Galvano-Fenton Engineering Solution with Spontaneous Catalyst’s Generation from Waste: Experimental Efficiency, Parametric Analysis and Modeling Interpretation Applied to a Clean Technology for Dyes Degradation in Water
Abstract
:1. Introduction
2. Results and Discussion
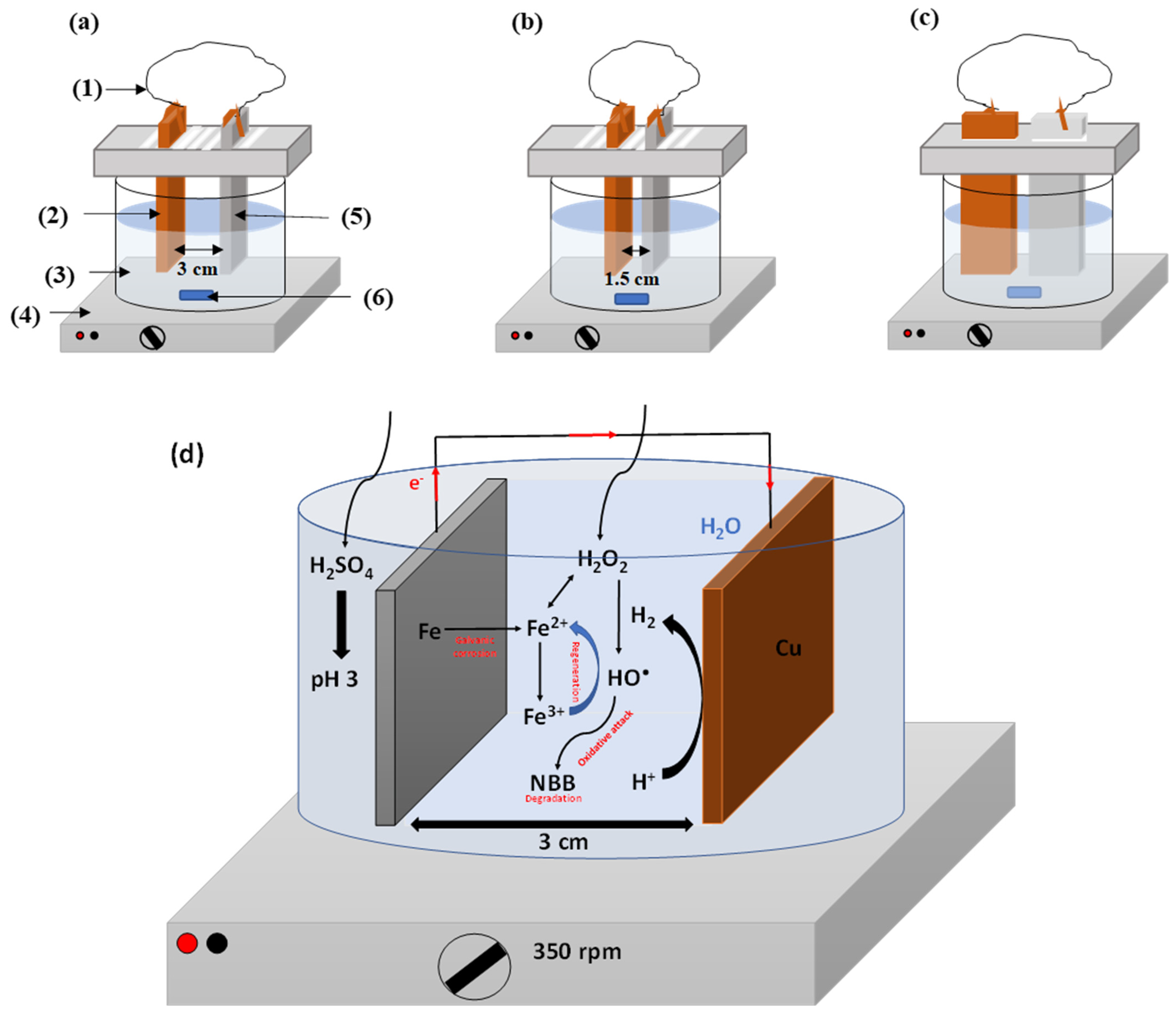
2.1. Selection of Operating Conditions
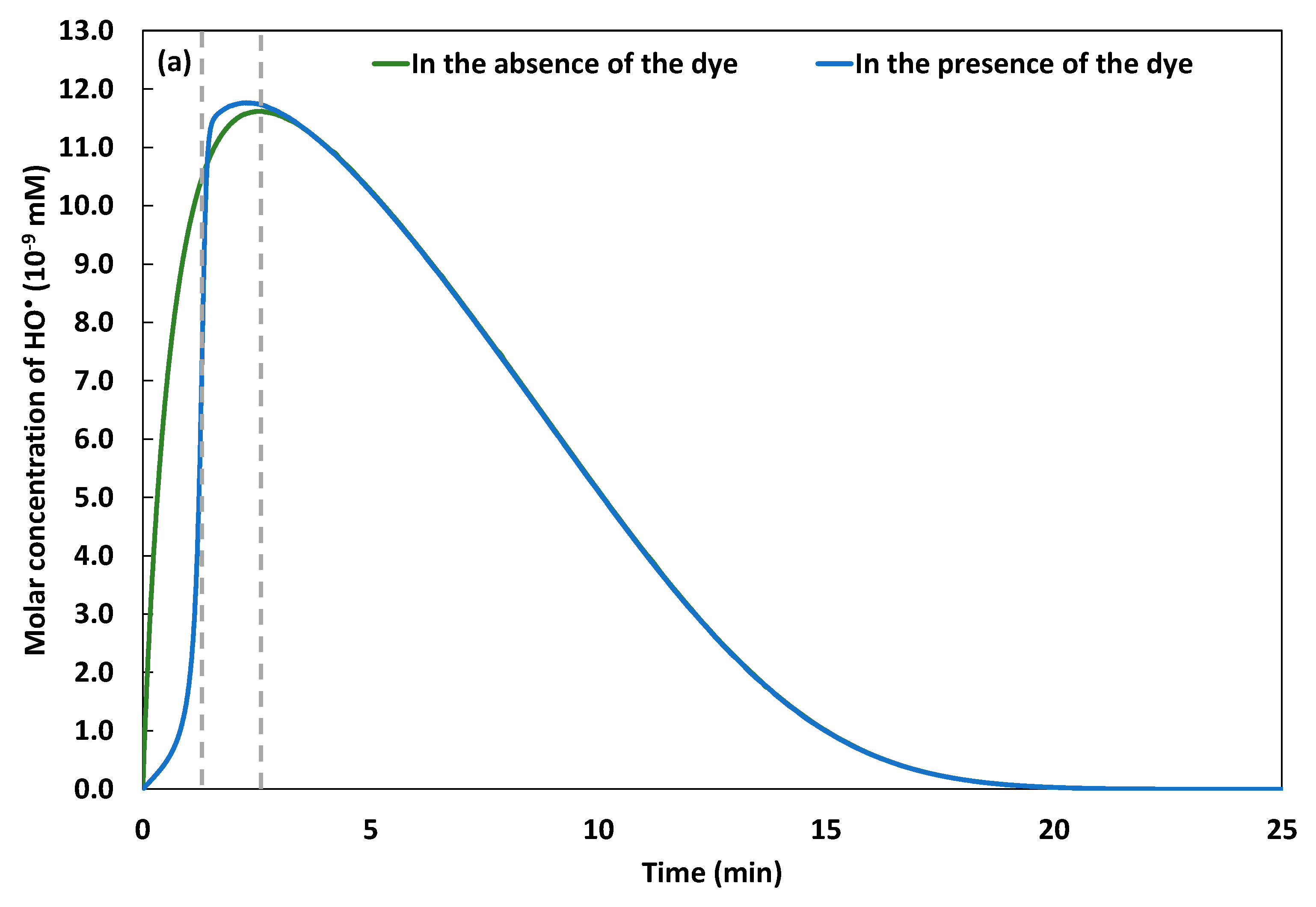
2.2. Monitoring of Fe(III) and Kinetics
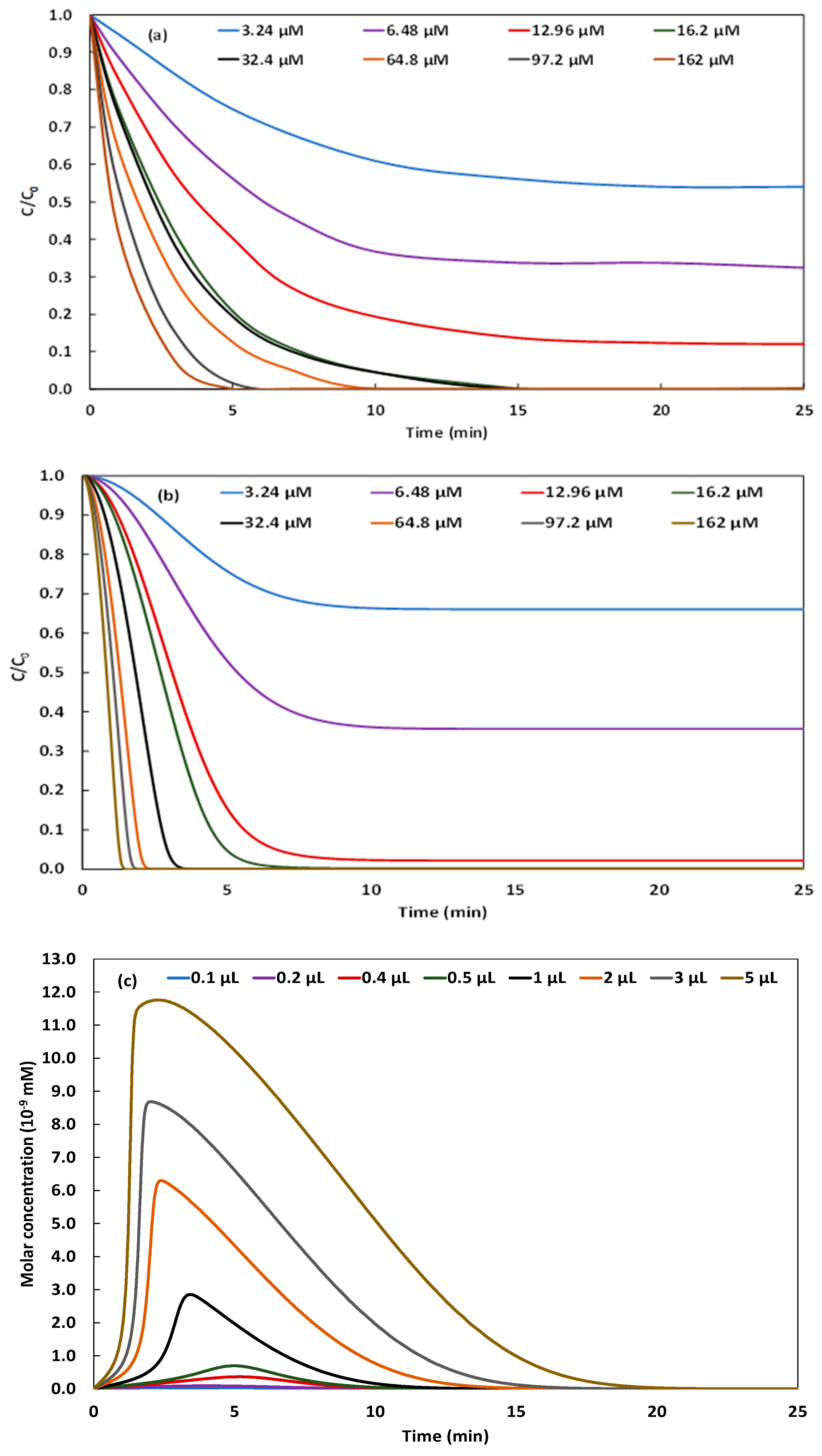
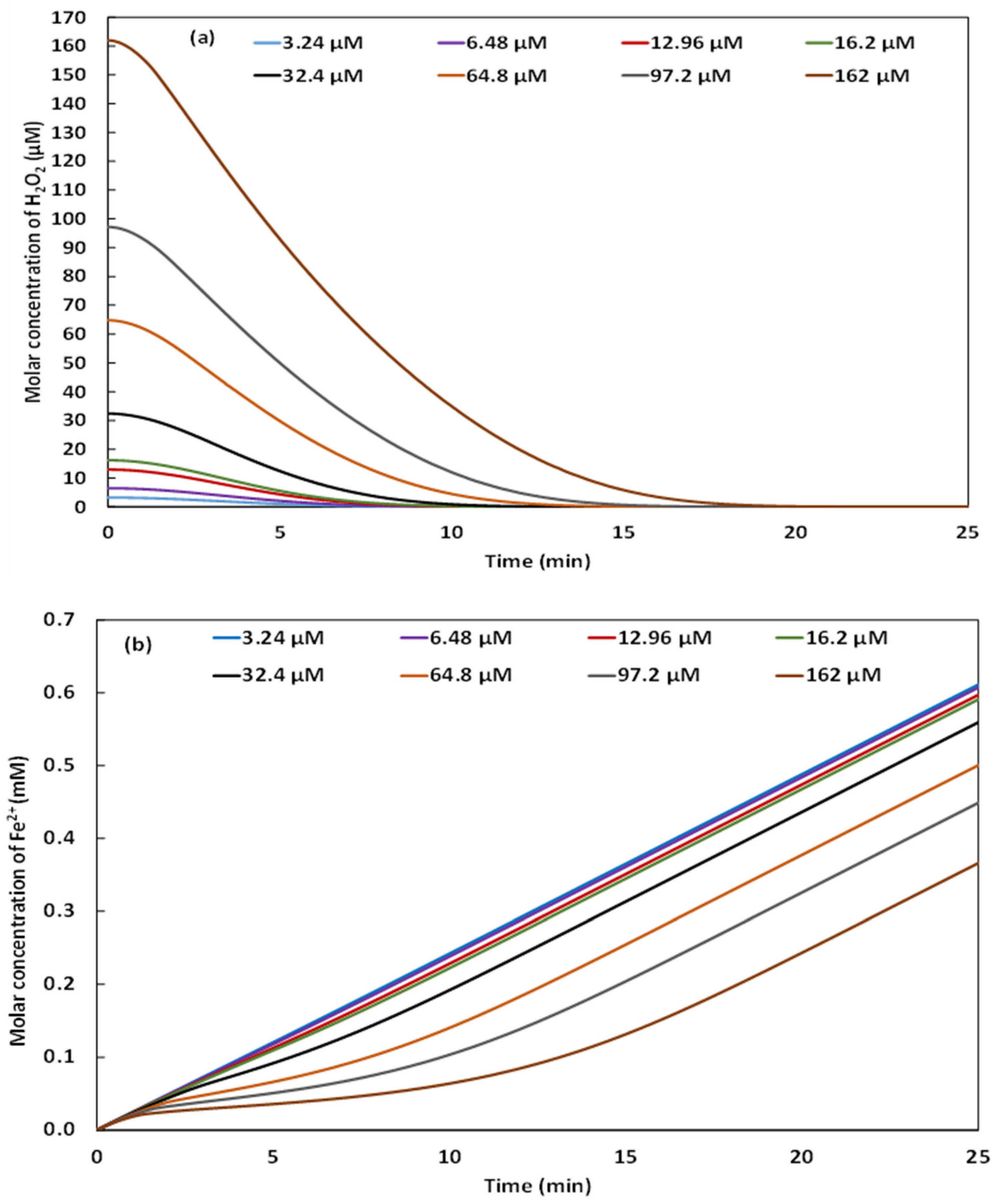
2.3. Effect of H2O2 Concentration
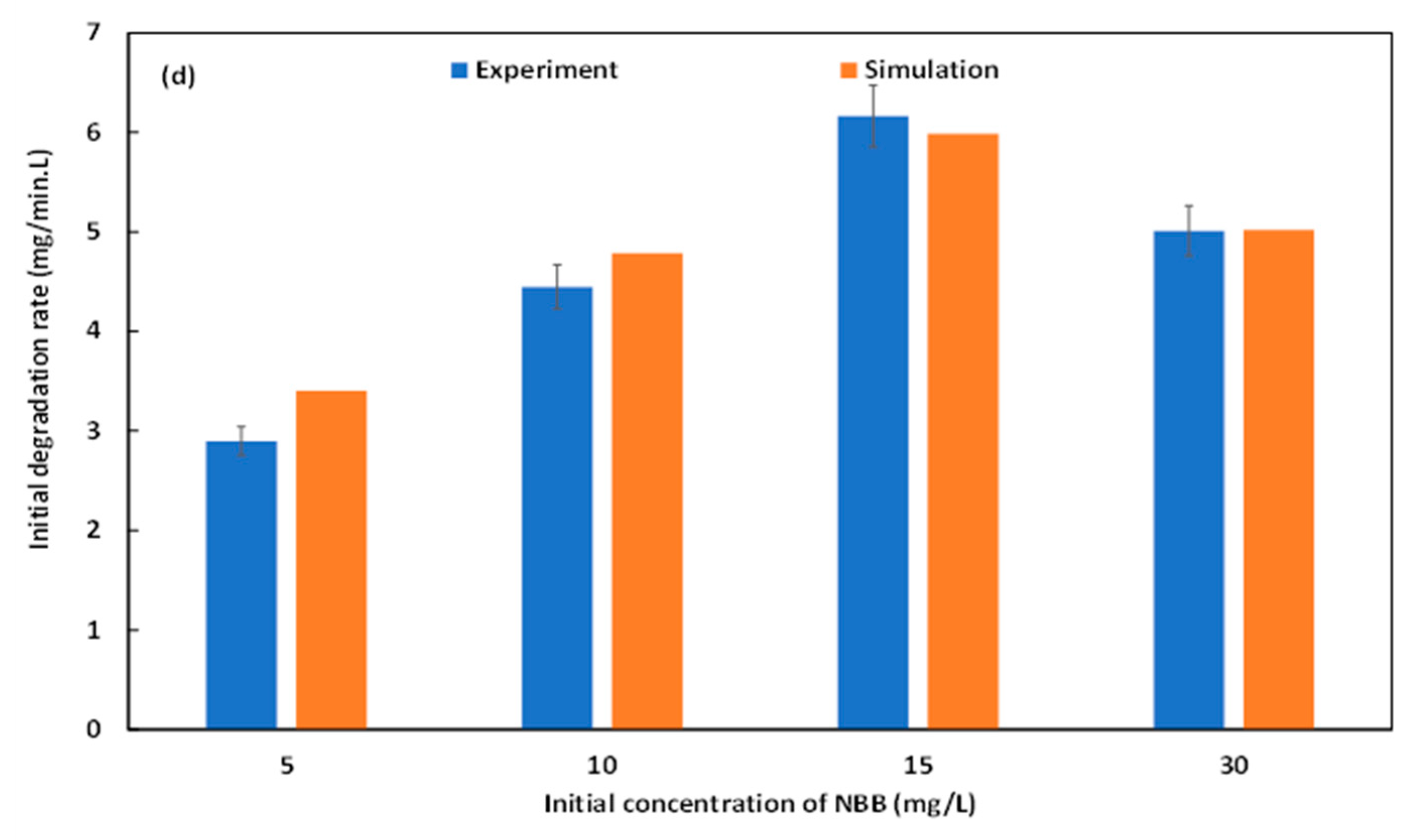
2.4. Effect of NBB Initial Concentration
3. Materials and Methods
3.1. Experimental Setup
3.2. Numerical Modeling and Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tröster, I.; Fryda, M.; Herrmann, D.; Schäfer, L.; Hänni, W.; Perret, A.; Blaschke, M.; Kraft, A.; Stadelmann, M. Electrochemical advanced oxidation process for water treatment using DiaChem® electrodes. Diam. Relat. Mater. 2002, 11, 640–645. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Von Sonntag, C. Advanced oxidation processes: Mechanistic aspects. Water Sci. Technol. 2008, 58, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L. Kinetic study of hydroxyl radical formation in a continuous hydroxyl generation system. RSC Adv. 2018, 8, 40632–40638. [Google Scholar] [CrossRef] [Green Version]
- Nurhayati, E. A Brief Review on Electro-generated Hydroxyl Radical for Organic Wastewater Mineralization. JSTL. 2012, 4, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Tarkwa, J.B.; Oturan, N.; Acayanka, E.; Laminsi, S.; Oturan, M.A. Photo-Fenton oxidation of Orange G azo dye: Process optimization and mineralization mechanism. Environ. Chem. Lett. 2019, 17, 473–479. [Google Scholar] [CrossRef]
- Kausley, S.B.; Desai, K.S.; Shrivastava, S.; Shah, P.R.; Patil, B.R.; Pandit, A.B. Mineralization of alkyd resin wastewater: Feasibility of different advanced oxidation processes. J. Environ. Chem. Eng. 2018, 6, 3690–3701. [Google Scholar] [CrossRef]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Borba, F.H.; Manetil, D.R. Performance evaluation of an integrated photo-Fenton - Electrocoagulation process applied to pollutant removal from tannery effluent in batch system. Chem. Eng. J. 2012, 197, 1–9. [Google Scholar] [CrossRef]
- He, Z.; Zhu, R.; Xu, X.; Song, S. Ozonation combined with sonolysis for degradation and detoxification of m-nitrotoluene in aqueous solution. Ind. Eng. Chem. Res. 2009, 48, 5578–5583. [Google Scholar] [CrossRef]
- He, Y.; Grieser, F.; Ashokkumar, M. The mechanism of sonophotocatalytic degradation of methyl orange and its products in aqueous solutions. Ultrason. Sonochem. 2011, 18, 974–980. [Google Scholar] [CrossRef]
- Kerboua, K.; Hamdaoui, O.; Alghyamah, A. Predicting the Sonochemical Efficiency for Water Decontamination: An Upscaled Numerical Approach. Chem. Eng. Technol. 2020, 44, 1–18. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Rawat, D.; Sharma, R.S.; Karmakar, S.; Singh Arora, L.; Mishra, V. Ecotoxic potential of a presumably non-toxic azo dye. Ecotoxicol. Environ. Saf. 2018, 148, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Bin Hussein, M.Z.; Zainal, Z.; Yahaya, A.H.; Binti Abd Aziz, A. Synthesis of layered organic-inorganic nanohybrid material: An organic dye, naphthol blue black in magnesium-aluminum layered double hydroxide inorganic lamella. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2002, 88, 98–102. [Google Scholar] [CrossRef]
- Dalhatou, S.; Petrier, C.; Laminsi, S.; Baup, S. Sonochemical removal of naphthol blue black azo dye: Influence of parameters and effect of mineral ions. Int. J. Environ. Sci. Technol. 2015, 12, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ferkous, H.; Hamdaoui, O.; Merouani, S. Sonochemical degradation of naphthol blue black in water: Effect of operating parameters. Ultrason. Sonochem. 2015, 26, 40–47. [Google Scholar] [CrossRef]
- Özcan, A.A.; Özcan, A. Investigation of applicability of Electro-Fenton method for the mineralization of naphthol blue black in water. Chemosphere 2018, 202, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Haddour, N.; Kharrat, R. Procédé de Traitement d’un Effluent Liquide Comprenant un Polluant Organique, Assurant la Production D’énergie Électrique et Dispositif Associé. France Patent FR3030480 (BOPI 2016-25), 24 June 2016. [Google Scholar]
- Gasmi, I.; Kerboua, K.; Haddour, N.; Hamdaoui, O.; Alghyamah, A.; Buret, F. Kinetic pathways of iron electrode transformations in Galvano-Fenton process: A mechanistic investigation of in-situ catalyst formation and regeneration. J. Taiwan Inst. Chem. Eng. 2020, 116, 81–91. [Google Scholar] [CrossRef]
- Gasmi, I.; Kerboua, K.; Haddour, N.; Hamdaoui, O.; Alghyamah, A.; Buret, F. The Galvano-Fenton process: Experimental insights and numerical mechanistic investigation applied to the degradation of acid orange 7. Electrochim. Acta. 2021, 373, 137897. [Google Scholar] [CrossRef]
- Kerboua, K.; Hamdaoui, O.; Haddour, N.; Alghyamah, A. Simultaneous Galvanic Generation of Fe2+ Catalyst and Spontaneous Energy Release in the Galvano-Fenton Technique: A Numerical Investigation of Phenol’s Oxidation and Energy production and saving. Catalysts 2021, 11, 943. [Google Scholar] [CrossRef]
- Gasmi, I.; Haddour, N.; Hamdaoui, O.; Kerboua, K.; Alghyamah, A.; Buret, F. A Novel Energy-from-Waste Approach for Electrical Energy Production by Galvano – Fenton Process. Molecules 2021, 26, 4013. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Raut, P.D. Treatment of textile wastewater by Fenton’s process as a Advanced Oxidation Process. IOSR J. Environ. Sci. Toxicol. Food. Technol. 2014, 8, 29–32. [Google Scholar] [CrossRef]
- Kaur, P.; Sangal, V.K.; Kushwaha, J.P. Parametric study of electro-Fenton treatment for real textile wastewater, disposal study and its cost analysis. Int. J. Environ. Sci. Technol. 2019, 16, 801–810. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Bayar, S.; Yilmaz, A.E.; Boncukcuoǧlu, R.; Baybars, A.F.; Muhtar, K.M. Effects of operational parameters on cadmium removal from aqueous solutions by electrochemical coagulation. Desalin. Water Treat. 2013, 51, 2635–2643. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsieh, Y.H.; Cheng, K.Y.; Hsieh, L.L.; Cheng, T.C.; Yao, K.S. Effect of pH on Fenton process using estimation of hydroxyl radical with salicylic acid as trapping reagent. Water Sci. Technol. 2008, 58, 873–879. [Google Scholar] [CrossRef]
- Burbano, A.A.; Dionysiou, D.D.; Suidan, M.T.; Richardson, T.L. Oxidation kinetics and effect of pH on the degradation of MTBE with Fenton reagent. Water Res. 2005, 39, 107–118. [Google Scholar] [CrossRef]
- Safarzadeh-Amiri, A.; Bolton, J.R.; Cater, S.R. The Use of Iron in Advanced Oxidation Processes. J. Adv. Oxid. Technol. 2017, 1, 18–26. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Diagrams for Iron At 25–300 °C. Corros. Sci. 1996, 38, 2121–3215. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. The mechanism of the fricke dosimeter. Int. J. Radiat. Phys. Chem. 1975, 7, 363–370. [Google Scholar] [CrossRef]
- Ahmad, Z. Corrosion Kinetics. In Principles of Corrosion Engineering and Corrosion Control, 2nd ed.; Elsevier: Amsetrdam, The Netherlands, 2019; Volume 1, pp. 57–119. [Google Scholar]
- Machulek, A., Jr.; Moraes, J.E.F.; Okano, L.T.; Silvério, C.A.; Quina, F.H. Photolysis of ferric ions in the presence of sulfate or chloride ions: Implications for the photo-Fenton process. Photochem. Photobiol. Sci. 2009, 8, 985–991. [Google Scholar] [CrossRef] [PubMed]
- De Laat, J.; Le, T.G. Effects of chloride ions on the iron (III)-catalyzed decomposition of hydrogen peroxide and on the efficiency of the Fenton-like oxidation process. Appl. Catal. B Environ. 2006, 66, 137–146. [Google Scholar] [CrossRef]
- Bray, W.C. The mechanism of reactions in aqueous solution Examples Involving Equilibria and Steady States. Chem. Rev. 1931, 10, 161–177. [Google Scholar] [CrossRef]
- De Laat, J.; Le, T.G. Kinetics and modeling of the Fe(III)/H2O2 system in the presence of sulfate in acidic aqueous solutions. Environ. Sci. Technol. 2005, 39, 1811–1818. [Google Scholar] [CrossRef]






| Reactions | |||
|---|---|---|---|
| I | Anodic reaction | ||
| II | Cathodic reaction | ||
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| 8 | |||
| 9 | |||
| 10 | |||
| 11 | |||
| 12 | |||
| 13 | |||
| 14 | |||
| 15 | |||
| 16 | |||
| 17 | |||
| 18 | |||
| 19 | |||
| 20 | |||
| 21 | |||
| 22 | |||
| 23 | |||
| 24 | |||
| 25 | |||
| 26 | |||
| 27 | |||
| 28 | |||
| 29 | |||
| 30 | |||
| 31 | |||
| 32 | |||
| 33 | |||
| 34 | |||
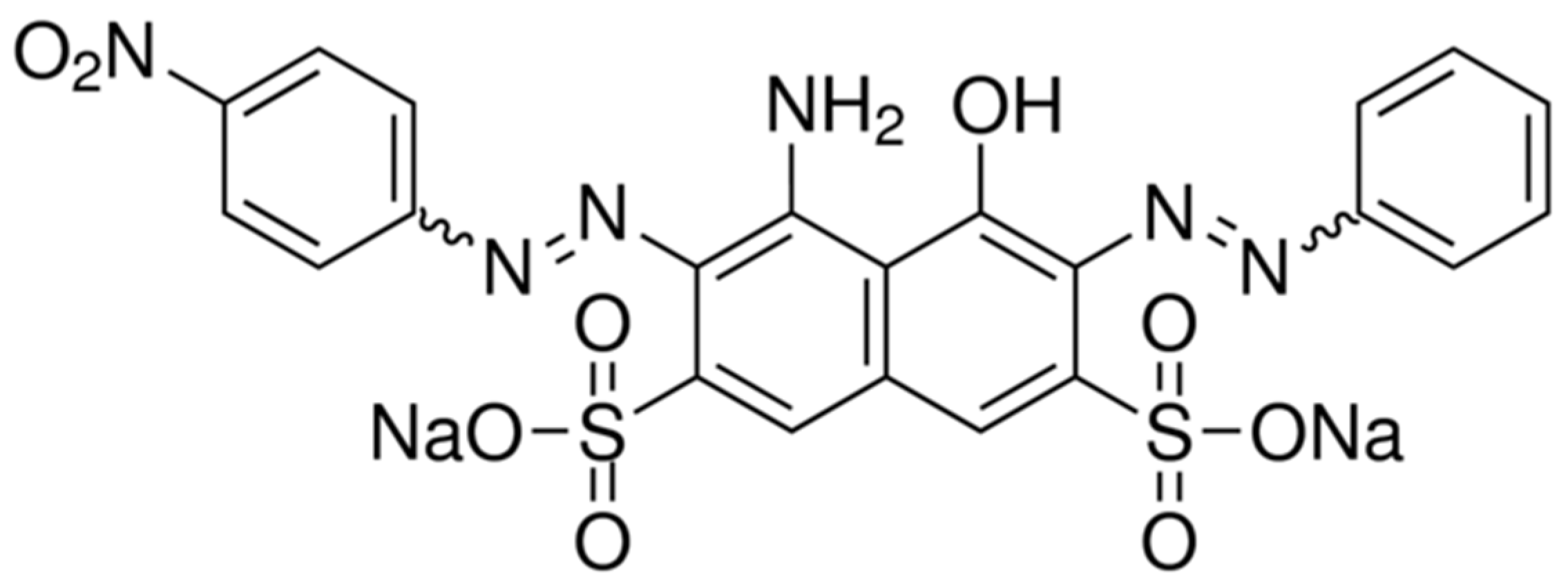
| NBB+ HO• → aniline + 4-nitroaniline + multi-substituted naphthalene ring | Initiation |
| Aniline → phenol | Propagation |
| Aniline → p-hydroquinone | |
| Phenol → p-hydroquinone | |
| p-hydroquinone → p-benzoquinone | |
| Phenol → catechol | |
| Phenol → resorcinol | |
| Catechol → 1, 2, 3 benzenetriol | |
| Catechol → 1, 2, 4 benzenetriol | |
| Resorcinol→ 1, 2, 3 benzenetriol | |
| Resorcinol→ 1, 2, 4 benzenetriol | |
| p-hydroquinone → maleic acid, butanedioic acid, butenedioic acid, glycolic acid, malic acid, glyceric acid and 3-hydroxypropanoic acid. | |
| p-benzoquinone → maleic acid, butanedioic acid, butenedioic acid, glycolic acid, malic acid, glyceric acid and 3-hydroxypropanoic acid. | |
| 1, 2, 3 benzenetriol → maleic acid, butanedioic acid, butenedioic acid, glycolic acid, malic acid, glyceric acid and 3-hydroxypropanoic acid. | |
| 1, 2, 4 benzenetriol → maleic acid, butanedioic acid, butenedioic acid, glycolic acid, malic acid, glyceric acid and 3-hydroxypropanoic acid. | |
| maleic acid, butanedioic acid, butenedioic acid, glycolic acid, malic acid, glyceric acid and 3-hydroxypropanoic acid → oxalic acid, oxamic acid | |
| oxalic acid, oxamic acid → carbon dioxide, water, ammonium, nitrate, sulfate | Termination |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferkous, H.; Kerboua, K.; Hamdaoui, O.; Haddour, N.; Alghyamah, A. Galvano-Fenton Engineering Solution with Spontaneous Catalyst’s Generation from Waste: Experimental Efficiency, Parametric Analysis and Modeling Interpretation Applied to a Clean Technology for Dyes Degradation in Water. Molecules 2021, 26, 5640. https://doi.org/10.3390/molecules26185640
Ferkous H, Kerboua K, Hamdaoui O, Haddour N, Alghyamah A. Galvano-Fenton Engineering Solution with Spontaneous Catalyst’s Generation from Waste: Experimental Efficiency, Parametric Analysis and Modeling Interpretation Applied to a Clean Technology for Dyes Degradation in Water. Molecules. 2021; 26(18):5640. https://doi.org/10.3390/molecules26185640
Chicago/Turabian StyleFerkous, Hamza, Kaouther Kerboua, Oualid Hamdaoui, Naoufel Haddour, and Abdulaziz Alghyamah. 2021. "Galvano-Fenton Engineering Solution with Spontaneous Catalyst’s Generation from Waste: Experimental Efficiency, Parametric Analysis and Modeling Interpretation Applied to a Clean Technology for Dyes Degradation in Water" Molecules 26, no. 18: 5640. https://doi.org/10.3390/molecules26185640
APA StyleFerkous, H., Kerboua, K., Hamdaoui, O., Haddour, N., & Alghyamah, A. (2021). Galvano-Fenton Engineering Solution with Spontaneous Catalyst’s Generation from Waste: Experimental Efficiency, Parametric Analysis and Modeling Interpretation Applied to a Clean Technology for Dyes Degradation in Water. Molecules, 26(18), 5640. https://doi.org/10.3390/molecules26185640








