
- Prins, J.B.; Walker, N.I.; Winterford, C.M.; Cameron, D.P. Apoptosis of human adipocytes in vitro. Biochem. Bioph. Res. Co. 1994, 201, 500–507. [Google Scholar] [CrossRef]
- Koyani, C.N.; Windischhofer, W.; Rossmann, C.; Jin, G.; Kickmaier, S.; Heinzel, F.R.; Groschner, K.; Alavian-Ghavanini, A.; Sattler, W.; Malle, E. 15-deoxy-Delta(1)(2),(1)(4)-PGJ(2) promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFalpha axis. Int. J. Cardiol. 2014, 173, 472–480. [Google Scholar] [CrossRef][Green Version]
- Fan, Q.; Chen, M.; Zuo, L.; Shang, X.; Huang, M.Z.; Ciccarelli, M.; Raake, P.; Brinks, H.; Chuprun, K.J.; Dorn, G.W.; et al. Myocardial ablation of G protein-coupled receptor kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. PLoS ONE 2013, 8, e66234. [Google Scholar] [CrossRef]
- Gao, C.; Wang, F.; Wang, Z.; Zhang, J.; Yang, X. Asiatic acid inhibits lactate-induced cardiomyocyte apoptosis through the regulation of the lactate signaling cascade. Int. J. Mol. Med. 2016, 38, 1823–1830. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhao, Y.; Liu, Y.; Ma, N.; Wang, C.; Zou, J.; Liu, Z.; Zhou, Z.; Han, D.; He, J.; et al. miR-483-3p regulates hyperglycaemia-induced cardiomyocyte apoptosis in transgenic mice. Biochem. Biophys. Res. Commun. 2016, 477, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, H.; He, W.; Kong, B.; Hu, H.; Fan, Y.; Liao, J.; Wang, L.; Mei, Y.; Liu, W.; et al. Regulator of G-protein signaling 5 protects cardiomyocytes against apoptosis during in vitro cardiac ischemia-reperfusion in mice by inhibiting both JNK1/2 and P38 signaling pathways. Biochem. Biophys. Res. Commun. 2016, 473, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ren, A.; Chen, J.; Li, H.; Wei, B.; Wang, J.; Azam, S.M.R.; Bhandari, B.; Zhou, C.; Ma, H. Effect of multi-mode dual-frequency ultrasound irradiation on the degradation of waxy corn starch in a gelatinized state. Food Hydrocoll. 2021, 113, 106440. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Liu, X.; Zhou, A.; Xiao, J.; Huang, K.; Chen, H.; Cao, Y. Optimization in continuous phase-transition extraction of crude flavonoids from finger citron fruit and evaluation on their antiaging activities. Food Sci. Nutr. 2020, 8, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rong, X.; Qin, J.; Wang, S. An improved two-step method for extraction and purification of primary cardiomyocytes from neonatal mice. J. Pharmacol. Toxicol. Methods 2020, 104, 106887. [Google Scholar] [CrossRef] [PubMed]
- Damrongrungruang, T.; Kitchindaopat, N.; Thanasothon, P.; Theeranut, K.; Tippayawat, P.; Ruangsuwan, C.; Suwannee, B. Effects of photodynamic therapy with azulene on peripheral blood mononuclear cell viability and singlet oxygen formation. Photodiagn. Photodyn. 2018, 24, 318–323. [Google Scholar] [CrossRef]
- Martinez Fernandez, A.; Regazzoni, L.; Brioschi, M.; Gianazza, E.; Agostoni, P.; Aldini, G.; Banfi, C. Pro-oxidant and pro-inflammatory effects of glycated albumin on cardiomyocytes. Free Radic. Biol. Med. 2019, 144, 245–255. [Google Scholar] [CrossRef]
- Deng, X.U.; Xia, K.E.; Chen, P.O.; Ali Sheikh, M.S.; Yang, D.F.; Li, S.M.; Yang, T.L. Reversion of left ventricle remodeling in spontaneously hypertensive rats by valsartan is associated with the inhibition of caspase-3, -8 and -9 activities. Biomed. Rep. 2015, 3, 533–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, N.; Zou, L.; Hu, M.; Zhang, M. Heme as a target for protection against doxorubicin-induced apoptosis in H9c2 cardiomyocytes. Cell. Stress. Chaperones. 2019, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]

| Groups | Extraction Rate (%) | |
|---|---|---|
| Cellulase mass percentage (%) | 0.5 | 1.80 ± 0.07 d |
| 1.0 | 2.63 ± 0.12 c | |
| 1.5 | 3.52 ± 0.15 b | |
| 2.0 | 5.81 ± 0.26 a | |
| 2.5 | 5.83 ± 0.23 a | |
| 3.0 | 5.92 ± 0.19 a | |
| Hydrolysis temperature (°C) | 30 | 3.75 ± 0.11 c |
| 35 | 4.82 ± 0.26 b | |
| 40 | 5.63 ± 0.28 a | |
| 45 | 5.65 ± 0.26 a | |
| 50 | 5.58 ± 0.19 a | |
| 55 | 5.49 ± 0.15 a | |
| Groups | Extraction Rate (%) | |
|---|---|---|
| Ultrasonic power (W) | 200 | 3.69 ± 0.11 c |
| 250 | 5.07 ± 0.15 b | |
| 300 | 6.15 ± 0.23 a | |
| 350 | 6.10 ± 0.18 a | |
| 400 | 5.91 ± 0.21 a | |
| 450 | 5.85 ± 0.17 a | |
| Ultrasonic time (min) | 20 | 4.27 ± 0.16 c |
| 30 | 5.12 ± 0.26 b | |
| 40 | 6.06 ± 0.21 a | |
| 50 | 6.17 ± 0.31 a | |
| 60 | 6.15 ± 0.16 a | |
| 70 | 6.23 ± 0.22 a | |
| Number | Cellulase Mass Percentage A (%) | Hydrolysis Temperature B (°C) | Ultrasonic Power C (W) | Ultrasonic Time D (S) | Extraction Rate (%) |
|---|---|---|---|---|---|
| 1 | 1.5 | 35 | 250 | 30 | 5.62 |
| 2 | 1.5 | 40 | 300 | 40 | 5.78 |
| 3 | 1.5 | 45 | 350 | 50 | 5.95 |
| 4 | 2.0 | 35 | 300 | 50 | 5.86 |
| 5 | 2.0 | 40 | 350 | 30 | 6.28 |
| 6 | 2.0 | 45 | 200 | 40 | 6.03 |
| 7 | 2.5 | 35 | 350 | 40 | 6.31 |
| 8 | 2.5 | 40 | 200 | 50 | 5.97 |
| 9 | 2.5 | 45 | 250 | 30 | 6.15 |
| K1 | 17.350 | 17.790 | 17.620 | 18.050 | |
| K2 | 18.170 | 18.030 | 17.790 | 18.120 | |
| K3 | 18.430 | 18.130 | 18.540 | 17.780 | |
| k1 | 5.783 | 5.930 | 5.873 | 6.017 | |
| k2 | 6.057 | 6.010 | 5.930 | 6.040 | |
| k3 | 6.143 | 6.043 | 6.180 | 5.927 | |
| R | 0.360 | 0.113 | 0.250 | 0.113 |
| Variance Source | Square Sum | Degrees of Freedom | Mean Square | F-Value | Significance |
|---|---|---|---|---|---|
| A | 0.212 | 2 | 0.106 | 10.406 | * |
| B | 0.020 | 2 | 0.010 | 1.000 | |
| C | 0.160 | 2 | 0.080 | 7.848 | * |
| D | 0.021 | 2 | 0.011 | 1.056 | |
| Error | 0.020 | 2 | 0.010 |
| Groups | Apoptosis Rate (%) |
|---|---|
| Control | 24.20 ± 0.67 e |
| H2O2 | 61.23 ± 1.81 a |
| FPMB-L | 40.80 ± 1.07 b |
| FPMB-M | 30.10 ± 0.88 c |
| FPMB-H | 26.60 ± 0.53 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Xu, J.; Chen, H.; Lv, W. Ultrasonic-Assisted Enzymolysis Extraction and Protective Effect on Injured Cardiomyocytes in Mice of Flavonoids from Prunus mume Blossom. Molecules 2021, 26, 5818. https://doi.org/10.3390/molecules26195818
Zhu S, Xu J, Chen H, Lv W. Ultrasonic-Assisted Enzymolysis Extraction and Protective Effect on Injured Cardiomyocytes in Mice of Flavonoids from Prunus mume Blossom. Molecules. 2021; 26(19):5818. https://doi.org/10.3390/molecules26195818
Chicago/Turabian StyleZhu, Shengnan, Jicheng Xu, Huizhi Chen, and Weiqiao Lv. 2021. "Ultrasonic-Assisted Enzymolysis Extraction and Protective Effect on Injured Cardiomyocytes in Mice of Flavonoids from Prunus mume Blossom" Molecules 26, no. 19: 5818. https://doi.org/10.3390/molecules26195818
APA StyleZhu, S., Xu, J., Chen, H., & Lv, W. (2021). Ultrasonic-Assisted Enzymolysis Extraction and Protective Effect on Injured Cardiomyocytes in Mice of Flavonoids from Prunus mume Blossom. Molecules, 26(19), 5818. https://doi.org/10.3390/molecules26195818






