Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades
Abstract
1. Introduction
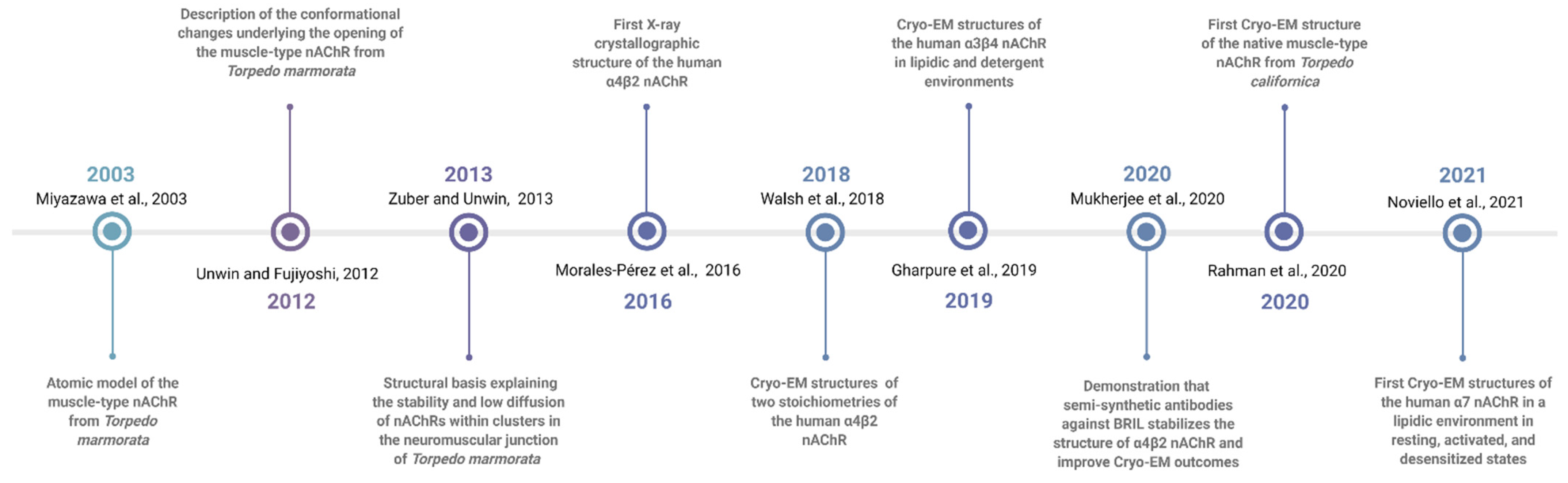
2. Historical Notes on nAChRs
3. nAChRs as Therapeutic Targets for Neurological Diseases
4. Attempts to Crystallize the Muscle-Type nAChR
5. Early Cryo-EM Structural Studies of nAChRs
6. The Challenge of Crystal Formation for nAChRs
7. Methods Historically Used for nAChR Solubilization
8. New Methods for nAChR Detergent Solubilization
9. Assessment of Purity and Stability of nAChR-DCs
10. X-ray and Cryo-EM Structural Studies of α4β2 nAChRs
11. The Nicotine Binding Site of the α4β2 nAChR
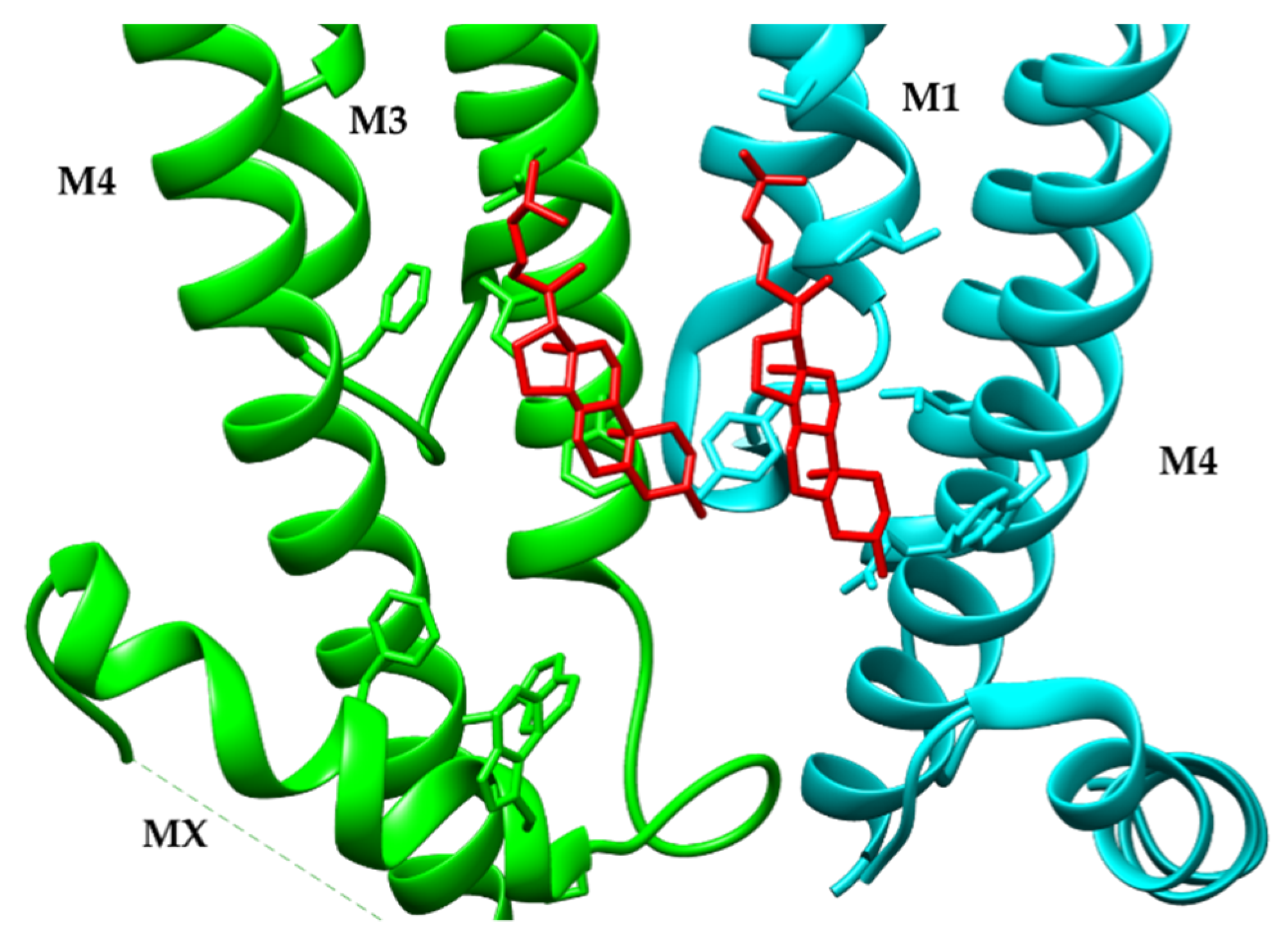
12. Sterol Binding Sites of the α4β2 nAChR
13. The Cryo-EM Structure of the α3β4 nAChR
14. The Cryo-EM Structure of the Neuronal α7 nAChR
15. The Most Recent Cryo-EM Structure of the Tc nAChR
16. Summary of nAChR Structures: Lessons Learned
- (1)
- Agonist and antagonist binding sites. The agonist binding site of nAChR is located and embodied within six canonical loops in the ECDs, in the interface between a primary subunit (loops A–C) and a complementary subunit (loops D–F). The emerging structures of the nAChRs have confirmed that the signature loop C, which undergoes a marked conformational change upon agonist binding, contains the agonist binding site. The α4β2 nAChR X-ray structure and the Cryo-EM structures for α4β2, α3β4, α7 and muscle-type Tm nAChRs confirm previous biophysical studies for the location of amino acid residues and glycans involved in agonist binding site. In particular, the structure of the muscle-type Tm nAChRs-Bgtx complex at 2.7 Å resolution confirms side-chain contacts in the orthosteric site (αTyr-93, αTyr-190, αTyr-198), and stacks in a cation-π sandwich between α Tyr-198 and Phe-32. Additionally, αTrp-149, γTrp-55, δTrp-57 participate in ligand binding. In addition, this structure shows a cation-π sandwich that corresponds to αTyr-198-Bgtx-R36-Bgtx-Phe-32, which presumably indicates the pivotal binding site for neurotoxins to nAChRs including α7 nAChR.
- (2)
- Structural basis for differences in agonist affinities. The Cryo-EM structure of the α4β2 nAChR uncovers two agonist binding sites, α4–β2 and β2–α4. The α4–β2 has the highest affinity for nicotine. Superposition of the α4β2 and α3β4 binding sites revealed that the side chains in contact with nicotine and their orientations are conserved. An outward shift in loop C upon nicotine binding results in a less compact agonist binding pocket of the α3β4 nAChR compared to α4β2 nAChR, which could explain the reduced affinity for nicotine in the α3β4 nAChR. Additionally, the less conserved loop E from the complementary face of the agonist binding site contains side chains that are close to the agonist could contribute to affinity ranges.
- (3)
- Two stoichiometries of the α4β2 nAChR. Cryo-EM data confirmed two stoichiometries (α4(2)β2(3)) and (α4(3)β2(2)) previously suggested by functional studies [174].
- (4)
- Permeation pathways. The structure of the muscle-type Tm nAChRs-Bgtx complex provides the best atomic resolution for the nAChR ion permeation pathway in the closed state. The permeation pathway consists of an electronegative extracellular vestibule and tightly closed ion channel pore with a polar intracellular domain. The M2 helices from each subunit have two hydrophobic constriction points, one at the extracellular side of the pore, at the 16′ and 9′ positions. These two gates at 16′ and 9′ positions are the main obstructions for hydrated ion permeation.
- (5)
- Gating mechanism. The Cryo-EM maps of the α7 nAChR bound to α-Bgtx, epibatidine + PNU-120596, and epibatidine alone in combination with an elegant electrophysiological characterization of the EM construct expressed in HEK293S GnTl- cells in the absence and presence ligands and prior to solubilization of the α7 nAChR with DDM, has provided the structural basis for the resting, activated and desensitized states. Molecular dynamics simulations were used to predict a model for the α7 nAChR gating mechanism.
- (6)
- The role of the latch turns in the α7 nAChR gating. Interestingly, the α7 nAChR structures revealed residues Pro-469 and Ala-467 in the latch turn are essential for coupling agonist binding and channel gating. The mechanism underlying the contribution of the latch turn on the nAChR ion channel gating remains to be elucidated.
- (7)
- Structural basis for the high calcium permeability of the α7 nAChR. A glutamate side-chain located in position 97 (Asp-97) in the extracellular vestibule that forms a salt bridge with residue K124 in the resting state is pivotal for the high calcium permeability. A 6.4 Å constriction of the ECD during agonist activation removes the salt bridge and reorients E97 into the center axis of the permeation pathway to secure calcium conductance. Interestingly, in the desensitized conformation the salt bridge is reestablished.
- (8)
- The role of transmembrane (M1, M3, and M4). Molecular dynamics simulations of the α7 nAChR structures suggest that the M1, M3, and M4 tilts during channel activation from 8 degrees (resting state) to 25 degrees (open channel state) and then relaxes to 15 degrees in the desensitized state. During channel activation, M4 slides up four residues, consistent with functional studies that proposed a spring model for the M4 by Otero-Cruz et al., 2007 [175]. The slide of the M4 lipid-exposed helix results in a shift of the M2 helix away from the pore axis resulting in channel opening. The role of M4 in channel gating have been previously described by structure-function studies (http://nachrs.org/ (accessed on 21 September 2021)). Along these lines, the upward shift of the M4 helix drags the MA TMD resulting in a subtle uncoiling of the M3-M4 loop which has may have consequences in channel activation and desensitization.
- (9)
- Structure of intracellular domains (ICDs). Structural information of the ICDs of the nAChRs has remained ambiguous, due to the need to delete a large portion of this domain and or modification with YFP or the thermostable BRIL protein. Nonetheless, the muscle-type Tm nAChRs-α-Bgtx complex provides structural information of a “native ICD” in the closed state. This structure shows a “hydrophobic plug” of EM density surrounded by hydrophobic residues, similar to the modified ICD of the α3β4 nAChRs. Molecular dynamics simulation of the α3β4 nAChR suggested that a lipid molecule is needed to stabilize the non-native ICD. The five MA helices of the muscle-type Tm nAChRs-Bgtx complex converge in a narrow constriction (~5.4 Å) at the cytosolic end, which permits mobilization of hydrated sodium ions. The five portals located at the interface of the MA helices in the α3β4 nAChRs contain fenestrations encompassed by acidic and polar side chains, providing the required architecture for the cation exit pathway.
17. nAChR Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Unwin, N. The Nicotinic Acetylcholine Receptor of the Torpedo Electric Ray. J. Struct. Biol. 1998, 121, 181–190. [Google Scholar] [CrossRef]
- Karlin, A.; Akabas, M. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron 1995, 15, 1231–1244. [Google Scholar] [CrossRef]
- Devillérs-Thiery, A.; Galzi, J.L.; Eiselé, J.L.; Bertrand, S.; Bertrand, D.; Changeux, J.P. Functional architecture of the nicotinic acetylcholine receptor: A prototype of ligand-gated ion channels. J. Membr. Biol. 1993, 136, 97–112. [Google Scholar] [CrossRef]
- Pedersen, S.; Bridgman, P.C.; Sharp, S.D.; Cohen, J.B. Identification of a cytoplasmic region of the Torpedo nicotinic acetylcholine receptor alpha-subunit by epitope mapping. J. Biol. Chem. 1990, 265, 569–581. [Google Scholar] [CrossRef]
- Czajkowski, C.; Karlin, A. Structure of the Nicotinic Receptor Acetylcholine-Binding Site. Identification of Acidic Residues in the Delta Subunit within 0.9 Nm of the 5 Alpha Subunit-Binding. J. Biol. Chem. 1995, 270, 3160–3164. [Google Scholar] [CrossRef]
- Czajkowski, C.; Karlin, A. Agonist binding site of Torpedo electric tissue nicotinic acetylcholine receptor. A negatively charged region of the delta subunit within 0.9 nm of the alpha subunit binding site disulfide. J. Biol. Chem. 1991, 266, 22603–22612. [Google Scholar] [CrossRef]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature 2016, 538, 411–415. [Google Scholar] [CrossRef]
- Karlin, A. Chemical Modification of the Active Site of the Acetylcholine Receptor. J. Gen. Physiol. 1969, 54, 245–264. [Google Scholar] [CrossRef]
- Patrick, J.; Lindstrom, J.; Culp, B.; Mcmillan, J. Studies on Purified Eel Acetylcholine Receptor and Anti-Acetylcholine Receptor Antibody. Proc. Natl. Acad. Sci. USA 1973, 70, 3334–3338. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Changeux, J. The Cholinergic Receptor Protein in Its Membrane Environment. Annu. Rev. Pharmacol. 1975, 15, 83–103. [Google Scholar] [CrossRef]
- Raftery, M.; Hunkapiller, M.; Strader, C.; Hood, L. Acetylcholine receptor: Complex of homologous subunits. Science 1980, 208, 1454–1456. [Google Scholar] [CrossRef]
- Noda, M.; Takahashi, H.; Tanabe, T.; Toyosato, M.; Furutani, Y.; Hirose, T.; Asai, M.; Inayama, S.; Miyata, T.; Numa, S. Primary Structure of Alpha-Subunit Precursor of Torpedo Californica Acetylcholine Receptor Deduced from CDNA Sequence. Nature 1982, 299, 793–797. [Google Scholar] [CrossRef]
- Ballivet, M.; Patrick, J.; Lee, J.; Heinemann, S. Molecular cloning of cDNA coding for the gamma subunit of Torpedo acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1982, 79, 4466–4470. [Google Scholar] [CrossRef]
- Karlin, A.; Cowburn, D. The Affinity-Labeling of Partially Purified Acetylcholine Receptor from Electric Tissue of Electrophorus. Proc. Natl. Acad. Sci. USA 1973, 70, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.N.; Karlin, A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J. Biol. Chem. 1986, 261, 8085–8088. [Google Scholar] [CrossRef]
- Galzi, J.L.; Revah, F.; Black, D.; Goeldner, M.; Hirth, C.; Changeux, J.P. Identification of a novel amino acid alpha-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. J. Biol. Chem. 1990, 265, 10430–10437. [Google Scholar] [CrossRef]
- Corringer, P.-J.; Galzi, J.-L.; Eiselé, J.-L.; Bertrand, S.; Changeux, J.-P.; Bertrand, D. Identification of a New Component of the Agonist Binding Site of the Nicotinic α7 Homooligomeric Receptor. J. Biol. Chem. 1995, 270, 11749–11752. [Google Scholar] [CrossRef]
- Changeux, J.-P. The TiPS lecture the nicotinic acetylcholine receptor: An allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol. Sci. 1990, 11, 485–492. [Google Scholar] [CrossRef]
- Patrick, J.; Lindstrom, J. Autoimmune Response to Acetylcholine Receptor. Science 1973, 180, 871–872. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Karlin, A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry 1978, 17, 2035–2038. [Google Scholar] [CrossRef]
- Lindstrom, J.; Merlie, J.; Yogeeswaran, G. Biochemical properties of acetylcholine receptor subunits from Torpedo californica. Biochemistry 1979, 18, 4465–4470. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.J.; Beeson, D.M.; Jackson, J.F.; Darlison, M.G.; Barnard, E.A. Differential expression of nicotinic acetylcholine receptor genes in innervated and denervated chicken muscle. EMBO J. 1987, 6, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Kuhse, J.; Schmieden, V.; Betz, H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 1990, 265, 22317–22320. [Google Scholar] [CrossRef]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, C.P. Nicotinic acetylcholine receptors in human genetic disease. Genet. Med. 2014, 16, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Clementi, F.; Fornasari, D.; Gotti, C. Neuronal nicotinic receptors, important new players in brain function. Eur. J. Pharmacol. 2000, 393, 3–10. [Google Scholar] [CrossRef]
- Nakaizumi, K.; Ouchi, Y.; Terada, T.; Yoshikawa, E.; Kakimoto, A.; Isobe, T.; Bunai, T.; Yokokura, M.; Suzuki, K.; Magata, Y. In vivo Depiction of α7 Nicotinic Receptor Loss for Cognitive Decline in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 1355–1365. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Y.; Gao, D.; Ye, J.; Wang, X.; Fang, L.; Wu, D.; Pi, G.; Lu, C.; Zhou, X.-W.; et al. Accumulation of human full-length tau induces degradation of nicotinic acetylcholine receptor α4 via activating calpain-2. Sci. Rep. 2016, 6, 27283. [Google Scholar] [CrossRef]
- Okada, H.; Ouchi, Y.; Ogawa, M.; Futatsubashi, M.; Saito, Y.; Yoshikawa, E.; Terada, T.; Oboshi, Y.; Tsukada, H.; Ueki, T.; et al. Alterations in α4β2 nicotinic receptors in cognitive decline in Alzheimer’s aetiopathology. Brain 2013, 136, 3004–3017. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Wicklund, L.; Lacor, P.N.; Klein, W.L.; Nordberg, A.; Marutle, A. Different β-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol. Aging 2012, 33, 825.e1–825.e13. [Google Scholar] [CrossRef]
- Kendziorra, K.; Wolf, H.; Meyer, P.M.; Barthel, H.; Hesse, S.; Becker, G.A.; Luthardt, J.; Schildan, A.; Patt, M.; Sorger, D.; et al. Decreased cerebral α4β2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer’s disease assessed with positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Mitsis, E.M.; Reech, K.M.; Bois, F.; Tamagnan, G.D.; MacAvoy, M.G.; Seibyl, J.P.; Staley, J.K.; Van Dyck, C.H. 123I-5-IA-85380 SPECT Imaging of Nicotinic Receptors in Alzheimer Disease and Mild Cognitive Impairment. J. Nucl. Med. 2009, 50, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Nathan, P.; Villemagne, V.; Mulligan, R.; Ellis, K.; Tochon-Danguy, H.; Chan, J.; O’Keefe, G.; Bradley, J.; Savage, G.; et al. The relationship between nicotinic receptors and cognitive functioning in healthy aging: An in vivo positron emission tomography (PET) study with 2-[(18)F]fluoro-A-85380. Synapse 2009, 63, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, S.D.; Jones, A.; Brown, L.; Sattelle, D.B. Nicotinic Acetylcholine Receptor Signalling: Roles in Alzheimer’s Disease and Amyloid Neuroprotection. Pharmacol. Rev. 2009, 61, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Villemagne, V.L.; Nathan, P.J.; Mulligan, R.S.; Gong, S.J.; Chan, G.; Sachinidis, J.; Keefe, G.J.; Pathmaraj, K.; Wesnes, K.; et al. Relationship between nicotinic receptors and cognitive function in early Alzheimer’s disease: A 2-[18F]fluoro-A-85380 PET study. Neurobiol. Learn. Mem. 2008, 90, 404–412. [Google Scholar] [CrossRef]
- Sabri, O.; Kendziorra, K.; Wolf, H.; Gertz, H.-J.; Brust, P. Acetylcholine receptors in dementia and mild cognitive impairment. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. S1), S30–S45. [Google Scholar] [CrossRef]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef]
- King, J.R.; Ullah, A.; Bak, E.; Jafri, M.S.; Kabbani, N. Ionotropic and Metabotropic Mechanisms of Allosteric Modulation of α7 Nicotinic Receptor Intracellular Calcium. Mol. Pharmacol. 2018, 93, 601–611. [Google Scholar] [CrossRef]
- Chrestia, J.F.; Bruzzone, A.; Esandi, M.D.C.; Bouzat, C. Tyrosine phosphorylation differentially fine-tunes ionotropic and metabotropic responses of human α7 nicotinic acetylcholine receptor. Cell. Mol. Life Sci. 2021, 78, 5381–5395. [Google Scholar] [CrossRef]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef]
- Báez-Pagán, C.A.; Delgado-Velez, M.; Lasalde-Dominicci, J.A. Activation of the Macrophage α7 Nicotinic Acetylcholine Receptor and Control of Inflammation. J. Neuroimmune Pharmacol. 2015, 10, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.L.; Porter, R.H. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem. Pharmacol. 2011, 82, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, B.; Kassai, F.; Szájli, Á.; Némethy, Z. α7 Nicotinic acetylcholine receptors and their role in cognition. Brain Res. Bull. 2013, 93, 86–96. [Google Scholar] [CrossRef]
- Wallace, T.; Bertrand, D. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem. Pharmacol. 2013, 85, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Dineley, K.T.; Pandya, A.A.; Yakel, J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015, 36, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Corradi, J.; Bouzat, C. Understanding the Bases of Function and Modulation of α7 Nicotinic Receptors: Implications for Drug Discovery. Mol. Pharmacol. 2016, 90, 288–299. [Google Scholar] [CrossRef]
- Cippitelli, A.; Wu, J.; Gaiolini, K.; Mercatelli, D.; Schoch, J.; Gorman, M.; Ramírez, A.; Ciccocioppo, R.; Khroyan, T.V.; Yasuda, D.; et al. AT-1001: A high-affinity α3β4 nAChR ligand with novel nicotine-suppressive pharmacology. Br. J. Pharmacol. 2015, 172, 1834–1845. [Google Scholar] [CrossRef]
- Walsh, R.M., Jr.; Roh, S.-H.; Gharpure, A.; Morales-Perez, C.L.; Teng, J.; Hibbs, R.E. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 2018, 557, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, A.; Teng, J.; Zhuang, Y.; Noviello, C.M.; Walsh, R.M., Jr.; Cabuco, R.; Howard, R.J.; Zaveri, N.T.; Lindahl, E.; Hibbs, R.E. Agonist Selectivity and Ion Permeation in the α3β4 Ganglionic Nicotinic Receptor. Neuron 2019, 104, 501–511.e6. [Google Scholar] [CrossRef]
- Hertling-Jaweed, S.; Bandini, G.; Müller-Fahrnow, A.; Dommes, V.; Hucho, F. Rapid preparation of the nicotinic acetylcholine receptor for crystallization in detergent solution. FEBS Lett. 1988, 241, 29–32. [Google Scholar] [CrossRef]
- Mitra, A.K.; McCarthy, M.P.; Stroud, R.M. Three-dimensional structure of the nicotinic acetylcholine receptor and location of the major associated 43-kD cytoskeletal protein, determined at 22 A by low dose electron microscopy and X-ray diffraction to 12.5 A. J. Cell Biol. 1989, 109, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Asmar-Rovira, G.A.; Asseo-García, A.M.; Quesada, O.; Hanson, M.A.; Cheng, A.; Nogueras, C.; Lasalde-Dominicci, J.A.; Stevens, R.C. Biophysical and Ion Channel Functional Characterization of the Torpedo californica Nicotinic Acetylcholine Receptor in Varying Detergent—Lipid Environments. J. Membr. Biol. 2008, 223, 13–26. [Google Scholar] [CrossRef][Green Version]
- Asmar-Rovira, G.A. Structural and Biophysical Characterization Studies of the Torpedo Nicotinic Acetylcholine Receptor (NAChR). Ph.D. Thesis, University of Puerto Rico, San Juan, Puerto Rico, 2007. [Google Scholar]
- Padilla-Morales, L.F.; Colón-Sáez, J.O.; González-Nieves, J.E.; Quesada-González, O.; Lasalde-Dominicci, J.A. Assessment of the functionality and stability of detergent purified nAChR from Torpedo using lipidic matrixes and macroscopic electrophysiology. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Morales, L.F.; Colón-Sáez, J.O.; González-Nieves, J.E.; Quesada-González, O.; Lasalde-Dominicci, J.A. Functionality and stability data of detergent purified nAChR from Torpedo using lipidic matrixes and macroscopic electrophysiology. Data Brief 2016, 6, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Dellisanti, C.D.; Yao, Y.; Stroud, J.; Wang, Z.-Z.; Chen, L. Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 Å resolution. Nat. Neurosci. 2007, 10, 953–962. [Google Scholar] [CrossRef]
- Miyazawa, A.; Fujiyoshi, Y.; Stowell, M.; Unwin, N. Nicotinic Acetylcholine Receptor at 4.6 A Resolution: Transverse Tunnels in the Channel Wall. J. Mol. Biol. 1999, 288, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N. Nicotinic Acetylcholine Receptor an 9 Å Resolution. J. Mol. Biol. 1993, 229, 1101–1124. [Google Scholar] [CrossRef]
- Unwin, N. Acetylcholine receptor channel imaged in the open state. Nature 1995, 373, 37–43. [Google Scholar] [CrossRef]
- Miyazawa, A.; Fujiyoshi, Y.; Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 2003, 423, 949–955. [Google Scholar] [CrossRef]
- Unwin, N.; Fujiyoshi, Y. Gating Movement of Acetylcholine Receptor Caught by Plunge-Freezing. J. Mol. Biol. 2012, 422, 617–634. [Google Scholar] [CrossRef]
- Zuber, B.; Unwin, N. Structure and superorganization of acetylcholine receptor-rapsyn complexes. Proc. Natl. Acad. Sci. USA 2013, 110, 10622–10627. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Erramilli, S.K.; Ammirati, M.; Alvarez, F.J.D.; Fennell, K.F.; Purdy, M.D.; Skrobek, B.M.; Radziwon, K.; Coukos, J.; Kang, Y.; et al. Synthetic antibodies against BRIL as universal fiducial marks for single−particle cryoEM structure determination of membrane proteins. Nat. Commun. 2020, 11, 1598. [Google Scholar] [CrossRef]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 2021, 184, 2121–2134.e13. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.; Hibbs, R.E. Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 2020, 106, 952–962.e5. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N. Protein-lipid architecture of a cholinergic postsynaptic membrane. IUCrJ 2020, 7, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Rickert, K.W.; Imperiali, B. Analysis of the conserved glycosylation site in the nicotinic acetylcholine receptor: Potential roles in complex assembly. Chem. Biol. 1995, 2, 751–759. [Google Scholar] [CrossRef]
- Buller, A.L.; White, M.M. Altered patterns of N-linked glycosylation of theTorpedo acetylcholine receptor expressed in Xenopus oocytes. J. Membr. Biol. 1990, 115, 179–189. [Google Scholar] [CrossRef]
- Gehle, V.M.; Sumikawa, K. Site-directed mutagenesis of the conserved N-glycosylation site on the nicotinic acetylcholine receptor subunits. Mol. Brain Res. 1991, 11, 17–25. [Google Scholar] [CrossRef]
- Le Maire, M.; Champeil, P.; Møller, J.V. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1508, 86–111. [Google Scholar] [CrossRef]
- Arnold, T.; Linke, D. The Use of Detergents to Purify Membrane Proteins. Curr. Protoc. Protein Sci. 2008, 53, 4.8.1–4.8.30. [Google Scholar] [CrossRef]
- Orwick-Rydmark, M.; Arnold, T.; Linke, D. The Use of Detergents to Purify Membrane Proteins. Curr. Protoc. Protein Sci. 2016, 84, 4.8.1–4.8.35. [Google Scholar] [CrossRef]
- Quesada, O.; González-Freire, C.; Ferrer, M.C.; Colón-Sáez, J.O.; Fernández-García, E.; Mercado, J.; Davila-Pagan, A.; Morales, R.; Lasalde-Dominicci, J.A. Uncovering the lipidic basis for the preparation of functional nicotinic acetylcholine receptor detergent complexes for structural studies. Sci. Rep. 2016, 6, 32766. [Google Scholar] [CrossRef]
- Edelstein, S.J.; Beyer, W.B.; Elderfrawi, A.T.; Elderfrawi, M. Molecular weight of the acetylcholine receptors of electric organs and the effect of Triton X-100. J. Biol. Chem. 1975, 250, 6101–6106. [Google Scholar] [CrossRef]
- Barrantes, F.J.; Mieskes, G.; Wallimann, T. A membrane-associated creatine kinase (EC 2.7.3.2) identified as an acidic species of the non-receptor, peripheral v-proteins in Torpedoacetylcholine receptor membranes. FEBS Lett. 1983, 152, 270–276. [Google Scholar] [CrossRef]
- Hucho, F.; Bandini, G.; Suarez-Isla, B.A. The Acetylcholine Receptor as Part of a Protein Complex in Receptor-Enriched Membrane Fragments from Torpedo californica Electric Tissue. Eur. J. Biol. Chem. 1978, 83, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Hernández, R.; Quesada, O.; Colón-Sáez, J.O.; Lasalde-Dominicci, J.A. Sequential purification and characterization of Torpedo californica nAChR-DC supplemented with CHS for high-resolution crystallization studies. Anal. Biochem. 2020, 610, 113887. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Hernández, R.; Quesada, O.; Lasalde-Dominicci, J.A. Biophysical characterization dataset of native nicotinic acetylcholine receptor in lipid-like detergent complexes. Data Brief 2020, 32, 106230. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Morales, L.F.; Morales-Pérez, C.L.; De La Cruz-Rivera, P.C.; Asmar-Rovira, G.; Báez-Pagán, C.A.; Quesada, O.; Lasalde-Dominicci, J.A. Effects of Lipid-Analog Detergent Solubilization on the Functionality and Lipidic Cubic Phase Mobility of the Torpedo californica Nicotinic Acetylcholine Receptor. J. Membr. Biol. 2011, 243, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Psaridi-Linardaki, L.; Mamalaki, A.; Remoundos, M.; Tzartos, S.J. Expression of Soluble Ligand- and Antibody-binding Extracellular Domain of Human Muscle Acetylcholine Receptor α Subunit in Yeast Pichia pastoris: Role of Glycosylation in Alpha-Bungarotoxin Binding. J. Biol. Chem. 2002, 277, 26980–26986. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Viroonchatapan, N.; Samson, A.; Chill, J.; Rothe, E.; Anglister, J.; Wang, Z.-Z. Yeast Expression and NMR Analysis of the Extracellular Domain of Muscle Nicotinic Acetylcholine Receptor α Subunit. J. Biol. Chem. 2002, 277, 12613–12621. [Google Scholar] [CrossRef]
- Wells, G.B. Structural Answers and Persistent Questions about How Nicotinic Receptors Work. Front. Biosci. 2008, 13, 5479–5510. [Google Scholar] [CrossRef] [PubMed]
- Vassylyeva, M.N.; Klyuyev, S.; Vassylyev, A.D.; Wesson, H.; Zhang, Z.; Renfrow, M.B.; Wang, H.B.; Higgins, N.P.; Chow, L.T.; Vassylyev, D.G. Efficient, ultra-high-affinity chromatography in a one-step purification of complex proteins. Proc. Natl. Acad. Sci. USA 2017, 114, E5138–E5147. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Blanchard, S.G.; Wu, W.; Miller, J.; Strader, C.D.; Hartig, P.; Moore, H.P.; Racs, J.; Raftery, M. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem. J. 1980, 185, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Waser, P.G.; Bodmer, D.M.; Hopff, W.H. Isolation and purification of acetylcholine receptor proteins by affinity chromatography. Eur. J. Pharmacol. Mol. Pharmacol. 1989, 172, 231–238. [Google Scholar] [CrossRef]
- Hamouda, A.K.; Chiara, D.C.; Blanton, M.P.; Cohen, J.B. Probing the Structure of the Affinity-Purified and Lipid-Reconstituted Torpedo Nicotinic Acetylcholine Receptor. Biochemistry 2008, 47, 12787–12794. [Google Scholar] [CrossRef] [PubMed]
- Ringler, P.; Kessler, P.; Ménez, A.; Brisson, A. Purification of the nicotinic acetylcholine receptor protein by affinity chromatography using a regioselectively modified and reversibly immobilized α-toxin from Naja nigricollis. Biochim. Biophys. Acta (BBA)-Biomembr. 1997, 1324, 37–46. [Google Scholar] [CrossRef][Green Version]
- Criado, M.; Eibl, H.; Barrantes, F.J. Functional properties of the acetylcholine receptor incorporated in model lipid membranes. Differential effects of chain length and head group of phospholipids on receptor affinity states and receptor-mediated ion translocation. J. Biol. Chem. 1984, 259, 9188–9198. [Google Scholar] [CrossRef]
- Criado, M.; Eibl, H.; Barrantes, F.J. Effects of lipids on acetylcholine receptor. Essential need of cholesterol for maintenance of agonist-induced state transitions in lipid vesicles. Biochemistry 1982, 21, 3622–3629. [Google Scholar] [CrossRef]
- Fong, T.M.; McNamee, M.G. Correlation between acetylcholine receptor function and structural properties of membranes. Biochemistry 1986, 25, 830–840. [Google Scholar] [CrossRef]
- Ochoa, E.L.; Dalziel, A.W.; McNamee, M.G. Reconstitution of acetylcholine receptor function in lipid vesicles of defined composition. Biochim. Biophys. Acta (BBA)-Biomembr. 1983, 727, 151–162. [Google Scholar] [CrossRef]
- Sobel, A.; Weber, M.; Changeux, J.-P. Large-Scale Purification of the Acetylcholine-Receptor Protein in Its Membrane-Bound and Detergent-Extracted Forms from Torpedo marmorata Electric Organ. Eur. J. Biol. Chem. 1977, 80, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Meunier, J.-C.; Changeux, J.-P. Progress in the purification of the cholinergic receptor protein from Electrophorus electricus by affinity chromatography. FEBS Lett. 1972, 28, 96–100. [Google Scholar] [CrossRef]
- Miledi, R.; Molinoff, P.; Potter, L.T. Isolation of the Cholinergic Receptor Protein of Torpedo Electric Tissue. Nature 1971, 229, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B.; Weber, M.; Huchet, M.; Changeux, J.-P. Purification fromTorpedo marmorataelectric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett. 1972, 26, 43–47. [Google Scholar] [CrossRef]
- Levinson, S.; Keynes, R. Isolation of acetylcholine receptors by chloroform-methanol extraction: Artifacts arising in use of Sephadex LH-20 columns. Biochim. Biophys. Acta (BBA)-Biomembr. 1972, 288, 241–247. [Google Scholar] [CrossRef]
- Schmidt, J.; Raftery, M.A. Use of affinity chromatography for acetylcholine receptor purification. Biochem. Biophys. Res. Commun. 1972, 49, 572–578. [Google Scholar] [CrossRef]
- Schmidt, J.; Raftery, M.A. Purification of acetylcholine receptors from Torpedo claifornica electroplax by affinity chromatography. Biochemistry 1973, 12, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Klett, R.P.; Fulpius, B.W.; Cooper, D.; Smith, M.; Reich, E.; Possani, L.D. The acetylcholine receptor. I. Purification and characterization of a macromolecule isolated from Electrophorus electricus. J. Biol. Chem. 1973, 248, 6841–6853. [Google Scholar] [CrossRef]
- Meunier, J.-C.; Sugiyama, H.; Cartaud, J.; Sealock, R.; Changeux, J.-P. Functional properties of the purified cholinergic receptor protein fromelectrophorus electricus. Brain Res. 1973, 62, 307–315. [Google Scholar] [CrossRef]
- Raftery, M. Isolation of acetylcholine receptor—α-Bungarotoxin complexes from Torpedo californica electroplax. Arch. Biochem. Biophys. 1973, 154, 270–276. [Google Scholar] [CrossRef]
- Heilbronn, E.; Mattson, C. The nicotinic cholinergic receptor protein: Improved purification method, preliminary amino acid composition and observed auto-immuno response. J. Neurochem. 1974, 22, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.-C.; Sealock, R.; Olsen, R.; Chanqeux, J.-P. Purification and Properties of the Cholinergic Receptor Protein from Electrophorus electricus Electric Tissue. Eur. J. Biol. Chem. 1974, 45, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Weill, C.L.; McNamee, M.G.; Karlin, A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem. Biophys. Res. Commun. 1974, 61, 997–1003. [Google Scholar] [CrossRef]
- Vandlen, R.L.; Wu, W.C.-S.; Eisenach, J.C.; Raftery, M.A. Studies of the composition of purified Torpedo californica acetylcholine receptor and of its subunits. Biochemistry 1979, 18, 1845–1854. [Google Scholar] [CrossRef]
- Kemp, G.; Morley, B.; Dwyer, D.; Bradley, R.J. Purification and Characterization of Nicotinic Acetylcholine Receptors from Muscle. Membr. Biochem. 1980, 3, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, J.; Anholt, R.; Einarson, B.; Engel, A.; Osame, M.; Montal, M. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J. Biol. Chem. 1980, 255, 8340–8350. [Google Scholar] [CrossRef]
- Gonzales-Ros, J.M.; Paraschos, A.; Farach, M.C.; Martinez-Carrion, M. Characterization of acetylcholine receptor isolated from Torpedo californica electroplax through the use of an easily removable detergent, β-d-octylglucopyranoside. Biochim. Biophys. Acta (BBA)-Biomembr. 1981, 643, 407–420. [Google Scholar] [CrossRef]
- Norman, R.I.; Mehraban, F.; Barnard, E.A.; Dolly, J.O. Nicotinic acetylcholine receptor from chick optic lobe. Proc. Natl. Acad. Sci. USA 1982, 79, 1321–1325. [Google Scholar] [CrossRef]
- Hopff, W.H.; Bodmer, D.M.; Waser, P.G. Affinity Chromatography of Cholinergic Receptor Proteins. J. Recept. Res. 1984, 4, 219–229. [Google Scholar] [CrossRef]
- Whiting, P.; Lindstrom, J.M. Purification and characterization of a nicotinic acetylcholine receptor from chick brain. Biochemistry 1986, 25, 2082–2093. [Google Scholar] [CrossRef]
- Kubalek, E.; Ralston, S.; Lindstrom, J.; Unwin, N. Location of subunits within the acetylcholine receptor by electron image analysis of tubular crystals from Torpedo marmorata. J. Cell Biol. 1987, 105, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, S.; Conti-Tronconi, B.; Racs, J.; Raftery, M.A. Isolation and characterization of nicotinic acetylcholine receptor-like protein from fetal calf thymus. J. Neurol. Sci. 1988, 87, 195–209. [Google Scholar] [CrossRef]
- Kapp, E.; Whiteley, C. An improved procedure for the isolation and purification of nicotinic acytylcholine receptor from Torpedo fuscomaculata electric organ. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1990, 1034, 29–38. [Google Scholar] [CrossRef]
- Nakayama, H.; Shirase, M.; Nakashima, T.; Kurogochi, Y.; Lindstrom, J.M. Affinity purification of nicotinic acetylcholine receptor from rat brain. Mol. Brain Res. 1990, 7, 221–226. [Google Scholar] [CrossRef]
- Dwork, A.J.; Desmond, J.T. Purification of a nicotinic acetylcholine receptor from rat brain by affinity chromatography directed at the acetylcholine binding site. Brain Res. 1991, 552, 119–123. [Google Scholar] [CrossRef]
- Chak, A.; Karlin, A. Purification and reconstitution of nicotinic acetylcholine receptor. Methods Enzymol. 1992, 207, 546–555. [Google Scholar] [CrossRef]
- Dacosta, C.J.; Ogrel, A.A.; McCardy, E.A.; Blanton, M.P.; Baenziger, J. Lipid-Protein Interactions at the Nicotinic Acetylcholine Receptor: A Functional Coupling between Nicotinic Receptors and Phosphatidic Acid-Containing Lipid Bilayers. J. Biol. Chem. 2002, 277, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Labriola, J.M.; Dacosta, C.J.; Wang, S.; Figeys, D.; Smith, J.C.; Sturgeon, R.M.; Baenziger, J.E. Phospholipase C Activity Affinity Purifies with the Torpedo Nicotinic Acetylcholine Receptor. J. Biol. Chem. 2010, 285, 10337–10343. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Worrell, B.T.; Stowell, M.H.; Hibbs, R.E. Purification of a native nicotinic receptor. Methods Enzymol. 2021, 653, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Wenz, C.; Marchetti-Deschmann, M.; Herwig, E.; Schröttner, E.; Allmaier, G.; Trojer, L.; Vollmer, M.; Rüfer, A. A fluorescent derivatization method of proteins for the detection of low-level impurities by microchip capillary gel electrophoresis. Electrophoresis 2010, 31, 611–617. [Google Scholar] [CrossRef]
- Bousse, L.; Mouradian, S.; Minalla, A.; Yee, H.; Williams, K.; Dubrow, R. Protein Sizing on a Microchip. Anal. Chem. 2001, 73, 1207–1212. [Google Scholar] [CrossRef]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.; Thian, F.S.; Kobilka, T.S.; Choi, H.-J.; Kuhn, P.; Weis, W.; Kobilka, B.K.; et al. High-Resolution Crystal Structure of an Engineered Human β2-Adrenergic G Protein-Coupled Receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef]
- Brisson, A.; Unwin, P.N.T. Quaternary structure of the acetylcholine receptor. Nature 1985, 315, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. Manipulation of Subunit Stoichiometry in Heteromeric Membrane Proteins. Structure 2016, 24, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Galzi, J.-L.; Changeux, J.-P. Neuronal nicotinic receptors: Molecular organization and regulations. Neuropharmacology 1995, 34, 563–582. [Google Scholar] [CrossRef]
- Zhong, W.; Gallivan, J.P.; Zhang, Y.; Li, L.; Lester, H.A.; Dougherty, D.A. From ab initio quantum mechanics to molecular neurobiology: A cation-π binding site in the nicotinic receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 12088–12093. [Google Scholar] [CrossRef]
- Xiu, X.; Puskar, N.L.; Shanata, J.; Lester, H.A.; Dougherty, D.A. Nicotine binding to brain receptors requires a strong cation-π interaction. Nature 2009, 458, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Sixma, T.K.; Smit, A.B. Acetylcholine Binding Protein (AChBP): A Secreted Glial Protein That Provides a High-Resolution Model for the Extracellular Domain of Pentameric Ligand-Gated Ion Channels. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 311–334. [Google Scholar] [CrossRef]
- Unwin, N. Refined Structure of the Nicotinic Acetylcholine Receptor at 4 Å Resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Jakobi, A.J.; Wilmanns, M.; Sachse, C. Model-based local density sharpening of cryo-EM maps. eLife 2017, 6, e27131. [Google Scholar] [CrossRef] [PubMed]
- Vilas, J.L.; Tagare, H.D.; Vargas, J.; Carazo, J.M.; Sorzano, C.O.S. Measuring local-directional resolution and local anisotropy in cryo-EM maps. Nat. Commun. 2020, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, N.; Nury, H.; Baaden, M.; Le Poupon, C.; Changeux, J.-P.; Delarue, M.; Corringer, P.-J. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 2009, 457, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hilf, R.J.C.; Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 2008, 452, 375–379. [Google Scholar] [CrossRef]
- Nakane, T.; Kotecha, A.; Sente, A.; McMullan, G.; Masiulis, S.; Brown, P.M.G.E.; Grigoras, I.T.; Malinauskaite, L.; Malinauskas, T.; Miehling, J.; et al. Single-Particle Cryo-EM at Atomic Resolution. Nature 2020, 587, 152–156. [Google Scholar] [CrossRef]
- Overduin, M.; Esmaili, M. Memtein: The fundamental unit of membrane-protein structure and function. Chem. Phys. Lipids 2019, 218, 73–84. [Google Scholar] [CrossRef]
- Kumar, P.; Cymes, G.D.; Grosman, C. Structure and function at the lipid–protein interface of a pentameric ligand-gated ion channel. Proc. Natl. Acad. Sci. USA 2021, 118, 2100164118. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.S.; Scott, S.; Masiulis, S.; De Colibus, L.; Pardon, E.; Steyaert, J.; Aricescu, A.R. Structural basis for GABAA receptor potentiation by neurosteroids. Nat. Struct. Mol. Biol. 2017, 24, 986–992. [Google Scholar] [CrossRef]
- Laverty, D.; Thomas, P.; Field, M.; Andersen, O.J.; Gold, M.G.; Biggin, P.C.; Gielen, M.; Smart, T.G. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol. 2017, 24, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.; Wang, H.H.; Blanton, M.P. Identifying the cholesterol binding domain in the nicotinic acetylcholine receptor with [125I]azido-cholesterol. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1414, 65–74. [Google Scholar] [CrossRef][Green Version]
- Rollema, H.; Chambers, L.; Coe, J.; Glowa, J.; Hurst, R.; Lebel, L.; Lu, Y.; Mansbach, R.; Mather, R.; Rovetti, C.; et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 2007, 52, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Glick, S.D.; Sell, E.M.; McCallum, S.E.; Maisonneuve, I.M. Brain regions mediating α3β4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur. J. Pharmacol. 2011, 669, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Margreiter, M.A.; Fuchs, J.E.; Von Grafenstein, S.; Tautermann, C.; Liedl, K.R.; Fox, T. Heteroaromatic π-Stacking Energy Landscapes. J. Chem. Inf. Model. 2014, 54, 1371–1379. [Google Scholar] [CrossRef]
- Eaton, J.B.; Peng, J.-H.; Schroeder, K.M.; George, A.A.; Fryer, J.D.; Krishnan, C.; Buhlman, L.; Kuo, Y.-P.; Steinlein, O.; Lukas, R.J. Characterization of Human α4β2-Nicotinic Acetylcholine Receptors Stably and Heterologously Expressed in Native Nicotinic Receptor-Null SH-EP1 Human Epithelial Cells. Mol. Pharmacol. 2003, 64, 1283–1294. [Google Scholar] [CrossRef]
- Whiteaker, P.; Sharples, C.G.; Wonnacott, S. Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: Pharmacological and spatial definition. Mol. Pharmacol. 1998, 53, 950–962. [Google Scholar] [PubMed]
- Corrie, L.W.; Stokes, C.; Wilkerson, J.L.; Carroll, F.I.; McMahon, L.R.; Papke, R.L. Nicotinic Acetylcholine Receptor Accessory Subunits Determine the Activity Profile of Epibatidine Derivatives. Mol. Pharmacol. 2020, 98, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Tuan, E.W.; Horti, A.G.; Olson, T.T.; Gao, Y.; Stockmeier, C.A.; Al-Muhtasib, N.; Dalley, C.B.; Lewin, A.E.; Wolfe, B.B.; Sahibzada, N.; et al. AT-1001 Is a Partial Agonist with High Affinity and Selectivity at Human and Rat α3β4 Nicotinic Cholinergic Receptors. Mol. Pharmacol. 2015, 88, 640–649. [Google Scholar] [CrossRef]
- McCarthy, M.; Moore, M. Effects of lipids and detergents on the conformation of the nicotinic acetylcholine receptor from Torpedo californica. J. Biol. Chem. 1992, 267, 7655–7663. [Google Scholar] [CrossRef]
- Barrantes, F. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res. Rev. 2004, 47, 71–95. [Google Scholar] [CrossRef]
- Martinez, K.L.; Gohon, Y.; Corringer, P.-J.; Tribet, C.; Mérola, F.; Changeux, J.-P.; Popot, J.-L. Allosteric transitions of Torpedo acetylcholine receptor in lipids, detergent and amphipols: Molecular interactions vs. physical constraints. FEBS Lett. 2002, 528, 251–256. [Google Scholar] [CrossRef]
- Baenziger, J.E.; Domville, J.A.; Therien, J.P.D. The Role of Cholesterol in the Activation of Nicotinic Acetylcholine Receptors. Curr. Top. Membr. 2017, 80, 95–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-S.; Cheng, H.; Jiang, Y.; Melcher, K.; Xu, H.E. Ion channels gated by acetylcholine and serotonin: Structures, biology, and drug discovery. Acta Pharmacol. Sin. 2015, 36, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Harkness, P.C. Assembly and trafficking of nicotinic acetylcholine receptors (Review). Mol. Membr. Biol. 2008, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor—The relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Lummis, S.C.R. 5-HT3 Receptors. Curr. Pharm. Des. 2006, 12, 3615–3630. [Google Scholar] [CrossRef]
- Hassaine, G.; Deluz, C.; Grasso, L.; Wyss, R.; Tol, M.B.; Hovius, R.; Graff, A.; Stahlberg, H.; Tomizaki, T.; Desmyter, A.; et al. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 2014, 512, 276–281. [Google Scholar] [CrossRef]
- Kesters, D.; Thompson, A.J.; Brams, M.; van Elk, R.; Spurny, R.; Geitmann, M.; Villalgordo, J.M.; Guskov, A.; Danielson, H.; Lummis, S.C.R.; et al. Structural basis of ligand recognition in 5-HT 3 receptors. EMBO Rep. 2013, 14, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, S.-X.; Bren, N.; Cheng, K.; Gomoto, R.; Chen, L.; Sine, S.M. Complex between α-bungarotoxin and an α7 nicotinic receptor ligand-binding domain chimaera. Biochem. J. 2013, 454, 303–310. [Google Scholar] [CrossRef]
- Li, S.-X.; Huang, S.; Bren, N.; Noridomi, K.; Dellisanti, C.D.; Sine, S.M.; Chen, L. Ligand-binding domain of an α7-nicotinic receptor chimera and its complex with agonist. Nat. Neurosci. 2011, 14, 1253–1259. [Google Scholar] [CrossRef]
- Wen, J.; Hung, A. Effects of C-Terminal Carboxylation on α-Conotoxin LsIA Interactions with Human α7 Nicotinic Acetylcholine Receptor: Molecular Simulation Studies. Mar. Drugs 2019, 17, 206. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Couto, A.R.; Price, K.L.; Mesoy, S.; Capes, E.; Lummis, S.C.R. The M4 Helix Is Involved in α7 nACh Receptor Function. ACS Chem. Neurosci. 2020, 11, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.N.; Labarca, C.; Davidson, N.; Lester, H. Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J. Gen. Physiol. 1992, 100, 373–400. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, T.M.; Adams, D.; Hille, B. The permeability of the endplate channel to organic cations in frog muscle. J. Gen. Physiol. 1980, 75, 469–492. [Google Scholar] [CrossRef] [PubMed]
- Jaiteh, M.; Taly, A.; Hénin, J. Evolution of Pentameric Ligand-Gated Ion Channels: Pro-Loop Receptors. PLoS ONE 2016, 11, e0151934. [Google Scholar] [CrossRef]
- Osaka, H.; Malany, S.; Kanter, J.R.; Sine, S.M.; Taylor, P. Subunit Interface Selectivity of the α-Neurotoxins for the Nicotinic Acetylcholine Receptor. J. Biol. Chem. 1999, 274, 9581–9586. [Google Scholar] [CrossRef] [PubMed]
- Di Scala, C.; Baier, C.J.; Evans, L.S.; Williamson, P.T.; Fantini, J.; Barrantes, F.J. Relevance of CARC and CRAC Cholesterol-Recognition Motifs in the Nicotinic Acetylcholine Receptor and Other Membrane-Bound Receptors. Curr. Top. Membr. 2017, 80, 3–23. [Google Scholar] [CrossRef]
- Baier, C.J.; Fantini, J.; Barrantes, F.J. Disclosure of cholesterol recognition motifs in transmembrane domains of the human nicotinic acetylcholine receptor. Sci. Rep. 2011, 1, 69. [Google Scholar] [CrossRef]
- Brannigan, G.; Hénin, J.; Law, R.; Eckenhoff, R.; Klein, M.L. Embedded cholesterol in the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 14418–14423. [Google Scholar] [CrossRef]
- Jones, O.T.; Eubanks, J.; Earnest, J.P.; McNamee, M.G. A minimum number of lipids are required to support the functional properties of the nicotinic acetylcholine receptor. Biochemistry 1988, 27, 3733–3742. [Google Scholar] [CrossRef]
- Rudell, J.; Borges, L.S.; Yarov-Yarovoy, V.; Ferns, M. The MX-Helix of Muscle nAChR Subunits Regulates Receptor Assembly and Surface Trafficking. Front. Mol. Neurosci. 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Rudell, J.; Borges, L.S.; Rudell, J.B.; Beck, K.A.; Ferns, M. Determinants in the β and δ Subunit Cytoplasmic Loop Regulate Golgi Trafficking and Surface Expression of the Muscle Acetylcholine Receptor. J. Biol. Chem. 2014, 289, 203–214. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, G.Y.; Sánchez-Padilla, J.; Ortiz-Acevedo, A.; Lizardi-Ortiz, J.; Salas-Vincenty, J.; Rojas, L.V.; Lasalde-Dominicci, J.A. Nicotine-induced Up-regulation and Desensitization of α4β2 Neuronal Nicotinic Receptors Depend on Subunit Ratio. J. Biol. Chem. 2004, 279, 38007–38015. [Google Scholar] [CrossRef]
- Otero-Cruz, J.D.; Báez-Pagán, C.A.; Caraballo-González, I.M.; Lasalde-Dominicci, J.A. Tryptophan-scanning Mutagenesis in the αM3 Transmembrane Domain of the Muscle-type Acetylcholine Receptor: A Spring Model Revealed. J. Biol. Chem. 2007, 282, 9162–9171. [Google Scholar] [CrossRef] [PubMed]
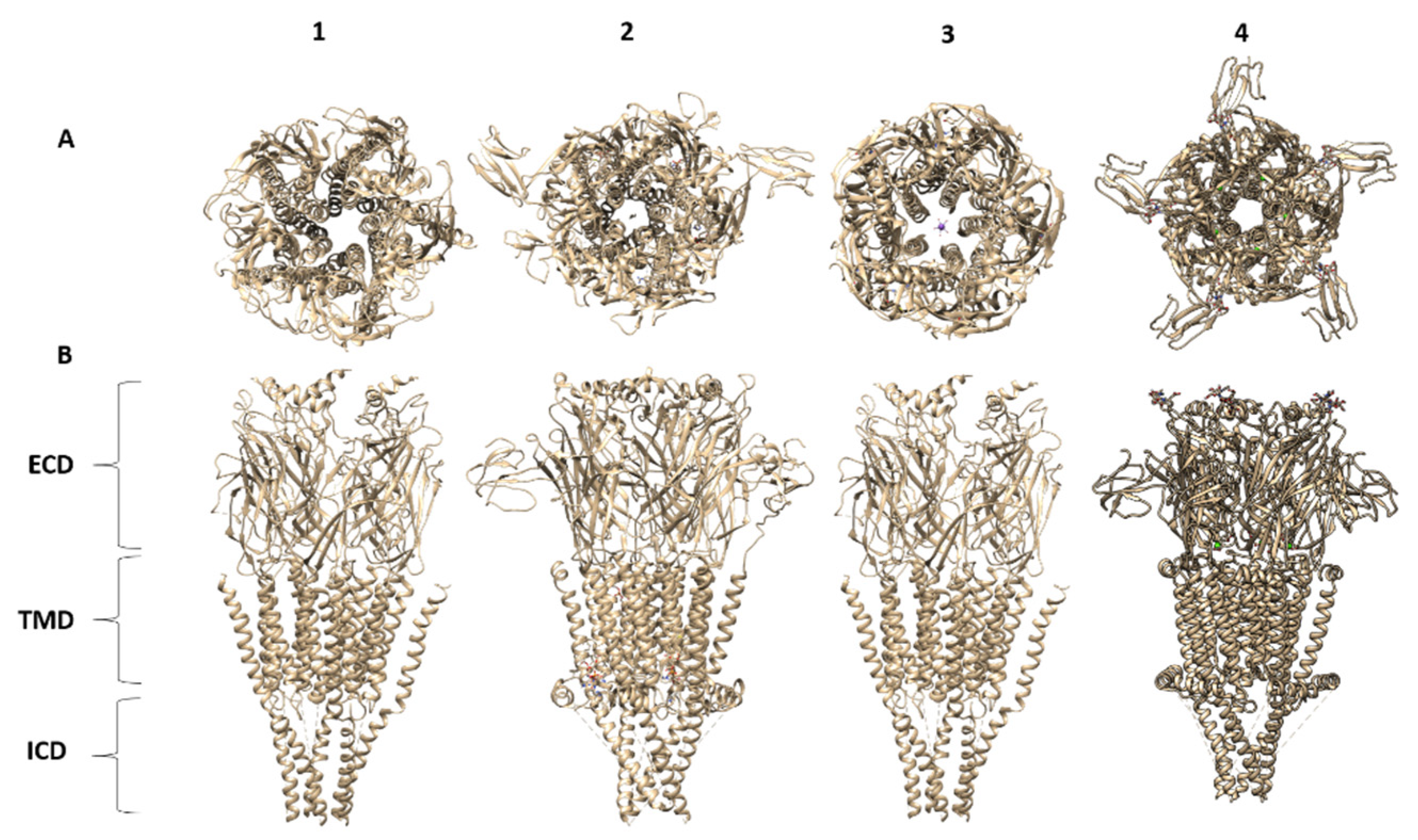
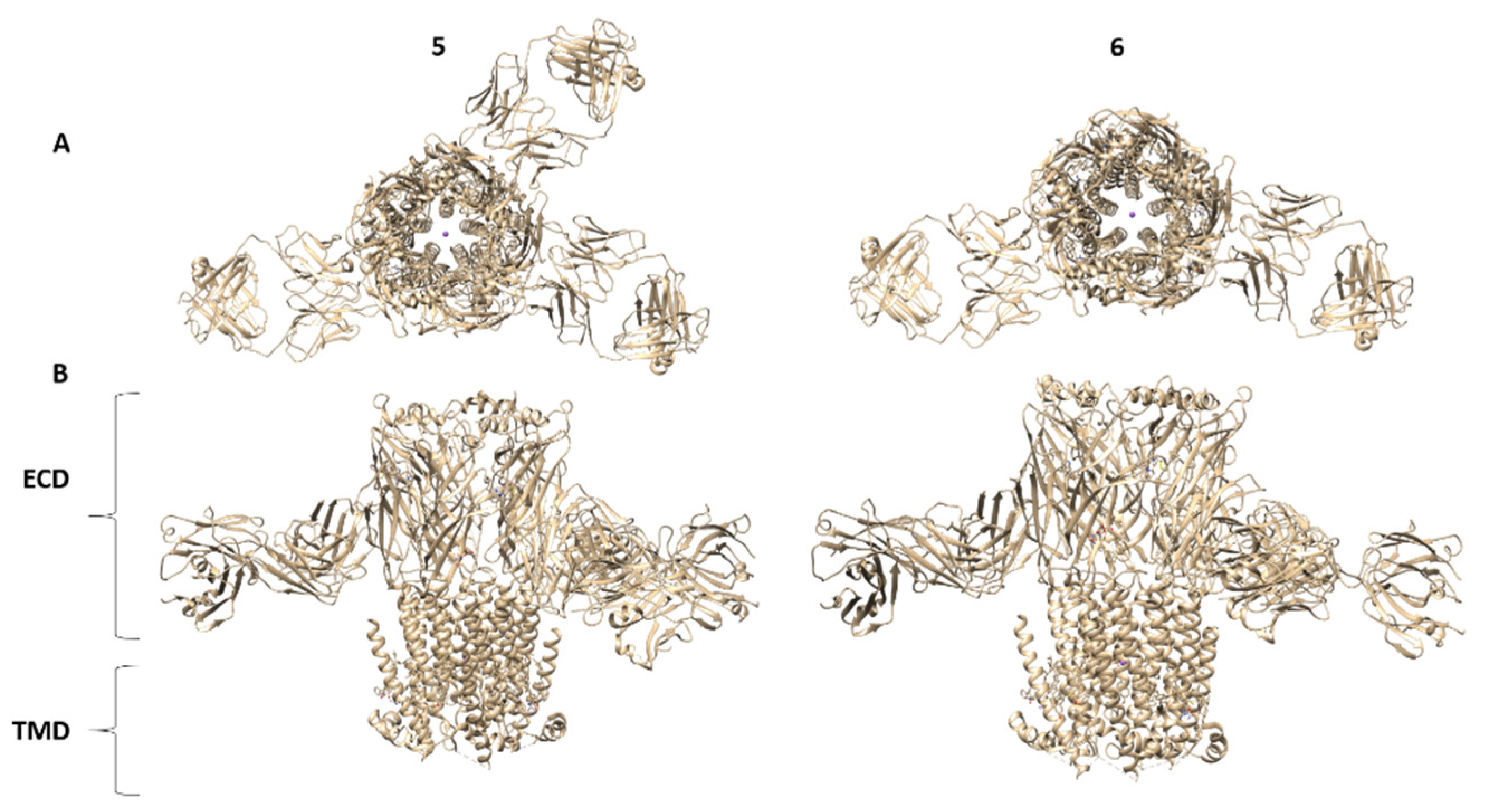
| 1 EMD | Protein Complex | Aggregation State | Reconstruction Method | Deposit Date | Resolution (Å) | Associated PDB | Organism | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1044 | Ion channel nAChR | Filament | Helical | 2003-04-03 | 4.00 | 1OED | Tm | [60] |
| 1044 | Ion channel nAChR | Helical array | Helical | 2004-12-17 | 4.00 | 2BG9 | Tm | [60] |
| 2071 | Ion channel nAChR | Particle | Single particle | 2012-04-12 | 6.2 | 4AQ5 | Tm | [61] |
| 2072 | Ion channel nAChR | Helical array | Helical | 2012-04-12 | 6.2 | 4AQ9 | Tm | [61] |
| 2376 | Ion channel nAChR/rapsyn | Particle | Tomography | 2013-05-19 | 50.0 | 4BOG | Tm | [62] |
| 2377 | Ion channel nAChR/rapsyn | Particle | Tomography | 2013-05-19 | 41.0 | 4BOI | Tm | [62] |
| 2378 | Ion channel nAChR/rapsyn | Tissue | Tomography | 2013-05-19 | 41.0 | 4BON | Tm | [62] |
| 2381 | Ion channel nAChR/rapsyn | Particle | Tomography | 2013-05-19 | 42.0 | 4BOO | Tm | [62] |
| 2382 | Ion channel nAChR/Rapsyn | Tissue | Tomography | 2013-05-19 | 42 | 4BOR | Tm | [62] |
| 2383 | Ion channel nAChR/Rapsyn | Particle | Tomography | 2013-05-19 | 42.0 | 4BOT | Tm | [62] |
| n/a | α4β2 | Crystal | X-Ray diffraction | 2016-7-20 | 3.94 | 5KXI | Hs | [7] |
| 7535 | 2α:3β/nicotine of α4β2 | Particle | Single particle | 2018-03-08 | 3.70 | 6CNJ | Hs, Mm | [48] |
| 7536 | 3α:2β2/nicotine of α4β2 | Particle | Single particle | 2018-03-08 | 3.90 | 6CNK | Hs, Mm | [48] |
| 20487 | α3β4/nicotine | Particle | Single particle | 2019-07-19 | 3.34 | 6PV7 | Hs, Mm, Ec O11 | [49] |
| 20488 | α3β4 ligand AT-1001/DDM | Particle | Single particle | 2019-07-19 | 3.87 | 6PV8 | Hs, Ec O11 | [49] |
| 20489 | α3β4/nicotine without CHS | Particle | Single particle | 2019-07-19 | 4.70 | n/a | Hs, Ec O11 | [49] |
| 20490 | α3β4/AT-1001/nanodiscs | Particle | Single particle | 2019-07-19 | 4.58 | n/a | Hs, Ec O11 | [49] |
| 20857 | α4β2/varenicline complex | Particle | Single particle | 2019-10-22 | 3.71 | 6UR8 | HS, Ec | [63] |
| 20863 | α4β2/varenicline complex/antibril | Particle | Single particle | 2019-10-26 | 3.87 | 6USF | Hs, Ec | [63] |
| 22983 | α7 nAChR/epibatidine and PNU-120596 | Particle | Single particle | 2021-03-17 | 2.70 | 7KOX | Hs, Ec | [64] |
| 22979 | α7 nAChR/α-Bgtx | Particle | Single particle | 2021-03-17 | 3.00 | 7KOO | Hs, Ec | [64] |
| 22980 | α7 nAChR/epibatidine | Particle | Single particle | 2021-03-17 | 3.60 | 7KOQ | Hs, Ec | [64] |
| 20928 | Native muscle-type nAChR/α-Bgtx | Particle | Single particle | 2019-11-06 | 2.69 | 6UWZ | Tc | [65] |
| 11239 | Ion channel nAChR | Filament | Helical | 2020-06-29 | 5.8 | n/a | Tm | [66] |
| Tissue Source | Quantity of Tissue | Purification Method | Detergent(s)/Solvent | Reported Yield | Ref. |
|---|---|---|---|---|---|
| 1Tm | n/a | Sephadex G-200/sucrose density gradient | Triton X-100 | n/a | [94] |
| Tm | 60 g | Sucrose density gradient | n/a | 8 mg (12%) | [95] |
| Ee | 5–20 g | Sephadex LH-20 columns | Chloroform-methanol extraction | n/a | [96] |
| Ee | 3–500 g | Sepharose 2B (affinity chromatography on a CT 5263 column) | Triton X-100 | 3–4 mg | [93] |
| Tc | n/a | Sepharose 6B activated with cyanogen bromide (CNBr)-affinity chromatography | Triton X-100 | 13 mg | [97] |
| Tc | 850 g | Sepharose 2B activated with CNBr -affinity chromatography | Triton X-100 | 30 mg (32%) | [98] |
| Ee | 800 g | Affinity chromatography containing covalently bound α-cobra neurotoxin followed by cyanogen bromide affinity chromatography and, finally, ion-exchange chromatography | Tween 80 | 8.5% | [99] |
| Ee | 3–500 g | Affinity chromatography and gel filtration using Sephadex G-75 to buffer exchange Triton X 100 to sodium cholate | Triton X-100 | 7–9 mg | [100] |
| Tc | 150 g | Affinity chromatography α-cobratoxin-Sepharose | Triton X-100 | n/a | [101] |
| Tm | n/a | Affinity chromatography neurotoxin-Sepharose 4B | Tween 20 | 0.4 mg/mL | [102] |
| Ee | 1000 g | Affinity chromatography (Sepharose 2B) and sucrose gradients, gel filtration using Sephadex G-75 for buffer exchange | Triton X-100 | 0.45 mg/mL (21%) | [103] |
| Tc | 600 g | Affinity chromatography | Triton X-100 | 115 µL/mL | [104] |
| Tm | 500–1000 g | Sucrose gradient and affinity chromatography | Triton X-100 | 50 mg | [92] |
| Tm | 800–900 g | Affinity chromatography, Sepharose 2B activated with CNBr | Triton X-100 | 78 mg | [105] |
| Sprague Dawley rats (skeletal muscle) | 300–500 g (2–10 rats) | Affinity chromatography, α-cobratoxin biospecific adsorption, ion-exchange chromatography, and gel filtration steps | Triton X-100 | 4.6–6.0 pM | [106] |
| Tc | 400 g | Affinity chromatography (α-cobratoxin) and Concanavalin A conjugated to beads to bind glycans | Sodium cholate | 20% | [107] |
| Tc | 60 mg of protein | Sucrose-density-gradient centrifugation and alkali treatment (pH 11.0) | Sodium cholate | # 100–150 nmol | [84] |
| Tc | n/a | Sucrose density gradient and α-cobra toxin affinity chromatography | Octylglucopyranoside and Triton X-100 | 85–90 mg | [108] |
| Optic lobes from whiteleg hornchicks | n/a | Affinity chromatography using α- Bgtx-Sepharose followed by a lentil lectin gel | Triton X-100 or Lubrol PX | 15–20% | [109] |
| Discopyge tschudii | 200 g | Sucrose gradient and affinity chromatography on Affi-Gel 401 using bromoacetylcholine as the ligand. | Sodium cholate | 2.30 mg (0.7%) | [91] |
| Tm | 100–200 g | Affinity chromatography, carbachol analog ligand | Triton X-100 | 0.2–4.5 nmol/mg (using α-Bgtx binding) | [110] |
| Chick brains | 300 g | Affinity chromatography: mAb 35 was coupled to Sepharose C14B | Triton X-100 | 0.165 mg | [111] |
| PC12 rat cell line | 100 g | Affinity chromatography: mAb 270-Sepharose | Triton X-100 | 0.216–0.245 mg/100 g rat brain | [111] |
| Tm | n/a | Affinity chromatography using α-cobratoxin covalently Sepharose 2B followed by CNBr | n/a | n/a | [112] |
| Tc | 100 g | Cibacron Blue Sepharose | β-octylglucopyranoside | $ 7 nmol | [50] |
| Fetal calf thymus | n/a | Affinity chromatography using α-cobratoxin-Sepharose after alkaline extraction | Triton X-100 | @ 11.34 μg/1059 g of the thymus | [113] |
| Tm | 100 g | Alkali treatment (pH 11.0), affinity chromatography (G = gallamine derivative N-(2-aminoethyl)-3,4,5-tris(2-triethylammonio-ethoxy)benzamide, C = N-(2-acetylamino- ethyl)-N-(2-aminoethyl)-N, N-dimethylammonium iodide hydroiodide, and D = N-(4-aminobenzyl)-N-dimethyl-N-(10-trimethyl-ammoniumdecyl) ammonium dichloride hydrochloride | Triton X-100 | Resin C = 3.0 mg (2%), Resin D = 3.5 mg (17%), Resin G = 6.5 mg (51%) | [85] |
| Tf | 120 g | Chromatofocusing, affinity chromatography using α-cobratoxin, and DEAE-Sepharose 6B | Triton X-100 | Chromatofocusing = 8.3 mg (12%), affinity chromatography using cobratoxin = 3.8 mg (5.6%), and DEAE-Sepharose 6B 2.5 mg (3%) | [114] |
| Wistar rat brains | 64 g | Affinity chromatography: DE-52 column and ACh-Affi-Gel | Lubrol PX | DE-52 = 43% and ACh-Affi-Gel 15% | [115] |
| Sprague Dawley rat brains | n/a | Affinity chromatography: Affi-Gel 401 | Triton X-100 | <0.01 mg/mL | [116] |
| Tc | 120 g | Affinity chromatography: Affi-Gel 10 | Triton X-100 | 30 mg | [117] |
| Tm | 50–1000 g | Affinity chromatography: The main column employed include reversible binding of Naja nigricollis by lysine-15 affinity chromatography. This column was compared with the following two affinity chromatography methods: First method: α-Bgtx from Bungarus multicinctus polymodified in various amines groups and covalently bound to a resin. Second method: α-cobratoxin from Naja naja kaouthia bound to a commercial agarose resin. | CHAPS (1%) | 80 pmol nAChR/mL of resin First method: 85 pmol nAChR/mL of resin 500 pmol nAChR/mL of resin | [87] |
| Tc | 100 g | Affinity-purified on a bromoacetylcholine bromide-derivatized Affi-Gel 102 column | Cholate | n/a | [118] |
| Tc | 5 mL | Sucrose gradient followed by affinity chromatography (using bromoacetylcholine bromide-derivatized Affi-Gel 10 column), and a PD-10 desalting column | Sodium cholate or Foscholine-12 or CHAPS or Anapoe-C12E9 or BigCHAP or Cymal-6 or DDM or LDAO or OG | n/a | [52] |
| Tc | n/a | Affinity chromatography using bromoacetylcholine bromide-derivatized Affi-Gel 10 column | Sodium cholate | n/a | [86] |
| Tc | 100 g | Affinity chromatography using bromoacetylcholine bromide-derivatized Affi-Gel 102 column | Cholate | n/a | [119] |
| Tc | 5 mL | Sucrose gradient followed by affinity chromatography (using bromoacetylcholine bromide-derivatized Affi-Gel 10 column), and a PD-10 desalting column | n-tetradecylphosphocholine, n-hexadecylphosphocholine f or 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine, 3-[(3-cholamidopropy) dimethylammonio]-2-hydroxy-1-propanesulfonate or (N,N′-bis-[3-D-gluconamidopropyl] cholamide), cymal-6, DDM, LDAO, and OG | 1–5 mg | [79] |
| Tm | 500–1000 g | Sucrose gradient | Triton X-100 | n/a | [62] |
| Tc | 60 g | Affinity purification using bromoacetylcholine bromide-derivatized Affi-Gel 10 column and a PD-10 desalting column | 1-palmitoyl-2-hydroxy-sn-glycero-3-phospho- | n/a | [54,55] |
| Tc | 50 g | Alkali treatment extraction at pH 11.0 followed by affinity purification (using 2-[aminobutanoyl)oxy]-N,N,N-trimethylethanaminium), and SEC | Triton X-100 and DDM | n/a | [65] |
| Tc | 20–40 g | Affinity purification (using bromoacetylcholine bromide-derivatized Affi-Gel 15 column), followed by Capto lentil lectin chromatography, and gel filtration | LysoFos Choline-16 and Cholesteryl hemisuccinate | 2–4 mg | [77,78] |
| Tc | 50 g | Alkali treatment extraction at pH 11.0 followed by affinity purification (using 2-[aminobutanoyl)oxy]-N,N,N-trimethylethanaminium), and SEC for characterization | Triton X-100 and DDM | n/a | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Vélez, M.; Quesada, O.; Villalobos-Santos, J.C.; Maldonado-Hernández, R.; Asmar-Rovira, G.; Stevens, R.C.; Lasalde-Dominicci, J.A. Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades. Molecules 2021, 26, 5753. https://doi.org/10.3390/molecules26195753
Delgado-Vélez M, Quesada O, Villalobos-Santos JC, Maldonado-Hernández R, Asmar-Rovira G, Stevens RC, Lasalde-Dominicci JA. Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades. Molecules. 2021; 26(19):5753. https://doi.org/10.3390/molecules26195753
Chicago/Turabian StyleDelgado-Vélez, Manuel, Orestes Quesada, Juan C. Villalobos-Santos, Rafael Maldonado-Hernández, Guillermo Asmar-Rovira, Raymond C. Stevens, and José Antonio Lasalde-Dominicci. 2021. "Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades" Molecules 26, no. 19: 5753. https://doi.org/10.3390/molecules26195753
APA StyleDelgado-Vélez, M., Quesada, O., Villalobos-Santos, J. C., Maldonado-Hernández, R., Asmar-Rovira, G., Stevens, R. C., & Lasalde-Dominicci, J. A. (2021). Pursuing High-Resolution Structures of Nicotinic Acetylcholine Receptors: Lessons Learned from Five Decades. Molecules, 26(19), 5753. https://doi.org/10.3390/molecules26195753





