Elucidation of the Structure of Lignin–Carbohydrate Complexes in Ginkgo CW-DHP by 13C-2H Dual Isotope Tracer
Abstract
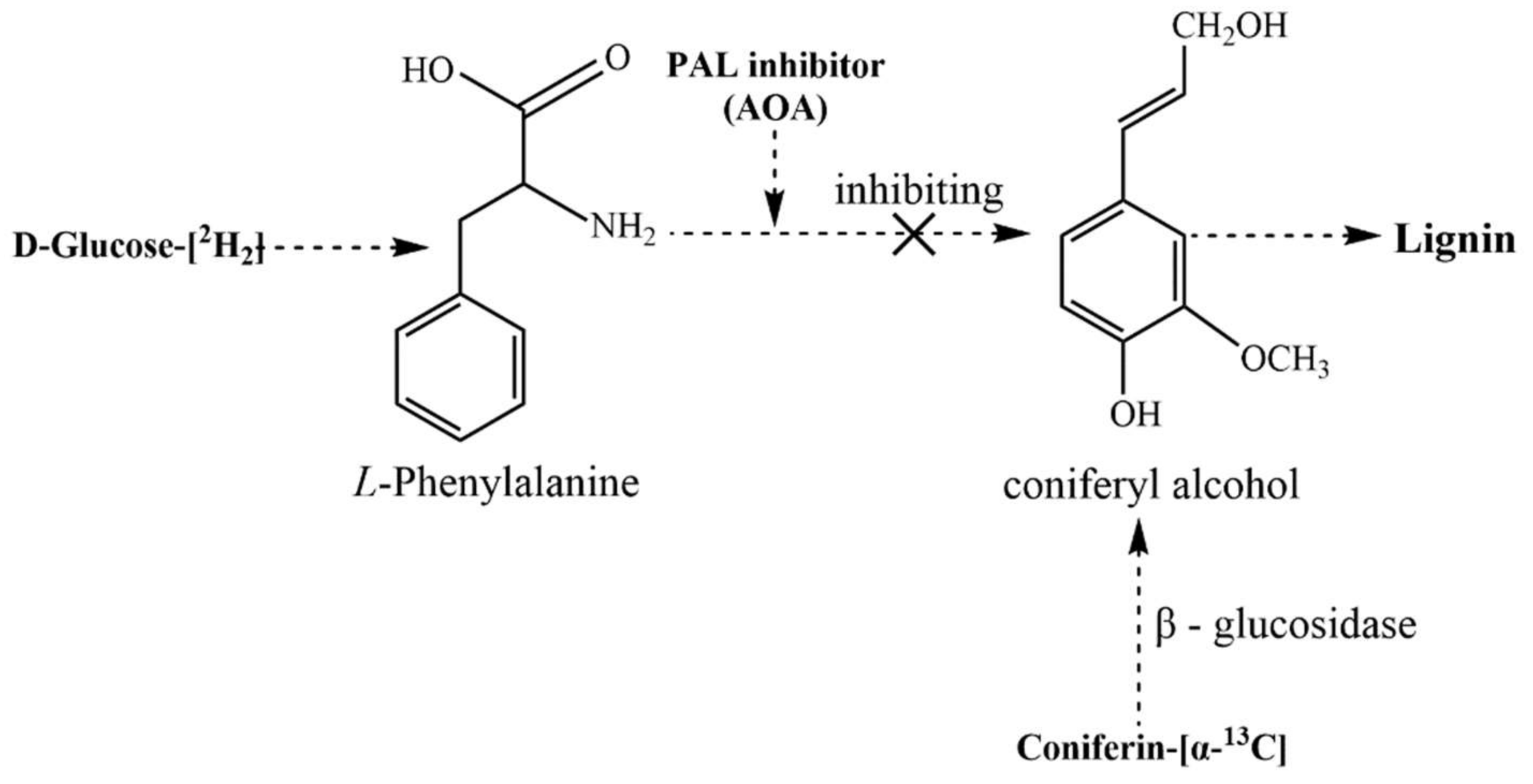
1. Introduction
2. Results and Discussion
2.1. Preparation of Ginkgo CW-DHP and Lignin–Carbohydrate Complex Fractions
2.2. Characterisation of Gingko CW-DHP
2.2.1. Abundance Characterization of 13C-2H-Enriched Ginkgo CW-DHP
2.2.2. Evaluation of the Lignification of Ginkgo CW-DHP
2.2.3. Solid-State CP/MAS 13C-NMR Analysis
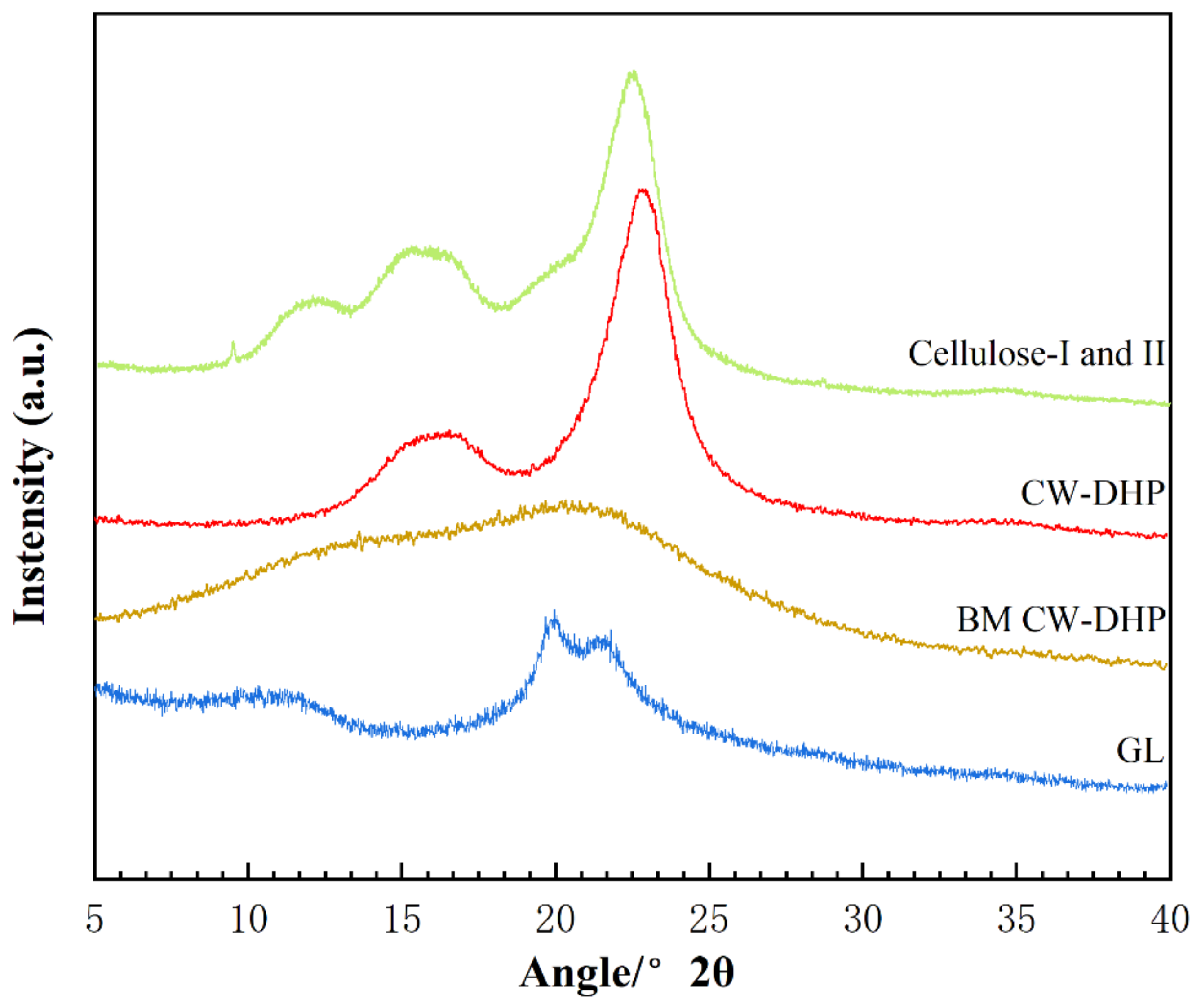
2.2.4. XRD Characterisation of CW-DHP and the Fractions
2.2.5. Molecular Weight Evaluation of LCC Fractions
2.3. Characterisation of the Glucan–Lignin Complex
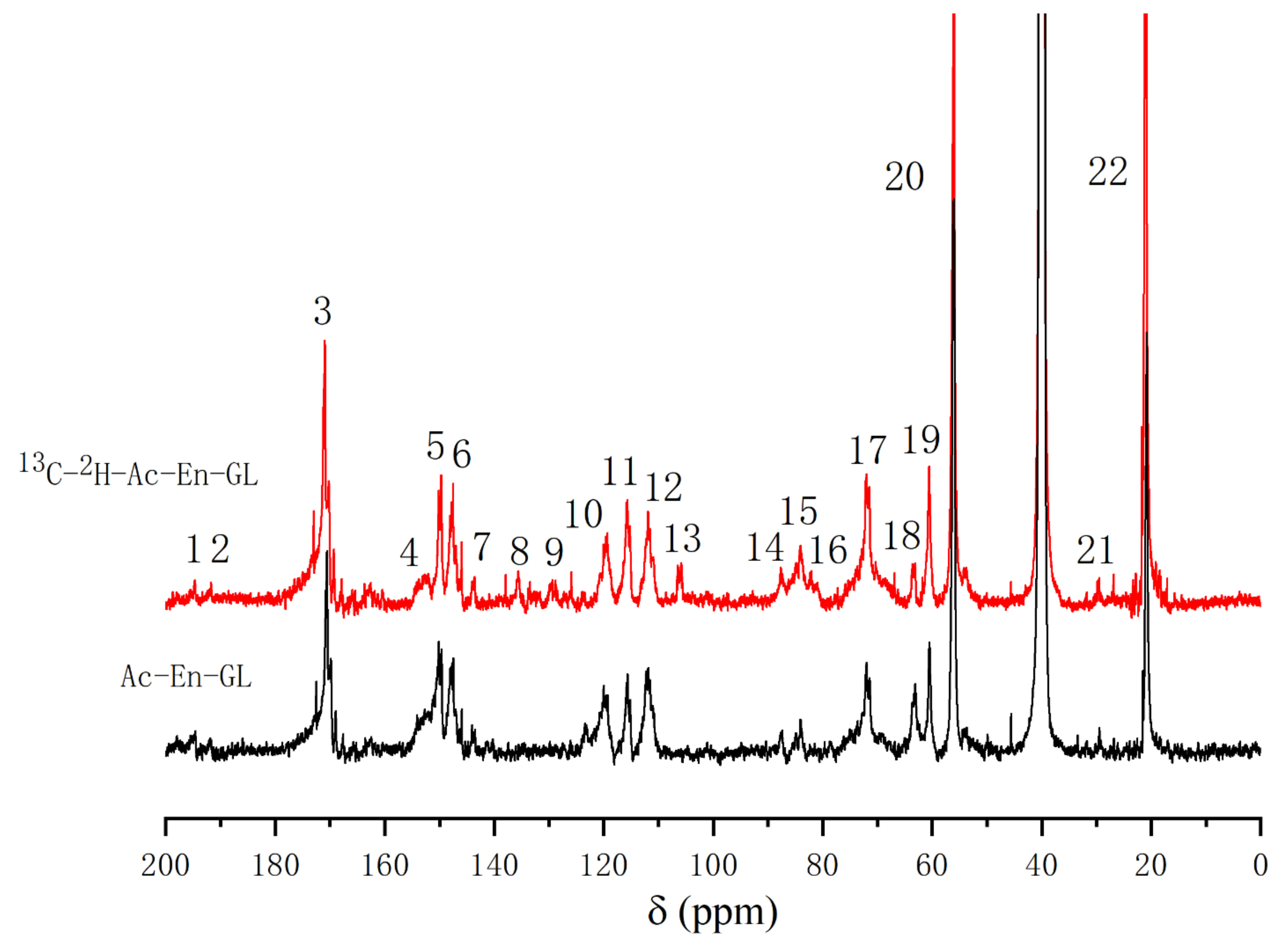
2.3.1. 13C-NMR Analysis of the Glucan–Lignin Complex (GL)
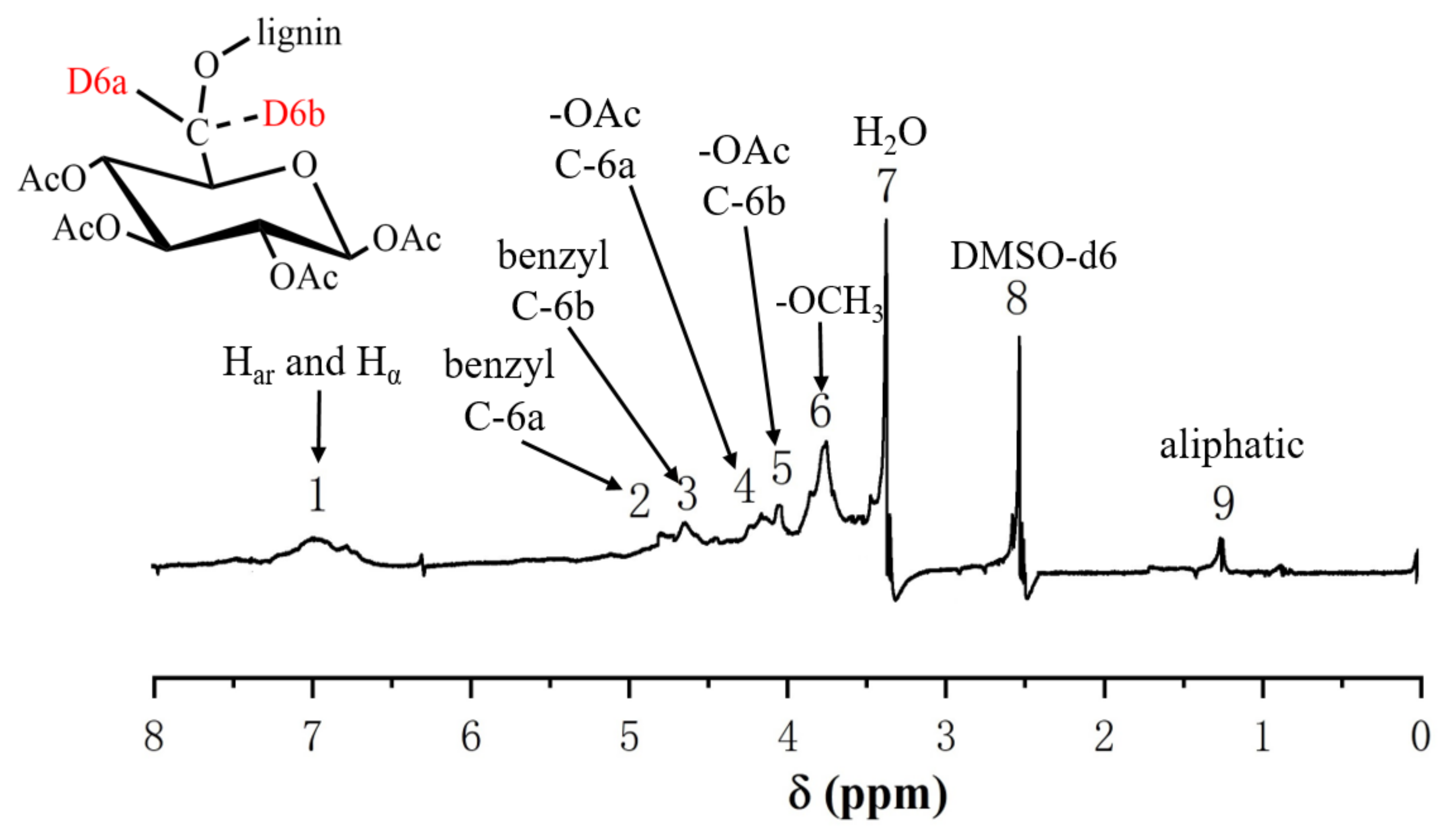
2.3.2. 1H-NMR Analysis of the GL
2.4. Characterisation of Glucomannan—Lignin Complex and Xylan—Lignin Complex
2.4.1. 13C-NMR Analysis of the Glucomannan—Lignin Complex (GML)
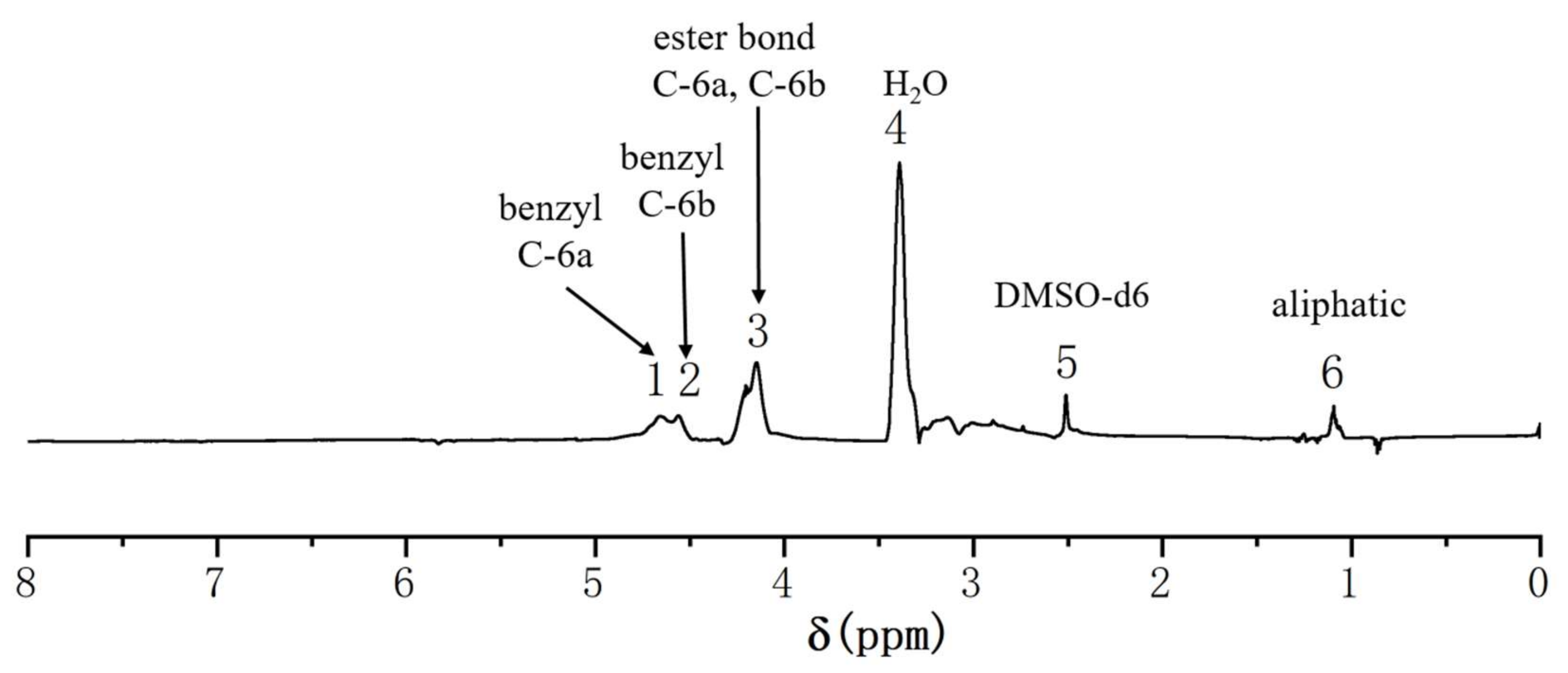
2.4.2. 1H-NMR Analysis of the GML
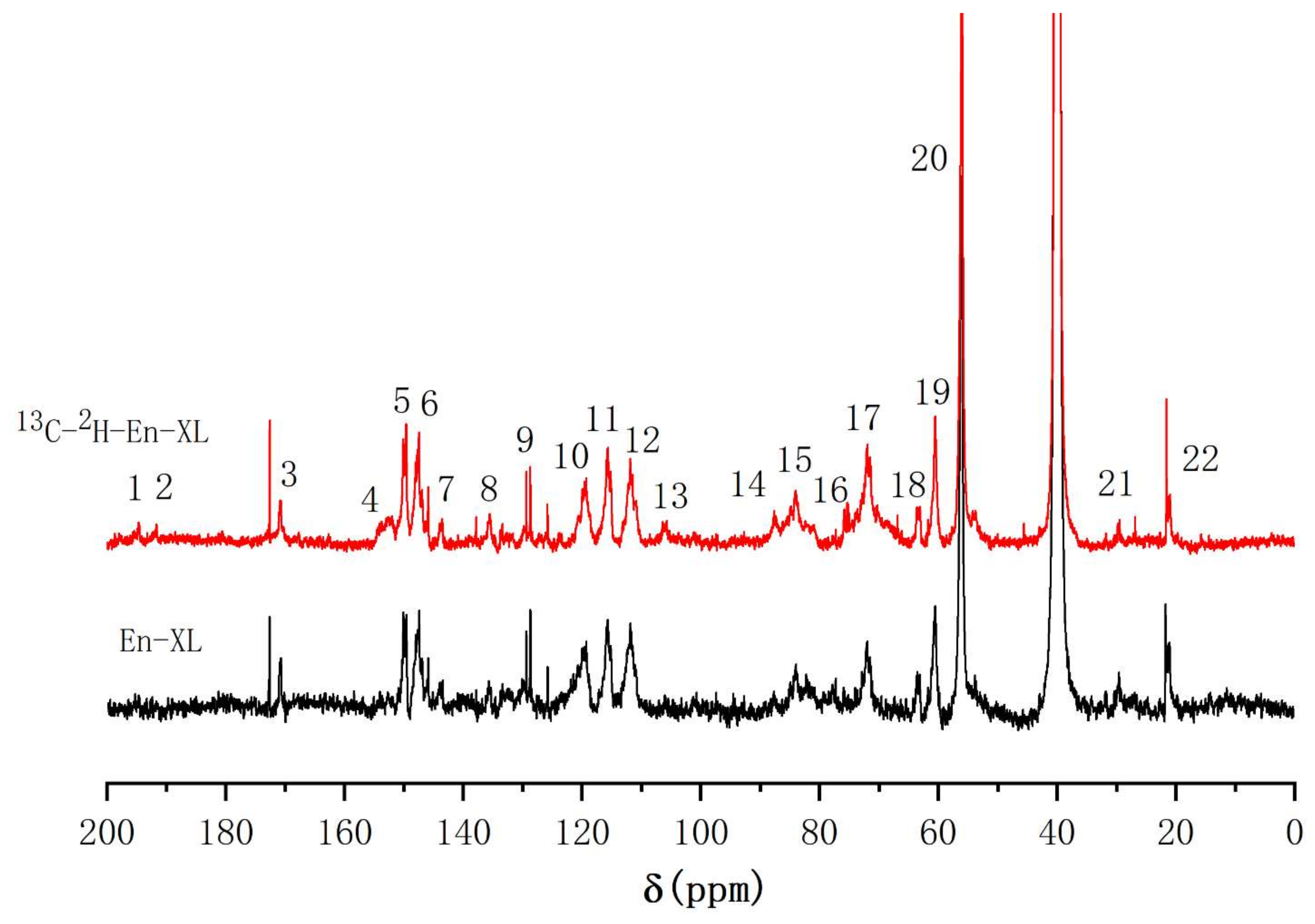
2.4.3. 13C-NMR Analysis of the Xylan—Lignin Complex
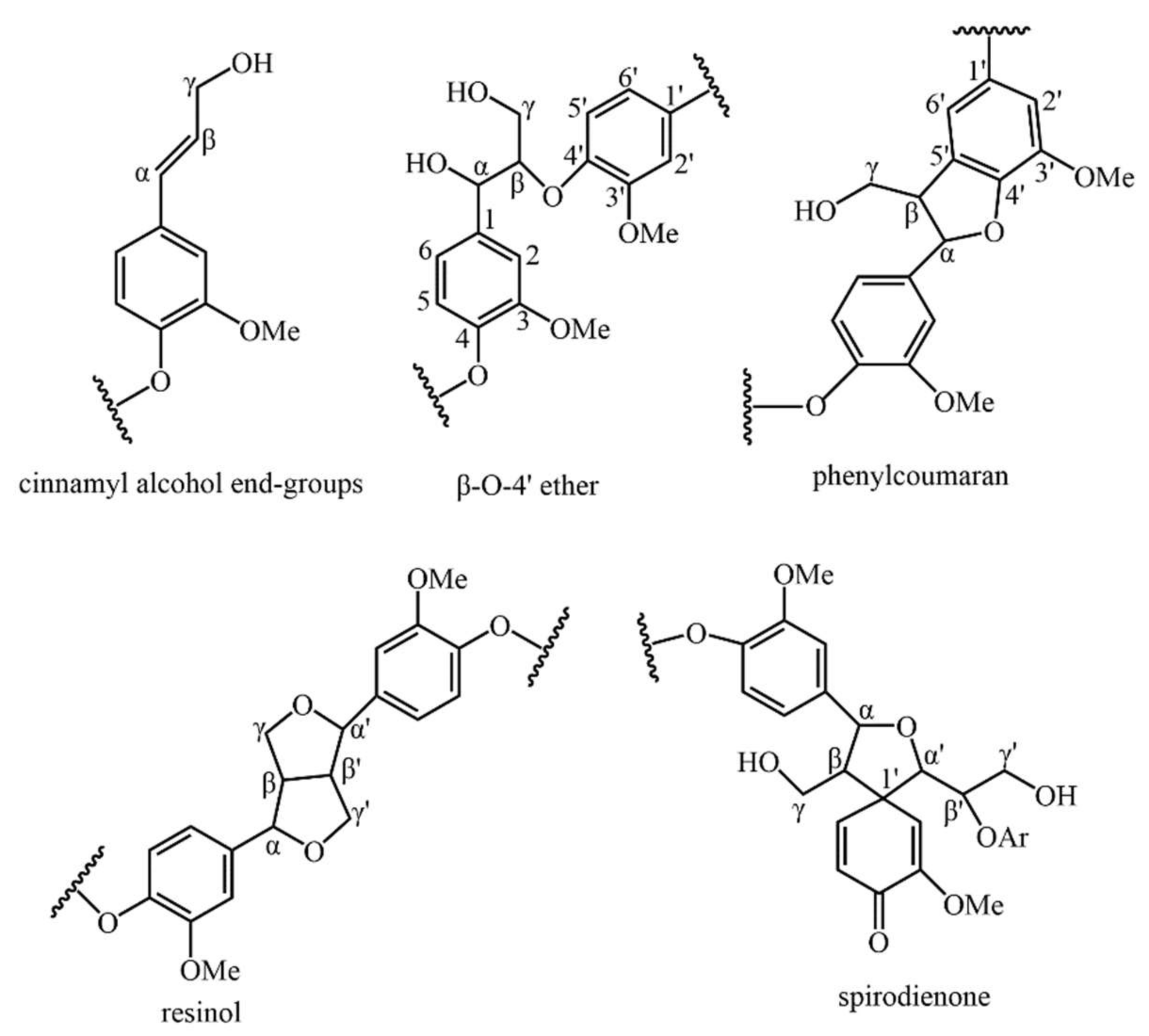
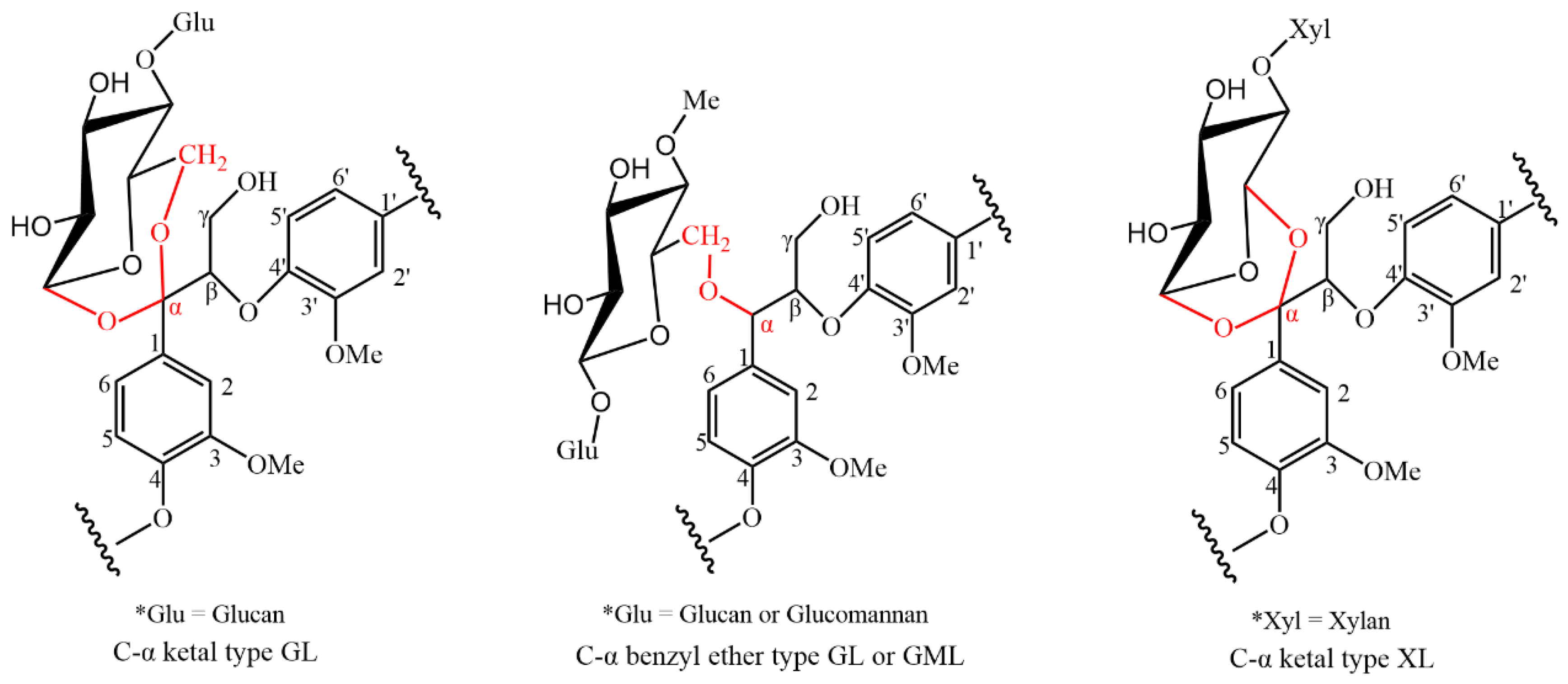
2.5. Chemical Structures
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ginkgo CW-DHP and Lignin–Carbohydrate Complex Fractions
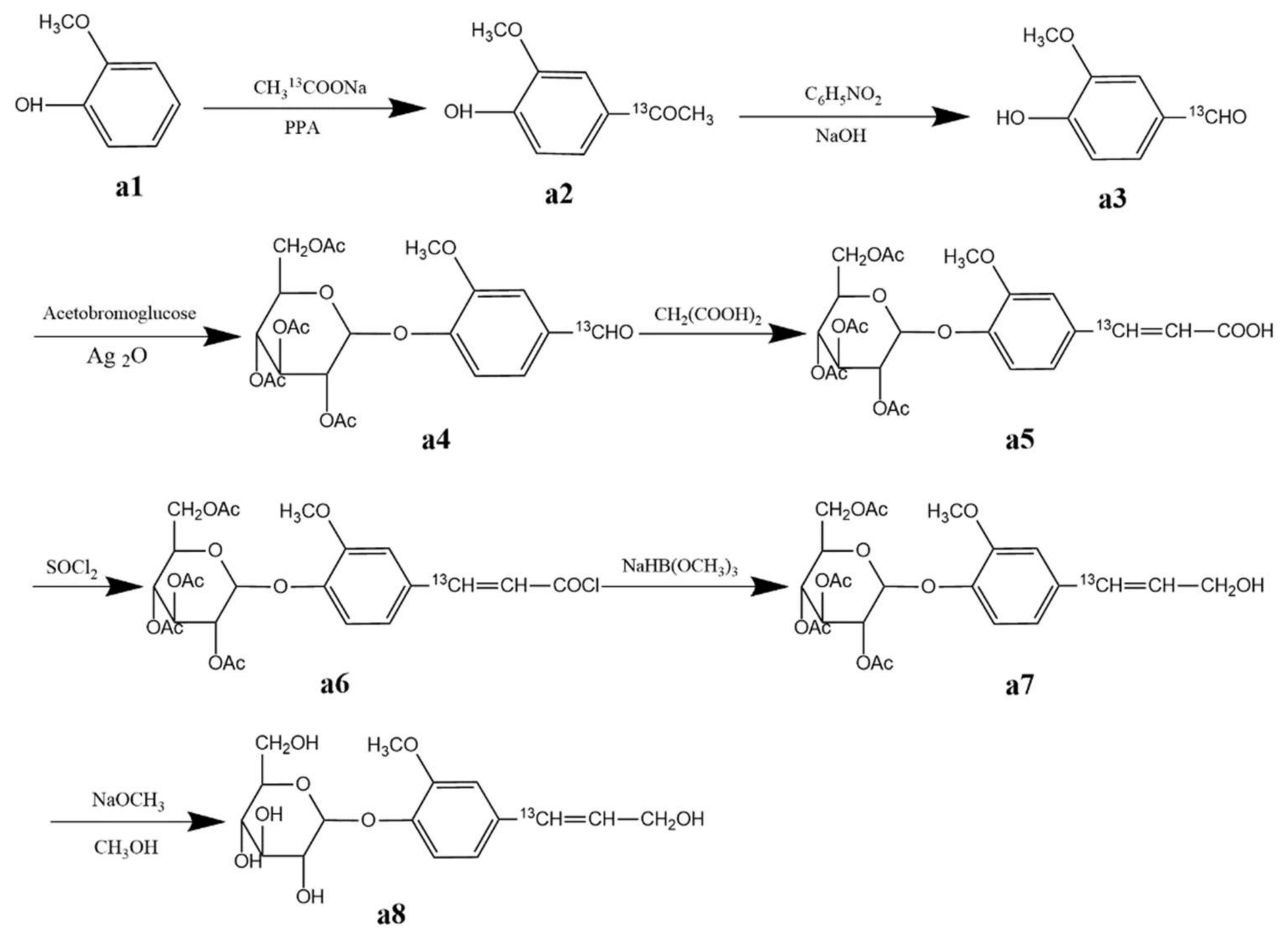
3.2.1. Synthesis of Coniferin
3.2.2. Preparation of Gingko CW-DHP
3.2.3. Preparation of Lignin–Carbohydrate Complex Fractions
3.2.4. Enzymatic Hydrolysis and Acetylation of LCC Fractions
3.3. Characterisation of Gingko CW-DHP
3.3.1. Determination of 13C and 2H Abundance
3.3.2. Determination of the Lignification of Ginkgo CW-DHP
3.3.3. Determination of Solid-State 13C-NMR Spectroscopy
3.3.4. Determination of XRD
3.3.5. Determination of Molecular Weights
3.3.6. Determination of 13C-NMR and 1H-NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Björkman, A. Studies on finely divided wood. Part 1. Extraction of lignin with neutral solvents. Sven. Papp. 1956, 59, 477–485. [Google Scholar]
- Koshijima, T.; Watanabe, T. Association between Lignin and Carbohydrates in Wood and Other Plant Tissues; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Gierer, J.; Wannström, S. The formation of ether bonds between lignins and carbohydrates during alkaline pulping processes. Holzforschung 1986, 40, 347–352. [Google Scholar] [CrossRef]
- Iverson, T.; Wannström, S. Lignin-carbohydrate bonds in a residual lignin isolated from pine kraft pulp. Holzforschung 1986, 40, 19–22. [Google Scholar] [CrossRef]
- Choi, J.W.; Choi, D.-H.; Faix, O. Characterization of lignin–carbohydrate linkages in the residual lignins isolated from chemical pulps of spruce (Picea abies) and beech wood (Fagus sylvatica). J. Wood Sci. 2007, 53, 309–313. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M.; Gilkes, N.; Kadla, J.; Maximenko, V.; Kubo, S.; Saddler, J. Inhibition of cellulase, xylanase and β-glucosidase activities by softwood lignin preparations. J. Biotechnol. 2006, 125, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The isolation, characterization and effect of lignin isolated from steam pretreated Douglas-fir on the enzymatic hydrolysis of cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Zheng, X.; Pu, Y.; Huang, F.; Meng, X.; Wu, W.; Ragauskas, A.; Yao, L. Comparative study of lignin characteristics from wheat straw obtained by soda AQ and kraft pretreatment and effect on the following enzymatic hydrolysis process. Bioresour. Technol. 2016, 207, 361–369. [Google Scholar] [CrossRef]
- Min, D.-Y.; Li, Q.; Chiang, V.; Jameel, H.; Chang, H.-M.; Lucia, L. The influence of lignin-carbohydrate complexes on the cellulase-mediated saccharification I: Transgenic black cottonwood (western balsam poplar, California poplar) P. trichocarpa including the xylan down-regulated and the lignin downregulated lines. Fuel 2014, 119, 207–213. [Google Scholar] [CrossRef]
- Min, D.-Y.; Yang, C.; Chiang, V.; Hasan, J.; Chang, H.-M. The influence of lignin-carbohydrate complexes on the cellulose-mediated saccharification II: Transgenic hybrid poplars (Populus nigra L. and Populus maximowiczii A.). Fuel 2014, 116, 56–62. [Google Scholar] [CrossRef]
- Xie, Y.; Yasuda, S.; Wu, H.; Liu, H. Analysis of the structure of lignin-carbohydrate complexes by the specific 13C tracer method. J. Wood Sci. 2000, 46, 130–136. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin-carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef]
- Nishimura, H.; Kamiya, A.; Nagata, T.; Katahira, M.; Watanabe, T. Direct evidence for a ether linkage between lignin and carbohydrates in wood cell walls. Sci. Rep. 2018, 8, 6538. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Chang, H.-M. A fraction of MWL with high concentration of lignin–carbohydrate linkages: Isolation and analysis with 2D NMR spectroscopic techniques. Holzforschung 2007, 61, 1–7. [Google Scholar] [CrossRef]
- Watanabe, T.; Koshijima, T. Evidence for an ester linkage between lignin and glucuronic acid in lignin-carbohydrate complexes by DDQ-Oxidation. Agric. Biol. Chem. 1988, 52, 2953–2955. [Google Scholar] [CrossRef]
- Eriksson, Ö.; Goring, D.A.I.; Lindgren, B.O. Structural studies on the chemical bonds between lignin and carbohydrates in spruce. Wood Sci. Technol. 1980, 14, 267–279. [Google Scholar] [CrossRef]
- Takakashi, N.; Koshijima, T. Ester linkages between lignin and glucuronoxylan in lignin-carbohydrate complex from beech (Fagus crenata) wood. Wood Sci. Technol. 1988, 22, 231–241. [Google Scholar] [CrossRef]
- Li, J.; Martin-Sampedro, R.; Pedrazzi, C.; Gellerstedt, G. Fractionation and characterization of lignin-carbohydrate complexes (LCCs) from eucalyptus fibers. Holzforschung 2011, 65, 43–50. [Google Scholar] [CrossRef]
- Du, X.; Gellerstedt, G.; Li, J. Universal fractionation of lignin–carbohydrate complexes (LCCs) from lignocellulosic biomass: An example using spruce wood. Plant J. 2013, 74, 328–338. [Google Scholar] [CrossRef]
- Du, X.; Pérez-Boada, M.; Fernández, C.; Rencoret, J.; del Río, J.C.; Jiménez-Barbero, J.; Li, J.; Gutiérrez, A.; Martínez, A. Analysis of lignin–carbohydrate and lignin–lignin linkages after hydrolase treatment of xylan–lignin, glucomannan–lignin and glucan–lignin complexes from spruce wood. Planta 2014, 239, 1079–1090. [Google Scholar] [CrossRef]
- Lewis, N.G.; Yamamoto, E.; Wooten, J.B.; Just, G.; Ohashi, H.; Towers, G.H.N. Monitoring biosynthesis of wheat cell-wall phenylpropanoids in situ. Science 1987, 237, 1344–1346. [Google Scholar] [CrossRef]
- Lewis, N.G.; Razal, R.A.; Dhara, K.P.; Yamamoto, E.; Bokelmann, G.H.; Wooten, J.B. Incorporation of [2-13C] ferulic acid, a lignin precursor, into Leucaena leucocephala and its analysis by solid state 13C NMR spectroscopy. J. Chem. Soc. Com. Chem. Commun. 1988, 24, 1626–1628. [Google Scholar] [CrossRef]
- Terashima, N.; Hafrén, J.; Westermark, U.; VanderHart, D.L. Structure of lignin in Ginkgo wood determined by a combination of specific 13C-enrichment technique and solid state NMR spectroscopy. In Proceedings of the 9th International Symposium on Wood and Pulping Chemistry, Montréal, QC, Canada, June 9–12 1997; pp. H1–H5. [Google Scholar]
- Terashima, N.; Atalla, R.H.; VanderHart, D.L. Solid state NMR spectroscopy of specifically 13C-enriched lignin in wheat straw from coniferin. Phytochemistry 1997, 46, 863–870. [Google Scholar] [CrossRef]
- Xie, Y.; Yasuda, S.; Terashima, N. Selective carbon 13 enrichment of side chain carbons of oleander lignin traced by carbon 13 nuclear magnetic resonance. Mokuzai Gakkaishi 1994, 40, 191–198. [Google Scholar]
- Xie, Y.; Liu, Y.; Jiang, C.; Wu, H. The Existence of Cellulose and Lignin Chemical Connections in Ginkgo Traced by 2H-13C Dual Isotopes. Bioresources 2020, 15, 9028–9044. [Google Scholar] [CrossRef]
- Hafrén, J.; Westermark, U.; Lennholm, H.; Terashima, N. Formation of 13C-Enriched Cell-Wall DHP Using Isolated Soft Xylem from Picea abies. Holzforschung 2002, 56, 585–591. [Google Scholar] [CrossRef]
- Xie, Y.; Terashima, N. Selective carbon 13-enrichment of side chain carbons of ginkgo lignin traced by carbon 13 nuclear magnetic resonance. Mokuzai Gakkaishi 1991, 37, 935–941. [Google Scholar]
- Xiang, S.; Xie, Y.; Yang, H.; Yao, L. Analysis of the association between cellulose and lignin by carbon 13 tracer method. Spectrosc. Spectr. Anal. 2013, 33, 2488–2491. [Google Scholar]
- Brunow, G.; Lundquist, K. Comparison of a synthetic dehydrogenation polymer of coniferyl alcohol with milled wood lignin from spruce, using 1H-NMR spectroscopy. Pap. Puu. 1980, 62, 669–672. [Google Scholar]
- Terashima, N.; Atalla, R.H.; Ralph, S.A.; Landucci, L.L.; Lapierre, C.; Monties, B. New preparations of lignin polymer models under conditions that approximate cell wall lignification. I. Synthesis of novel lignin polymer models and their structural characterization by 13C-NMR. Holzforschung 1995, 49, 521–527. [Google Scholar] [CrossRef]
- Terashima, N.; Atalla, R.H.; Ralph, S.A.; Landucci, L.L.; Lapierre, C.; Monties, B. New preparations of lignin polymer models under conditions that approximate cell wall lignification. II. Structural characterization of the models by thioacidolysis. Holzforschung 1996, 50, 9–14. [Google Scholar] [CrossRef]
- Terashima, N.; Hafrén, J.; Westermark, U. Preparation of 13C-enriched DHP on unlignified spruce cell walls (in Japanese). In Proceedings of the Annual Meeting of the Japan Wood Research Society, Shizuoka, Japan, 3–5 April 1998; Volume 3, p. 389. [Google Scholar]
- Terashima, N.; Hafrén, J.; Westermark, U.; VanderHart, D.L. Nondestructive analysis of lignin structure by NMR spectroscopy of specifically 13C-enriched lignins. Part 1. Solid state study of ginkgo wood. Holzforschung 2002, 56, 43–50. [Google Scholar] [CrossRef]
- Sipilä, J.; Brunow, G. On the mechanism of formation of non-cyclic benzyl ethers during lignin biosynthesis. Part 4. The reactions of a β-O-4 type quinone methide with carboxylic acids in the presence of phenols. The formation and stability of benzyl esters between lignin and carbohydrates. Holzforschung 1991, 45, 9–14. [Google Scholar]
- Jacques, D.; Haslam, E.; Bedford, G.R.; Greatbanks, D. Plant proanthocyanidins. Part II. Proanthocyanidin-A2 and its derivatives. J. Chem. Soc. Perkin Trans. 1974, 1, 2663–2671. [Google Scholar] [CrossRef]
- Lüdemann, H.D.; Nimz, H. Carbon-13 nuclear magnetic resonance spectra of lignins. Biochem. Biophys. Res. Commun. 1973, 52, 1162–1169. [Google Scholar] [CrossRef]
- Henrique, M.A.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Alves Gurgel, L.V.; Silva Barud, H.; Morais, L.C.; Pasquinia, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crop. Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef]
- Ahmed-Haras, M.R.; Kao, N.; Ward, L. Single-step heterogeneous catalysis production of highly monodisperse spherical nanocrystalline cellulose. Int. J. Biol. Macromol. 2020, 154, 246–255. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Jin, E.; Guo, J.; Yang, F.; Zhu, Y.; Song, J.; Jin, Y.; Orlando, J.R. On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr. Polym. 2016, 143, 327–335. [Google Scholar] [CrossRef]
- Meier, H. Barium hydroxide as a selective precipitating agent for hemicelluloses. Acta. Chem. Scand. 1958, 12, 144–146. [Google Scholar] [CrossRef][Green Version]
- Taneda, H.; Nakano, J.; Hosoya, S.; Chang, H.-M. Stability of α-ether type model compounds during chemical pulping processes. J. Wood Chem. Technol. 1987, 7, 485–497. [Google Scholar]
- Yoshihiro, N.; Hiroshi, O.; Hiroshi, M. 1H-NMR studies of (6r)-and (6s)-deuterated d-hexoses: Assignment of the preferred rotamers about C5-C6 bond of D-glucose and D-galactose derivatives in solutions. Tetrahedron Lett. 1984, 25, 1575–1578. [Google Scholar]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.-M.; Jameel, H. Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Deus, C.; Friebolin, H. Partiell acetylierte cellulose-synthese und bestimmung der substituentenverteilung mit hilfe der 1H NMR-spektroskopie. Makromol. Chem. 1991, 192, 75–83. [Google Scholar] [CrossRef]
- Hikichi, K.; Kakuta, Y.; Katoh, T. 1H NMR study on substituent distribution of cellulose diacetate. Polym. J. 1995, 27, 659–663. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Kamitakahara, H.; Takano, T.; Nakatsubo, F. Fractionation and characterization of lignin carbohydrate complexes (LCCs) of Eucalyptus globulus in residues left after MWL isolation. Part I: Analyses of hemicellulose-lignin fraction (HC-L). Holzforschung 2012, 66, 459–465. [Google Scholar] [CrossRef]
- Grasdalen, H.; Painter, T. NMR studies of composition and sequence in legume-seed galactomannans. Carbohydr. Res. 1980, 81, 59–66. [Google Scholar] [CrossRef]
- Bi, H.; Gao, T.; Li, Z.; Ji, L.; Yang, W.; Iteku Jeff, B.; Liu, E.; Zhou, Y. Structural elucidation and antioxidant activity of a water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries). Food Chem. 2013, 138, 1470–1475. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Kamitakahara, H.; Takano, T. Fractionation and characterization of lignin-carbohydrate complexes (LCCs) of Eucalyptus globulus in residues left after MWL isolation. Part II: Analyses of xylan-lignin fraction (X-L). Holzforschung 2013, 67, 629–642. [Google Scholar] [CrossRef]
- Yao, L.; Chen, C.; Zheng, X.; Peng, Z.; Yang, H.; Xie, Y. Determination of Lignin-Carbohydrate Complexes Structure of Wheat Straw using Carbon-13 Isotope as a Tracer. Bioresources 2016, 11, 6692–6707. [Google Scholar] [CrossRef]
- Slater, W.G.; Beevers, H. Utilization of D-Glucuronate by Corn Coleoptiles. Plant Physiol. 1958, 33, 146–151. [Google Scholar] [CrossRef]
- Bailey, R.W.; Hassid, W.Z. Xylan synthesis from uridine-diphosphate-d-xylose by particulate preparations from immature corncobs. Proc. Natl. Acad. Sci. USA 1966, 56, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Terashima, N.; Ralph, S.A.; Landucci, L.L. New facile syntheses of monolignol glucosides; p-glucocoumaryl alcohol, coniferin and syringin. Holzforschung 1996, 50, 151–155. [Google Scholar]
- Terashima, N.; Koa, C.; Matsushita, Y.; Westermark, U. Monolignol glucosides as intermediate compounds in lignin biosynthesis. Revisiting the cell wall lignification and new 13C-tracer experiments with Ginkgo biloba and Magnolia liliiflora. Holzforschung 2016, 70, 801–810. [Google Scholar] [CrossRef]
- Imai, T.; Terashima, N. Determination of the distribution and reaction of polysaccharides in wood cell walls by the isotope tracer technique. IV. Selective radio-labeling of xylan in magnolia (Magnolia kobus) and visualization of its distribution in differentiating xylem by microautoradiography. Mokuzai Gakkaishi 1992, 38, 693–699. [Google Scholar]
- Iiyama, K.; Wallis, A.F.A. An improved acetyl bromide procedure for determining lignin in woods and wood pulps. Wood Sci. Technol. 1988, 22, 271–280. [Google Scholar] [CrossRef]
- Parkås, J.; Paulsson, M.; Westermark, U.; Terashima, N. Solid State NMR Analysis of β-13C-Enriched Lignocellulosic Material During Light-Induced Yellowing. Holzforschung 2001, 55, 276–282. [Google Scholar] [CrossRef]





| Sample | 13C(VPDB) | 13Cα/12Cα (%) | D(VSMOW) | D6/H6 (%) |
|---|---|---|---|---|
| A | −27.18 (±2.21) | 1.08 | −132.90 (±4.20) | 0.01 |
| B | 81.69 (±5.46) | 7.10 | 1902.75 (±30.25) | 0.37 |
| Samples | Lignin Contents | ||
|---|---|---|---|
| Cambial Tissues | CW-DHP | Increase | |
| unenriched sample | 14.47% (±0.12%) | 19.74% (±0.16%) | 5.42% |
| 13C-2H-enriched sample | 14.47% (±0.12%) | 19.89% (±0.14%) | 5.27% |
| Shift (δ, ppm) | Assignments | Ratio of Integrated Area |
|---|---|---|
| 140.5–124.7 | -CαH = CH- of coniferyl alcohol | 10.8% |
| 100.5–110.2 | ketal linkages in carbohydrates and lignin C-α | 7.6% |
| 93.1–80.7 | β-5, β-β and Cα-O-R (R was glycosyl) | 29.1% |
| 80.1–67.9 | Cα in β-O-4 | 39.6% |
| 67.9–58.0 | Cα in β-1 | 12.9% |
| Samples | Mw (g/mol) | Mn (g/mol) | Mw/Mn |
|---|---|---|---|
| Ac-GL | 3.9 × 105 | 3.2 × 105 | 1.22 |
| Ac-En-GL | 1.7 × 104 | 6.4 × 103 | 2.62 |
| Ac-GML | 2.7 × 104 | 1.5 × 104 | 1.80 |
| XL | 9.9 × 103 | 6.3 × 103 | 1.57 |
| En-XL | 5.3 × 103 | 3.2 × 103 | 1.65 |
| Signal | δ13C (ppm) | Assignments | |
|---|---|---|---|
| Ac-En-GL | |||
| 13C-Enriched | Control | ||
| 1 | 194.6 | 194.3 | α-CO, and α-CHO in vanillin |
| 2 | 191.5 | 191.7 | α-CHO |
| 3 | 170.7 | 169.9 | -C = O in acetyl group |
| 4 | 153.0 | 153.2 | C-4 in G, α-etherified; C-α in cinnamaldehyde |
| 5 | 150.1 | 150.6 | C-3 in G, α-etherified; C-4 in G, non-etherified |
| 6 | 147.5 | 148.5 | C-3 in G, non-etherified |
| 7 | 143.7 | 143.3 | C-α in cinnamic acid; C-4 in phenylcoumaran substructures |
| 8 | 135.6 | - | C-1 in G, non-etherified |
| 9 | 129.7 | - | C-1 in G with C-α in -CαH = CH- of coniferyl alcohols |
| 10 | 119.5 | 119.3 | C-6 in G, non-etherified |
| 11 | 115.7 | 115.7 | C-6 in β-5; C-5 in G, non-etherified |
| 12 | 111.5 | 111.3 | C-2 in G, non-etherified |
| 13 | 105.8 | - | C-α with ketal linkages; C-1 in glucose |
| 14 | 87.4 | 87.2 | C-α in phenylcoumaran |
| 15 | 84.0 | 84.1 | C-β in β-arylether; C-α in pinoresinol |
| 16 | 82.5 | - | C-α etherified to glucan |
| 17 | 72.1 | 72.5 | C-α in β-arylether; C-6 in glucan with ether linkage; C-2 in glucose |
| 18 | 63.6 | 63.3 | C-γ in phenylcoumaran |
| 19 | 60.5 | 61.1 | C-γ in β-arylether; C-6 in glucose |
| 20 | 56.4 | 56.2 | -OCH3 |
| 21 | 29.5 | 29.3 | Unknown |
| 22 | 21.3 | 21.9 | -CH3 in acetyl group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Liu, Y.; Cui, S.; Xie, Y. Elucidation of the Structure of Lignin–Carbohydrate Complexes in Ginkgo CW-DHP by 13C-2H Dual Isotope Tracer. Molecules 2021, 26, 5740. https://doi.org/10.3390/molecules26195740
Zhang K, Liu Y, Cui S, Xie Y. Elucidation of the Structure of Lignin–Carbohydrate Complexes in Ginkgo CW-DHP by 13C-2H Dual Isotope Tracer. Molecules. 2021; 26(19):5740. https://doi.org/10.3390/molecules26195740
Chicago/Turabian StyleZhang, Kai, Yanchao Liu, Sheng Cui, and Yimin Xie. 2021. "Elucidation of the Structure of Lignin–Carbohydrate Complexes in Ginkgo CW-DHP by 13C-2H Dual Isotope Tracer" Molecules 26, no. 19: 5740. https://doi.org/10.3390/molecules26195740
APA StyleZhang, K., Liu, Y., Cui, S., & Xie, Y. (2021). Elucidation of the Structure of Lignin–Carbohydrate Complexes in Ginkgo CW-DHP by 13C-2H Dual Isotope Tracer. Molecules, 26(19), 5740. https://doi.org/10.3390/molecules26195740







