Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part
Abstract
1. Introduction
2. Results and Discussion
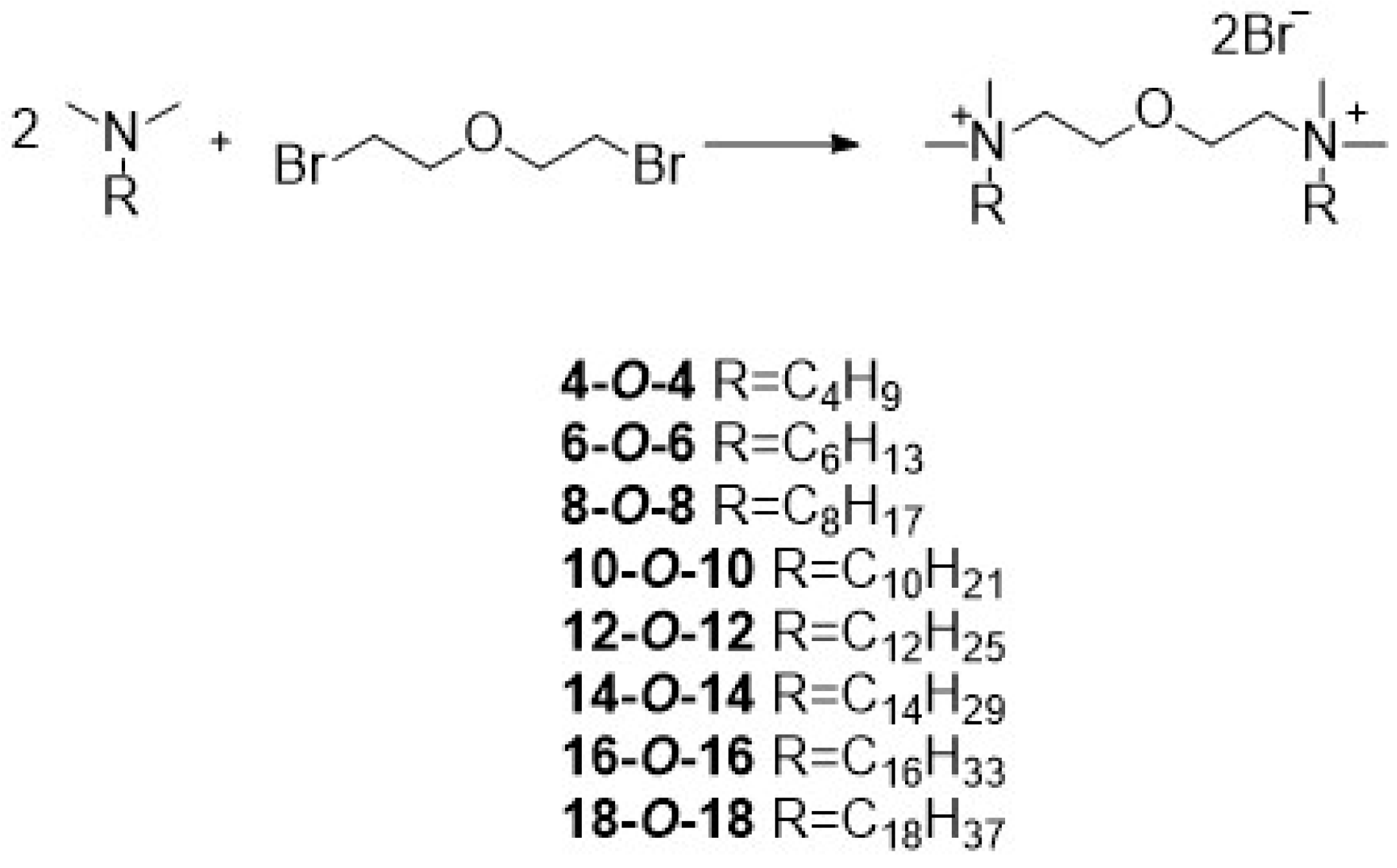
2.1. Synthesis and Analysis
2.2. Surface Properties of Gemini Surfactants
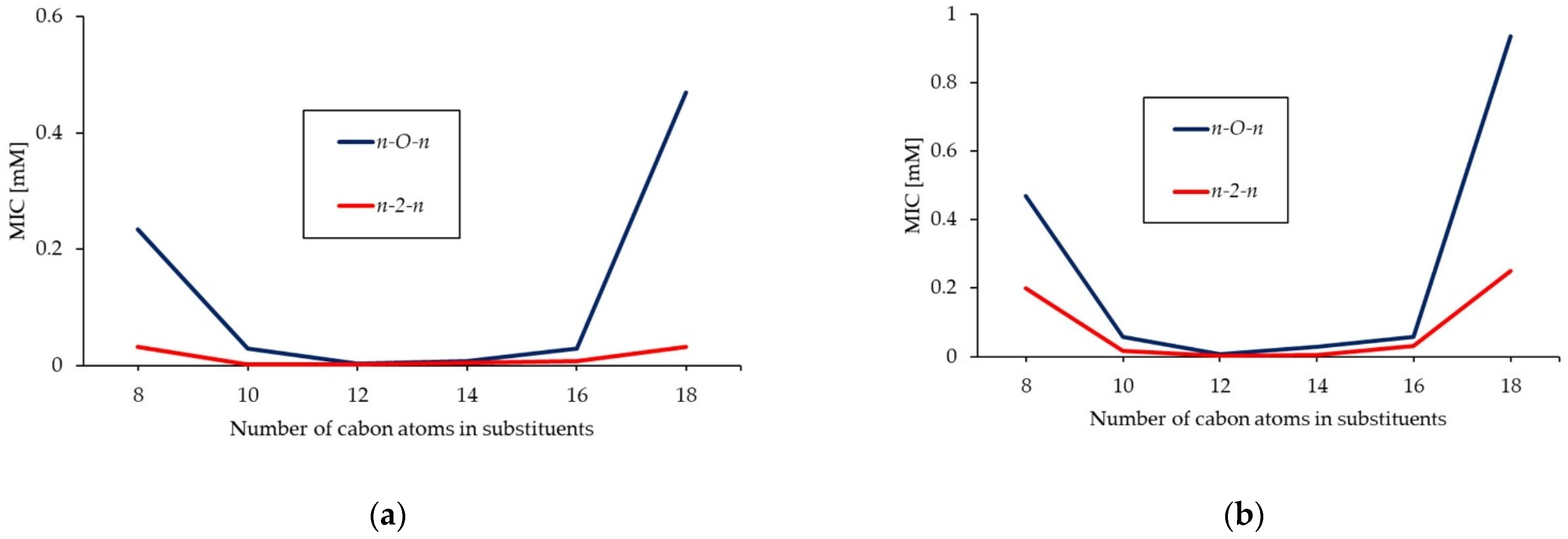
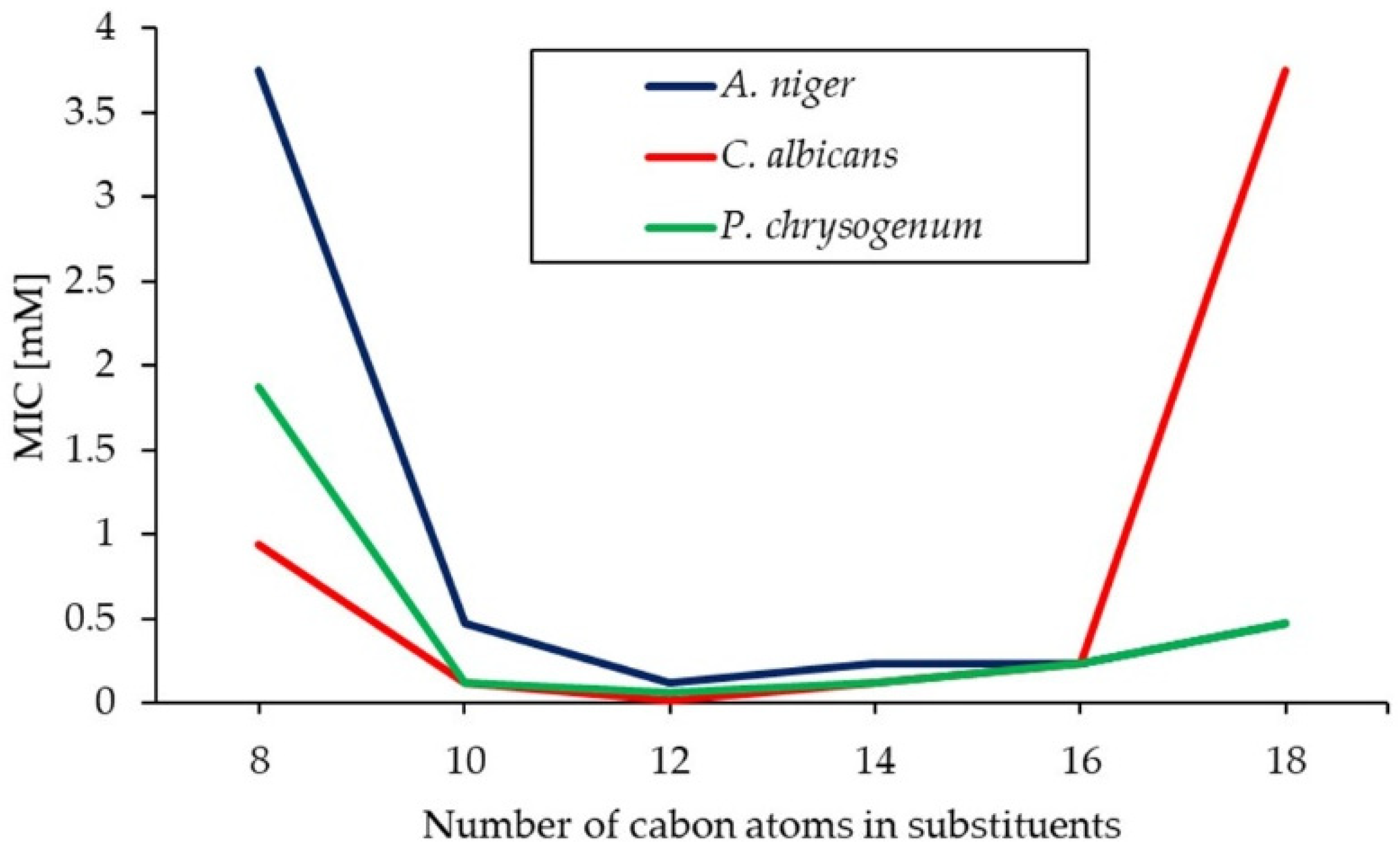
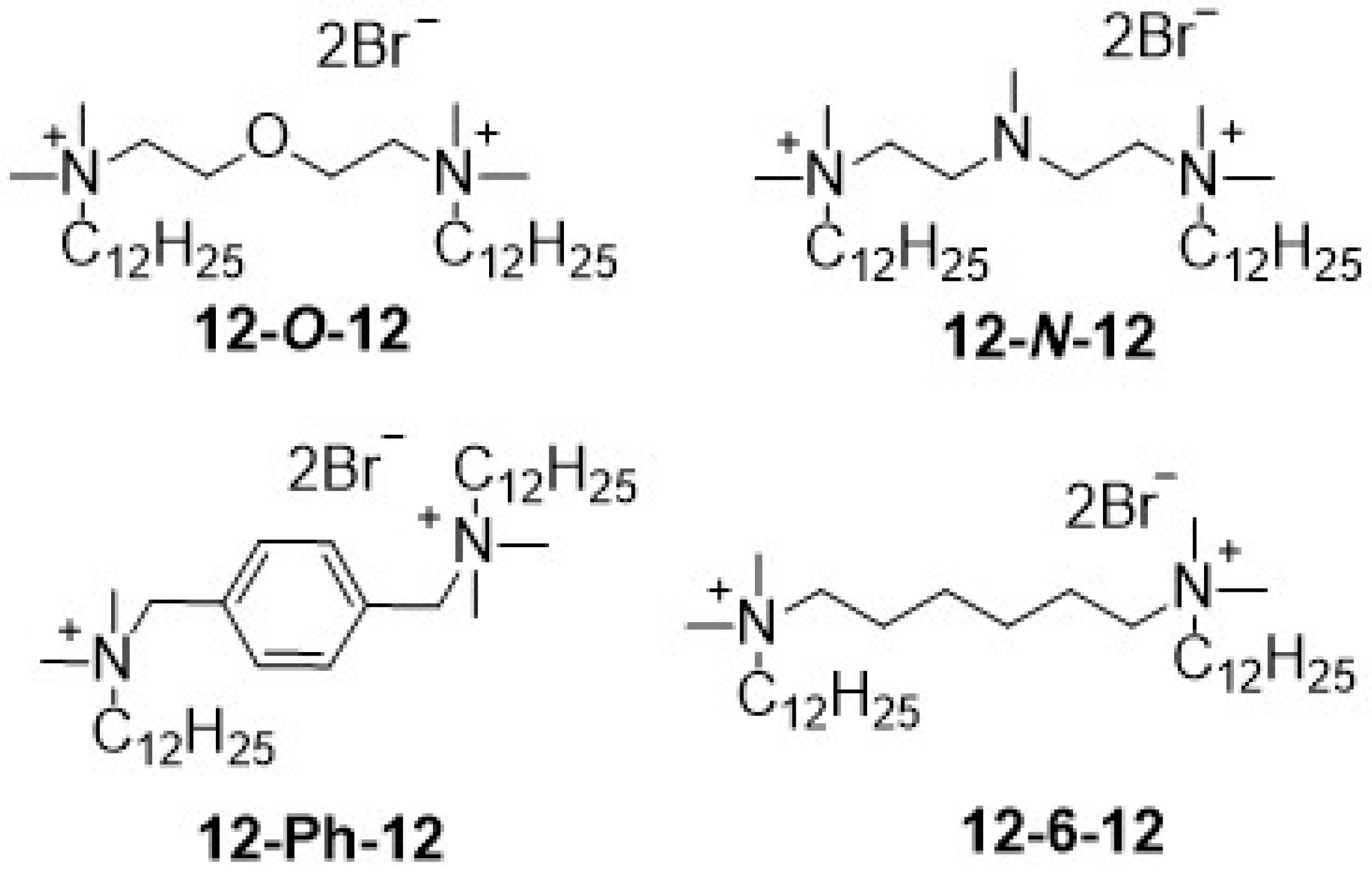
2.3. Antimicrobial Activity of Gemini Surfactants
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Analytical Methods
3.4. Conductivity Measurement
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Multifunctional Gemini Surfactants: Structure, Synthesis, Properties and Applications. In Application and Characterization of Surfactants; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Biocides Market Size, Share & Trends Analysis Report by Product (Halogen Compounds, Quaternary Ammonium Compounds), by Application (Paints & Coatings, Water Treatment), by Region, And Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/biocides-industry (accessed on 1 September 2021).
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Rodríguez-Verdugo, A.; Lozano-Huntelman, N.; Cruz-Loya, M.; Savage, V.; Yeh, P. Compounding Effects of Climate Warming and Antibiotic Resistance. iScience 2020, 23, 101024. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Reuter, G. Disinfection and hygiene in the field of food of animal origin. Int. Biodeterior. Biodegrad. 1998, 41, 209–215. [Google Scholar] [CrossRef]
- Russell, A. Introduction of biocides into clinical practice and the impact on antibiotic-resistant bacteria. J. Appl. Microbiol. 2002, 92, 121S–135S. [Google Scholar] [CrossRef] [PubMed]
- Russell, A. Biocide use and antibiotic resistance: The relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 2003, 3, 794–803. [Google Scholar] [CrossRef]
- Vereshchagin, A.; Frolov, N.; Egorova, K.; Seitkalieva, M.; Ananikov, V. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Brycki, B. Gemini Alkylammonium Salts as Biodeterioration Inhibitors. Pol. J. Microbiol. 2010, 59, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Domagk, G. Eine neue Klasse von Desinfektionsmitteln. DMW - Dtsch. Med. Wochenschr. 1935, 61, 829–832. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini-surfactants: Synthesis and properties. J. Am. Chem. Soc. 1991, 113, 1451–1452. [Google Scholar] [CrossRef]
- Alami, E.; Beinert, G.; Marie, P.; Zana, R. Alkanediyl- α,ω.-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 1993, 9, 1465–1467. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini Surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Buse, J.; Badea, I.; Verrall, R.E.; El-Aneed, A. Tandem Mass Spectrometric Analysis of the Novel Gemini Surfactant Nanoparticle Families G12-s and G18:1-s. Spectrosc. Lett. 2010, 43, 447–457. [Google Scholar] [CrossRef]
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self-assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Rosen, M.J.; Tracy, D.J. Gemini surfactants. J. Surfactants Deterg. 1998, 1, 547–554. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents - influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.; Rizvanova, L.Z.; Konovalov, A.I. Geminal alkylammonium surfactants: Aggregation properties and catalytic activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- He, S.; Chen, H.; Guo, Z.; Wang, B.; Tang, C.; Feng, Y. High-concentration silver colloid stabilized by a cationic gemini surfactant. Colloids Surfaces A: Physicochem. Eng. Asp. 2013, 429, 98–105. [Google Scholar] [CrossRef]
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N-dodecylammonium bromide). J. Mol. Liq. 2016, 221, 1086–1096. [Google Scholar] [CrossRef]
- Dani, U.; Bahadur, A.; Kuperkar, K. Micellization, Antimicrobial Activity and Curcumin Solubilization in Gemini Surfactants: Influence of Spacer and Non-Polar Tail. Colloid Interface Sci. Commun. 2018, 25, 22–30. [Google Scholar] [CrossRef]
- Minbiole, K.P.; Jennings, M.C.; Ator, L.E.; Black, J.W.; Grenier, M.C.; LaDow, J.E.; Caran, K.; Seifert, K.; Wuest, W. From antimicrobial activity to mechanism of resistance: The multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron 2016, 72, 3559–3566. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Yuan, H.; Yin, J.; Hu, M. Antibacterial Mechanism of Octamethylene-1,8-Bis(Dodecyldimethylammonium Bromide) Against E. coli. J. Surfactants Deterg. 2017, 20, 717–723. [Google Scholar] [CrossRef]
- Maneedaeng, A.; Phoemboon, S.; Chanthasena, P.; Chudapongse, N. Synthesis, interfacial properties, and antimicrobial activity of a new cationic gemini surfactant. Korean J. Chem. Eng. 2018, 35, 2313–2320. [Google Scholar] [CrossRef]
- Łudzik, K.; Kustrzepa, K.; Kowalewicz-Kulbat, M.; Kontek, R.; Kontek, B.; Wróblewska, A.; Jozwiak, M.; Lulo, D. Antimicrobial and Cytotoxic Properties of Bisquaternary Ammonium Bromides of Different Spacer Length. J. Surfactants Deterg. 2018, 21, 91–99. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Guz-Regner, K.; Dworniczek, E. Antibacterial activity of gemini quaternary ammonium salts. FEMS Microbiol. Lett. 2013, 350, 190–198. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Din, K.-U.; Beg, M. Ester-bonded cationic gemini surfactants: Assessment of their cytotoxicity and antimicrobial activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar] [CrossRef]
- Machuca, L.M.; Reno, U.; Plem, S.C.; Gagneten, A.M.; Murguía, M.C. N-Acetylated Gemini Surfactants: Synthesis, Surface-Active Properties, Antifungal Activity, and Ecotoxicity Bioassays. Adv. Chem. Eng. Sci. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.M.; Aiad, I.; Moustafa, H.; Hamed, A. Amidoamine Gemini surfactants based dimethylamino propyl amine: Preparation, characterization and evaluation as biocide. J. Mol. Liq. 2015, 212, 907–914. [Google Scholar] [CrossRef]
- Taleb, K.; Mohamed-Benkada, M.; Benhamed, N.; Saidi-Besbes, S.; Grohens, Y.; Derdour, A. Benzene ring containing cationic gemini surfactants: Synthesis, surface properties and antibacterial activity. J. Mol. Liq. 2017, 241, 81–90. [Google Scholar] [CrossRef]
- Hsu, L.-H.; Kwaśniewska, D.; Wang, S.-C.; Shen, T.-L.; Wieczorek, D.; Chen, Y.-L. Gemini quaternary ammonium compound PMT12-BF4 inhibits Candida albicans via regulating iron homeostasis. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Winnicki, K.; Łudzik, K.; Żabka, A.; Polit, J.T.; Zawisza, A.; Maszewski, J. Anti-algal activity of the 12-5-12 gemini surfactant results from its impact on the photosynthetic apparatus. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2014, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ly-Chatain, M.H.; Moussaoui, S.; Vera, A.; Rigobello, V.; Demarigny, Y. Antiviral effect of cationic compounds on bacteriophages. Front. Microbiol. 2013, 4, 46. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Másson, M.; Kristinsson, K.G.; Hjálmarsdóttir, M.A.; Hilmarsson, H.; Loftsson, T. Soft Antimicrobial Agents: Synthesis and Activity of Labile Environmentally Friendly Long Chain Quaternary Ammonium Compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Bolton, S.L.; Kotwal, G.; Harrison, M.A.; Law, S.E.; Harrison, J.A.; Cannon, J.L. Sanitizer Efficacy against Murine Norovirus, a Surrogate for Human Norovirus, on Stainless Steel Surfaces when Using Three Application Methods. Appl. Environ. Microbiol. 2012, 79, 1368–1377. [Google Scholar] [CrossRef]
- Thevenin, T.; Lobert, P.E.; Hober, D. Inactivation of Coxsackievirus B4, Feline Calicivirus and Herpes Simplex Virus Type 1: Unexpected Virucidal Effect of a Disinfectant on a Non-Enveloped Virus Applied onto a Surface. Intervirology 2013, 56, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Schrank, C.L.; Minbiole, K.P.C.; Wuest, W.M. Are Quaternary Ammonium Compounds, the Workhorse Disinfectants, Effective against Severe Acute Respiratory Syndrome-Coronavirus-2? ACS Infect. Dis. 2020, 6, 1553–1557. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.A.; Guastavino, J.F.; Nicollier, R.A.; Lancelle, M.V.; Russell-White, K.; Murguía, M.C. Synthesis and Properties of Cleavable Quaternary Ammonium Compounds. J. Oleo Sci. 2021, 70, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Szulc, A. Gemini Alkyldeoxy-D-Glucitolammonium Salts as Modern Surfactants and Microbiocides: Synthesis, Antimicrobial and Surface Activity, Biodegradation. PLoS ONE 2014, 9, e84936. [Google Scholar] [CrossRef] [PubMed]
- Kaczerewska, O.; Brycki, B.; Ribosa, I.; Comelles, F.; Garcia, M.T. Cationic gemini surfactants containing an O-substituted spacer and hydroxyethyl moiety in the polar heads: Self-assembly, biodegradability and aquatic toxicity. J. Ind. Eng. Chem. 2018, 59, 141–148. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Garcia, R.L.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Effectiveness of O -bridged cationic gemini surfactants as corrosion inhibitors for stainless steel in 3 M HCl: Experimental and theoretical studies. J. Mol. Liq. 2018, 249, 1113–1124. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B. Efficiency of cationic gemini surfactants with 3-azamethylpentamethylene spacer as corrosion inhibitors for stainless steel in hydrochloric acid. J. Mol. Liq. 2017, 247, 6–13. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Leiva-Garcia, R.; Akid, R.; Brycki, B.; Kowalczyk, I.; Pospieszny, T. Heteroatoms and π electrons as favorable factors for efficient corrosion protection. Mater. Corros. 2019, 70, 1099–1110. [Google Scholar] [CrossRef]
- Pakiet, M.; Kowalczyk, I.; Garcia, R.L.; Akid, R.; Brycki, B. Cationic clevelable surfactants as highly efficient corrosion inhibitors of stainless steel AISI 304: Electrochemical study. J. Mol. Liq. 2020, 315, 113675. [Google Scholar] [CrossRef]
- Kaczerewska, O.; Martins, R.; Figueiredo, J.; Loureiro, S.; Tedim, J. Environmental behaviour and ecotoxicity of cationic surfactants towards marine organisms. J. Hazard. Mater. 2020, 392, 122299. [Google Scholar] [CrossRef]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Biodegradability and aquatic toxicity of quaternary ammonium-based gemini surfactants: Effect of the spacer on their ecological properties. Chemosphere 2016, 154, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Waligórska, M.; Szulc, A. The biodegradation of monomeric and dimeric alkylammonium surfactants. J. Hazard. Mater. 2014, 280, 797–815. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Anwar, S.; Ansari, F.; Bhat, I.A.; Kabir-Ud-Din, K.-U.-D. Bio-physicochemical analysis of ethylene oxide-linked diester-functionalized green cationic gemini surfactants. RSC Adv. 2016, 6, 21697–21705. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and aquatic toxicity of new cleavable betainate cationic oligomeric surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- Tehrani, A.; Oskarsson, H.; van Ginkel, C.; Holmberg, K. Cationic ester-containing gemini surfactants: Chemical hydrolysis and biodegradation. J. Colloid Interface Sci. 2007, 312, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, A.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Physical−Chemical Properties. Langmuir 2010, 26, 9276–9282. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Bagha, A.; Holmberg, K.; van Ginkel, C.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interface Sci. 2015, 449, 72–79. [Google Scholar] [CrossRef]
- Bergero, M.; Liffourrena, A.; Opizzo, B.; Fochesatto, A.; Lucchesi, G. Immobilization of a microbial consortium on Ca-alginate enhances degradation of cationic surfactants in flasks and bioreactor. Int. Biodeterior. Biodegrad. 2017, 117, 39–44. [Google Scholar] [CrossRef]
- Bergero, M.F.; Lucchesi, G.I. Degradation of cationic surfactants using immobilized bacteria: Its effect on adsorption to activated sludge. J. Biotechnol. 2018, 272–273, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Szulc, A.; Babkova, M. Synthesis of Silver Nanoparticles with Gemini Surfactants as Efficient Capping and Stabilizing Agents. Appl. Sci. 2020, 11, 154. [Google Scholar] [CrossRef]
- Mondal, M.H.; Malik, S.; Roy, A.; Saha, R.; Saha, B. Modernization of surfactant chemistry in the age of gemini and bio-surfactants: A review. RSC Adv. 2015, 5, 92707–92718. [Google Scholar] [CrossRef]
- Mondal, M.H.; Roy, A.; Malik, S.; Ghosh, A.; Saha, B. Review on chemically bonded geminis with cationic heads: Second-generation interfactants. Res. Chem. Intermed. 2016, 42, 1913–1928. [Google Scholar] [CrossRef]
- Hauner, I.M.; Deblais, A.; Beattie, J.K.; Kellay, H.; Bonn, D. The Dynamic Surface Tension of Water. J. Phys. Chem. Lett. 2017, 8, 1599–1603. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-Active Properties and Antimicrobial Study of Conventional Cationic and Synthesized Symmetrical Gemini Surfactants. J. Surfactants Deterg. 2011, 15, 107–115. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y. Aggregation behavior of gemini surfactants and their interaction with macromolecules in aqueous solution. Phys. Chem. Chem. Phys. 2011, 13, 1939–1956. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Szulc, A.; Koenig, H.; Kowalczyk, I.; Pospieszny, T.; Górka, S. Effect of the alkyl chain length on micelle formation for bis(N-alkyl-N,N-dimethylethylammonium)ether dibromides. Comptes Rendus Chim. 2019, 22, 386–392. [Google Scholar] [CrossRef]
- Chlebicki, J.; Węgrzyńska, J.; Wilk, K.A. Surface-active, micellar, and antielectrostatic properties of bis-ammonium salts. J. Colloid Interface Sci. 2008, 323, 372–378. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, I.; Paul, B.K.; Ghosh, S. Physicochemical Behaviors of Cationic Gemini Surfactant (14-4-14) Based Microheterogeneous Assemblies. Langmuir 2014, 30, 12483–12493. [Google Scholar] [CrossRef]
- Pisárčik, M.; Devínsky, F. Surface tension study of cationic gemini surfactants binding to DNA. Open Chem. 2014, 12, 577–585. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, R. Synthesis, Structural Properties and Applications of Gemini Surfactants: A Review. Tenside Surfactants Deterg. 2009, 46, 373–382. [Google Scholar] [CrossRef]
- Kumar, N.; Tyagi, R. Dimeric Surfactants: Promising Ingredients of Cosmetics and Toiletries. Cosmetics 2013, 1, 3–13. [Google Scholar] [CrossRef]
- Banno, T.; Kawada, K.; Matsumura, S. Creation of Novel Green and Sustainable Gemini-Type Cationics Containing Carbonate Linkages. J. Surfactants Deterg. 2010, 13, 387–398. [Google Scholar] [CrossRef]
- Kirby, A.J.; Camilleri, P.; Engberts, J.B.F.N.; Feiters, M.C.; Nolte, R.; Söderman, O.; Bergsma, M.; Bell, P.C.; Fielden, M.L.; Rodríguez, C.L.G.; et al. Gemini Surfactants: New Synthetic Vectors for Gene Transfection. Angew. Chem. Int. Ed. 2003, 42, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Bhadani, A.; Singh, S. Novel Gemini Pyridinium Surfactants: Synthesis and Study of Their Surface Activity, DNA Binding, and Cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Kowalczyk, I.; Szulc, A.M.; Brycka, J.A. Quaternary Alkylammonium Salts as Cleaning and Disinfectant Agents. Tenside Surfactants Deterg. 2018, 55, 432–438. [Google Scholar] [CrossRef]
- Chang, H.; Cui, Y.; Wang, Y.; Li, G.; Gao, W.; Li, X.; Zhao, X.; Wei, W. Wettability and adsorption of PTFE and paraffin surfaces by aqueous solutions of biquaternary ammonium salt Gemini surfactants with hydroxyl. Colloids Surfaces A: Physicochem. Eng. Asp. 2016, 506, 416–424. [Google Scholar] [CrossRef]
- Alimohammadi, M.H.; Javadian, S.; Gharibi, H.; Tehrani-Bagha, A.R.; Alavijeh, M.R.; Kakaei, K. Aggregation behavior and intermicellar interactions of cationic Gemini surfactants: Effects of alkyl chain, spacer lengths and temperature. J. Chem. Thermodyn. 2012, 44, 107–115. [Google Scholar] [CrossRef]
- Grosmaire, L.; Chorro, M.; Chorro, C.; Partyka, S.; Zana, R. Alkanediyl-α,ω-Bis(dimethylalkylammonium Bromide) Surfactants: 9. Effect of the spacer carbon number and temperature on the enthalpy of micellization. J. Colloid Interface Sci. 2002, 246, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.; Kaczerewska, O.; Ribosa, I.; Brycki, B.; Materna, P.; Drgas, M. Hydrophilicity and flexibility of the spacer as critical parameters on the aggregation behavior of long alkyl chain cationic gemini surfactants in aqueous solution. J. Mol. Liq. 2017, 230, 453–460. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Studies on the physicochemical properties of synthesized tailor-made gemini surfactants for application in enhanced oil recovery. J. Mol. Liq. 2018, 258, 211–224. [Google Scholar] [CrossRef]
- Laschewsky, A.; Wattebled, L.; Arotcarena, M.; Habib-Jiwan, J.-L.; Rakotoaly, R.H. Synthesis and Properties of Cationic Oligomeric Surfactants. Langmuir 2005, 21, 7170–7179. [Google Scholar] [CrossRef] [PubMed]
- Pisárčik, M.; Polakovičová, M.; Markuliak, M.; Lukáč, M.; Devínsky, F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules 2019, 24, 1481. [Google Scholar] [CrossRef] [PubMed]
- Mivehi, L.; Bordes, R.; Holmberg, K. Adsorption of Cationic Gemini Surfactants at Solid Surfaces Studied by QCM-D and SPR: Effect of the Rigidity of the Spacer. Langmuir 2011, 27, 7549–7557. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Jiang, Y.; Wang, Y.; Ju, H.; Geng, T. Studies on physicochemical properties of three Gemini surfactants with different spacer groups. J. Mol. Liq. 2021, 325, 115039. [Google Scholar] [CrossRef]
- Islam, M.S.; Shortall, S.M.; Mekhail, G.M.; Callender, S.P.; Madkhali, O.; Bharwani, Z.; Ayyash, D.; Kobernyk, K.; Wettig, S.D. Effect of counterions on the micellization and monolayer behaviour of cationic gemini surfactants. Phys. Chem. Chem. Phys. 2017, 19, 10825–10834. [Google Scholar] [CrossRef]
- Jiang, N.; Li, P.; Wang, Y.; Wang, J.; Yan, H.; Thomas, R.K. Micellization of Cationic Gemini Surfactants with Various Counterions and Their Interaction with DNA in Aqueous Solution. J. Phys. Chem. B 2004, 108, 15385–15391. [Google Scholar] [CrossRef]
- Junior, P.B.; Tiera, V.A.; Tiera, M.J. A fluorescence probe study of gemini surfactants in aqueous solution: A comparison between n-2-n and n-6-n series of the alkanediyl- α,ω -bis(dimethylalkylammonium bromides). Eclética Química J. 2007, 32, 47–54. [Google Scholar] [CrossRef][Green Version]
- Zana, R.; Lévy, H. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) surfactants (dimeric surfactants) Part 6. CMC of the ethanediyl-1,2-bis(dimethylalkylammonium bromide) series. Colloids Surfaces A: Physicochem. Eng. Asp. 1997, 127, 229–232. [Google Scholar] [CrossRef]
- Dam, T.; Engberts, J.; Karthäuser, J.; Karaborni, S.; van Os, N. Synthesis, surface properties and oil solubilisation capacity of cationic gemini surfactants. Colloids Surfaces A: Physicochem. Eng. Asp. 1996, 118, 41–49. [Google Scholar] [CrossRef]
- Oda, R.; Candau, S.J.; Huc, I. Gemini surfactants, the effect of hydrophobic chain length and dissymmetry. Chem. Commun. 1997, 21, 2105–2106. [Google Scholar] [CrossRef]
- Kim, T.-S.; Kida, T.; Nakatsuji, Y.; Hirao, T.; Ikeda, I. Surface-active properties of novel cationic surfactants with two alkyl chains and two ammonio groups. J. Am. Oil Chem. Soc. 1996, 73, 907–911. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S.; Azov, V. Gemini Surfactants with Acetylenic Spacers. Langmuir 2000, 16, 2062–2067. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Wang, J.; Wang, Y.; Yan, H.; Thomas, R.K. Odd/Even Effect in the Chain Length on the Enthalpy of Micellization of Gemini Surfactants in Aqueous Solution. Langmuir 2005, 21, 6703–6706. [Google Scholar] [CrossRef] [PubMed]
- Zana, R. Alkanediyl-α,ω-bis(dimethylalkylammonium bromide) Surfactants: 10. Behavior in aqueous solution at concentrations below the critical micellization concentration: An electrical conductivity study. J. Colloid Interface Sci. 2002, 246, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Koziróg, A.; Kowalczyk, I.; Pospieszny, T.; Materna, P.; Marciniak, J. Synthesis, Structure, Surface and Antimicrobial Properties of New Oligomeric Quaternary Ammonium Salts with Aromatic Spacers. Molecules 2017, 22, 1810. [Google Scholar] [CrossRef] [PubMed]
- Laatiris, A.; El Achouri, M.; Infante, M.R.; Bensouda, Y. Antibacterial activity, structure and CMC relationships of alkanediyl α,ω-bis(dimethylammonium bromide) surfactants. Microbiol. Res. 2008, 163, 645–650. [Google Scholar] [CrossRef]
- Łudzik, K.; Kustrzepa, K.; Piekarski, H. Thermodynamics of Micelle Formation of Gemini Surfactants Hexylene-1,6-bis(dimethyloctylammonium bromide) and Dodecylene-1,12-bis(dimethyloctylammonium bromide) by Electric Conductance Mesurements. J. Chem. Eng. Data 2014, 59, 4165–4172. [Google Scholar] [CrossRef]
- Kowalczyk, I.; Pakiet, M.; Szulc, A.; Koziróg, A. Antimicrobial Activity of Gemini Surfactants with Azapolymethylene Spacer. Molecules 2020, 25, 4054. [Google Scholar] [CrossRef] [PubMed]
- Egorova, E.M.; Kaba, S.I. The effect of surfactant micellization on the cytotoxicity of silver nanoparticles stabilized with aerosol-OT. Toxicol. In Vitro 2019, 57, 244–254. [Google Scholar] [CrossRef]
- Singer, M.M.; George, S.; Tjeerdema, R.S. Relationship of Some Physical Properties of Oil Dispersants and Their Toxicity to Marine Organisms. Arch. Environ. Contam. Toxicol. 1995, 29, 33–38. [Google Scholar] [CrossRef]
- Devinsky, F.; Lacko, I.; Mlynarčik, D.; Račanský, V.; Krasnec, L. Relationship Between Critical Micelle Concentrations and Minimum Inhibitory Concentrations for Some Non-Aromatic Quaternary Ammonium Salts and Amine Oxides. Tenside Surfactants Deterg. 1985, 22, 10–15. [Google Scholar] [CrossRef]
- Králová, K.; Kallová, J.; Loos, D.; Devínsky, F. Correlation between biological activity and the structure of N,N′-bis(alkyldimethyl)-1,6-hexanediammonium dibromides. Antibacterial activity and inhibition of photochemical activity of chloroplasts. Die Pharm. 1994, 49, 857–858. [Google Scholar]
- Banno, T.; Toshima, K.; Kawada, K.; Matsumura, S. Synthesis and Properties of Gemini-type Cationic Surfactants Containing Carbonate Linkages in the Linker Moiety Directed Toward Green and Sustainable Chemistry. J. Surfactants Deterg. 2009, 12, 249–259. [Google Scholar] [CrossRef]
- Caillier, L.; de Givenchy, E.T.; Levy, R.; Vandenberghe, Y.; Geribaldi, S.; Guittard, F. Polymerizable semi-fluorinated gemini surfactants designed for antimicrobial materials. J. Colloid Interface Sci. 2009, 332, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Paniak, T.J.; Jennings, M.C.; Shanahan, P.C.; Joyce, M.D.; Santiago, C.N.; Wuest, W.; Minbiole, K.P. The antimicrobial activity of mono-, bis-, tris-, and tetracationic amphiphiles derived from simple polyamine platforms. Bioorganic Med. Chem. Lett. 2014, 24, 5824–5828. [Google Scholar] [CrossRef]
- Mlynarcik, D.; Lacko, I.; Devinsky, F. Antimicrobial effect of bis-quaternary ammonium salts derived from 1,3-propanediamine. Cell. Mol. Life Sci. 1979, 35, 1044–1045. [Google Scholar] [CrossRef]
- Xu, D.; Ni, X.; Zhang, C.; Mao, J.; Song, C. Synthesis and properties of biodegradable cationic gemini surfactants with diester and flexible spacers. J. Mol. Liq. 2017, 240, 542–548. [Google Scholar] [CrossRef]
- Massi, L.; Guittard, F.; Levy, R.; Géribaldi, S. Enhanced activity of fluorinated quaternary ammonium surfactants against Pseudomonas aeruginosa. Eur. J. Med. Chem. 2009, 44, 1615–1622. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, S.; Yu, J.; Chen, X.; Lei, Q.; Fang, W. Antibacterial Activity, in Vitro Cytotoxicity, and Cell Cycle Arrest of Gemini Quaternary Ammonium Surfactants. Langmuir 2015, 31, 12161–12169. [Google Scholar] [CrossRef]
- Imam, T.; Devinsky, F.; Lacko, I.; Mlynarcik, D.; Krasnec, L. Preparation And Antimicrobial Activity Of Some New Bisquaternary Ammonium Salts. Pharmazie 1983, 38, 308–310. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Koziróg, A. Synthesis, Molecular Structure, Spectral Properties and Antifungal Activity of Polymethylene-α,ω-bis(N,N- dimethyl-N-dodecyloammonium Bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Martín, V.I.; de la Haba, R.R.; Ventosa, A.; Congiu, E.; Ortega-Calvo, J.J.; Moyá, M.L. Colloidal and biological properties of cationic single-chain and dimeric surfactants. Colloids Surf. B Biointerfaces 2014, 114, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Sun, X.; Zou, Q.; Zhang, J.; Ni, H. Antibacterial Activities of Five Cationic Gemini Surfactants with Ethylene Glycol Bisacetyl Spacers. J. Surfactants Deterg. 2014, 17, 1089–1097. [Google Scholar] [CrossRef]
- Zhu, H.-L.; Hu, Z.-Y.; Ma, X.-M.; Wang, J.-L.; Cao, D.-L. Synthesis, Surface and Antimicrobial Activities of Cationic Gemini Surfactants with Semi-Rigid Spacers. J. Surfactants Deterg. 2016, 19, 265–274. [Google Scholar] [CrossRef]
- Jennings, M.C.; Ator, L.E.; Paniak, T.J.; Minbiole, K.P.C.; Wuest, W.M. Biofilm-Eradicating Properties of Quaternary Ammonium Amphiphiles: Simple Mimics of Antimicrobial Peptides. ChemBioChem 2014, 15, 2211–2215. [Google Scholar] [CrossRef]
- Tatsumi, T.; Zhang, W.; Nakatsuji, Y.; Miyake, K.; Matsushima, K.; Tanaka, M.; Furuta, T.; Ikeda, I. Preparation, surface-active properties, and antimicrobial activities of bis(alkylammonium) dichlorides having a butenylen or a butynylene spacer. J. Surfactants Deterg. 2001, 4, 271–277. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Dworniczek, E.; Olejniczak, T. The Influence of Biodegradable Gemini Surfactants, N,N′-bis(1-Decyloxy-1-Oxopronan-2-yl)-N,N,N′,N′ Tetramethylpropane-1,3-Diammonium Dibromide and N,N′-bis(1-Dodecyloxy-1-Oxopronan-2-yl) N,N,N′,N′-Tetramethylethane-1,2-Diammonium Dibromide, on Fungal Biofilm and Adhesion. J. Oleo Sci. 2015, 64, 527–537. [Google Scholar] [CrossRef]
- Ding, Z.; Fang, S. Synthesis, Surface and Antimicrobial Activities of Novel Cationic Gemini Surfactants. J. Surfactants Deterg. 2015, 18, 1051–1057. [Google Scholar] [CrossRef]
- Maillard, J.-Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16S–27S. [Google Scholar] [CrossRef]
- Mlynarcik, D.; Sirotková, L.; Devínsky, F.; Masárová, Ľ.; Pikulíková, A.; Lacko, I. Potassium leakage from Escherichia coli cells treated by organic ammonium salts. J. Basic Microbiol. 1992, 32, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Devínsky, F.; Kopecka-Leitmanová, A.; Šeršeň, F.; Balgavý, P. Cut-off Effect in Antimicrobial Activity and in Membrane Perturbation Efficiency of the Homologous Series of N,N-Dimethylalkylamine Oxides. J. Pharm. Pharmacol. 2011, 42, 790–794. [Google Scholar] [CrossRef]
- Balgavý, P.; Devínsky, F. Cut-off effects in biological activities of surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Pakiet, M.; Tedim, J.; Kowalczyk, I.; Brycki, B. Functionalised novel gemini surfactants as corrosion inhibitors for mild steel in 50 mM NaCl: Experimental and theoretical insights. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123699. [Google Scholar] [CrossRef]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
| Gemini | -OCH2 | -N+CH2CH2O- | -N+CH2- | -N+(CH3)2 | -N+CH2CH2- | -CH2(CH2)xCH3 | -CH2CH3 |
|---|---|---|---|---|---|---|---|
| 4-O-4 | 4.14 (4H) | 3.83 (4H) | 3.53 (4H) | 3.29 (12H) | 1.74 (4H) | 1.44 (4H) | 1.01 (6H) |
| 6-O-6 | 4.18 (4H) | 3.85 (4H) | 3.51 (4H) | 3.32 (12H) | 1.75 (4H) | 1.37 (12H) | 0.90 (6H) |
| 8-O-8 | 4.35 (4H) | 4.04 (4H) | 3.65 (4H) | 3.46 (12H) | 1.73 (4H) | 1.47–1.19 (20H) | 0.88 (6H) |
| 10-O-10 | 4.36 (4H) | 4.04 (4H) | 3.65 (4H) | 3.46 (12H) | 1.73 (4H) | 1.48–1.14 (28H) | 0.88 (6H) |
| 12-O-12 | 4.35 (4H) | 4.04 (4H) | 3.63 (4H) | 3.46 (12H) | 1.73 (4H) | 1.45–1.17 (36H) | 0.88 (6H) |
| 14-O-14 | 4.36 (4H) | 4.04 (4H) | 3.65 (4H) | 3.46 (12H) | 1.73 (4H) | 1.49–1.08 (44H) | 0.88 (6H) |
| 16-O-16 | 4.36 (4H) | 4.05 (4H) | 3.64 (4H) | 3.46 (12H) | 1.73 (4H) | 1.47–1.10 (52H) | 0.88 (6H) |
| 18-O-18 | 4.16 (4H) | 3.84 (4H) | 3.49 (4H) | 3.29 (12H) | 1.72 (4H) | 1.46–1.03 (60H) | 0.88 (6H) |
| Gemini | -N+CH2CH2O- | -OCH2- | -N+CH2- | -N+CH3 | -N+CH2(CH2)x- | -CH2CH3 | -CH2CH3 |
|---|---|---|---|---|---|---|---|
| 4-O-4 | 65.61 | 64.42 | 63.98 | 51.36 | 24.42 | 19.39 | 13.41 |
| 6-O-6 | 65.88 | 64.47 | 63.96 | 51.40 | 31.10, 25.70, 22.55 | 22.20 | 13.69 |
| 8-O-8 | 65.76 | 64.56 | 63.89 | 51.58 | 31.57, 29.18, 28.98, 26.23, 22.83 | 22.48 | 13.97 |
| 10-O-10 | 65.83 | 64.60 | 63.96 | 51.61 | 31.74, 29.38, 29.35, 29.25, 29.15, 26.28, 22.87 | 22.55 | 13.99 |
| 12-O-12 | 65.81 | 64.57 | 63.94 | 51.60 | 31.82, 29.53, 29.46, 29.38, 29.28, 29.26, 26.27, 22.87 | 22.60 | 14.05 |
| 14-O-14 | 65.87 | 64.61 | 64.00 | 51.64 | 31.85, 29.62, 29.60, 29.59, 29.56, 29.48, 29.40, 29.29, 26.31, 22.90 | 22.61 | 14.04 |
| 16-O-16 | 65.92 | 64.59 | 64.03 | 51.57 | 31.82, 29.61, 29.58, 29.56, 29.54, 29.45, 29.37, 29.25, 26.25, 22.83 | 22.58 | 14.00 |
| 18-O-18 | 65.65 | 64.56 | 63.76 | 51.37 | 31.84, 29.63, 29.61, 29.58, 29.52, 29.48, 29.40, 29.28, 26.31, 22.85 | 22.60 | 14.03 |
| Gemini Surfactants | CMC (mM) | β | ΔG0mic (kJ/mol) |
|---|---|---|---|
| 4-O-4 | 158.5 ± 3.5 | 0.31 | −11.8 |
| 6-O-6 | 81.1 ± 2.0 | 0.34 | −12.2 |
| 8-O-8 | 21.1 ± 0.9 | 0.34 | −17.8 |
| 10-O-10 | 6.59 ± 0.16 | 0.51 | −26.9 |
| 12-O-12 | 1.047 ± 0.006 | 0.71 | −42.9 |
| 14-O-14 | 0.201 ± 0.002 | 0.58 | −47.3 |
| 16-O-16 | 0.032 ± 0.002 | 0.44 | −49.9 |
| 18-O-18 | 0.033 ± 0.001 | 0.58 | −56.9 |
| Gemini Surfactants | MIC (mM) | ||||
|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | A. niger | P. chrysogenum | |
| 4-O-4 | >3.75 | >3.75 | >3.75 | >3.75 | >3.75 |
| 6-O-6 | 1.875 | 1.875 | >3.75 | >3.75 | >3.75 |
| 8-O-8 | 0.234 | 0.469 | 0.937 | 3.75 | 1.875 |
| 10-O-10 | 0.0293 | 0.058 | 0.117 | 0.469 | 0.117 |
| 12-O-12 | 0.0037 | 0.0073 | 0.0146 | 0.117 | 0.058 |
| 14-O-14 | 0.0073 | 0.0293 | 0.117 | 0.234 | 0.117 |
| 16-O-16 | 0.0293 | 0.058 | 0.234 | 0.234 | 0.234 |
| 18-O-18 | 0.469 | 0.937 | >3.75 | 0.469 | 0.469 |
| Gemini Surfactants | MIC (mM) | ||||
|---|---|---|---|---|---|
| S. aureus | E. coli | C. albicans | A. niger | P. chrysogenum | |
| 12-O-12 | 0.0037 1 | 0.0073 1 | 0.0146 1 | 0.117 1 | 0.058 1 |
| 12-N-12 | 0.003 2 | 0.007 2 | 0.023 2 | 0.116 2 | - |
| 12-Ph-12 | 0.0122 3 | 0.0244 3 | 0.0122 3 | 0.0976 3 | 0.0488 3 |
| 12-6-12 | 0.0036 4 | - | 0.015 5 | 0.12 5 | 0.06 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brycki, B.E.; Szulc, A.; Kowalczyk, I.; Koziróg, A.; Sobolewska, E. Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules 2021, 26, 5759. https://doi.org/10.3390/molecules26195759
Brycki BE, Szulc A, Kowalczyk I, Koziróg A, Sobolewska E. Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules. 2021; 26(19):5759. https://doi.org/10.3390/molecules26195759
Chicago/Turabian StyleBrycki, Bogumil Eugene, Adrianna Szulc, Iwona Kowalczyk, Anna Koziróg, and Ewelina Sobolewska. 2021. "Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part" Molecules 26, no. 19: 5759. https://doi.org/10.3390/molecules26195759
APA StyleBrycki, B. E., Szulc, A., Kowalczyk, I., Koziróg, A., & Sobolewska, E. (2021). Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules, 26(19), 5759. https://doi.org/10.3390/molecules26195759








