Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Experimental Mice
2.3. Cell Isolation and Culture
2.4. Transfection with siRNA
2.5. Total RNA Extraction and the RT-qPCR Assay
2.6. Protein Extraction and Western Blot Assay
2.7. Cell Proliferation Assay
2.8. Apoptosis Assay
2.9. Cell Cycle Assay
2.10. Statistical Analysis
3. Results
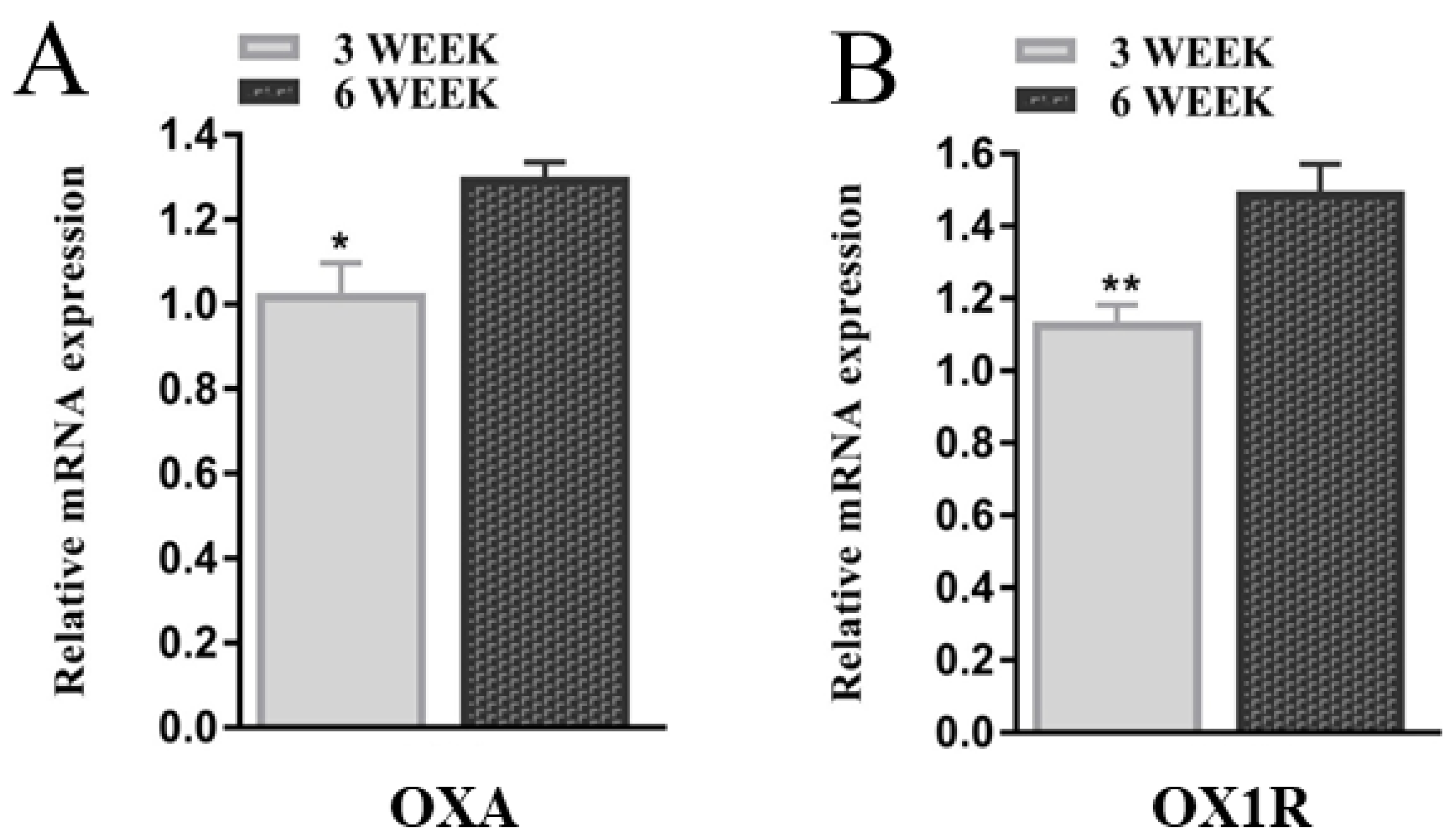
3.1. Orexin-A via OX1R Regulates Mouse GCs during Different Developmental Stages
3.2. The Expression of OX1R Was Efficiently Knocked down by Short Interfering RNA (siRNA) in Mouse GCs
3.3. Downregulation of OX1R Promoted Apoptosis in Mouse GCs
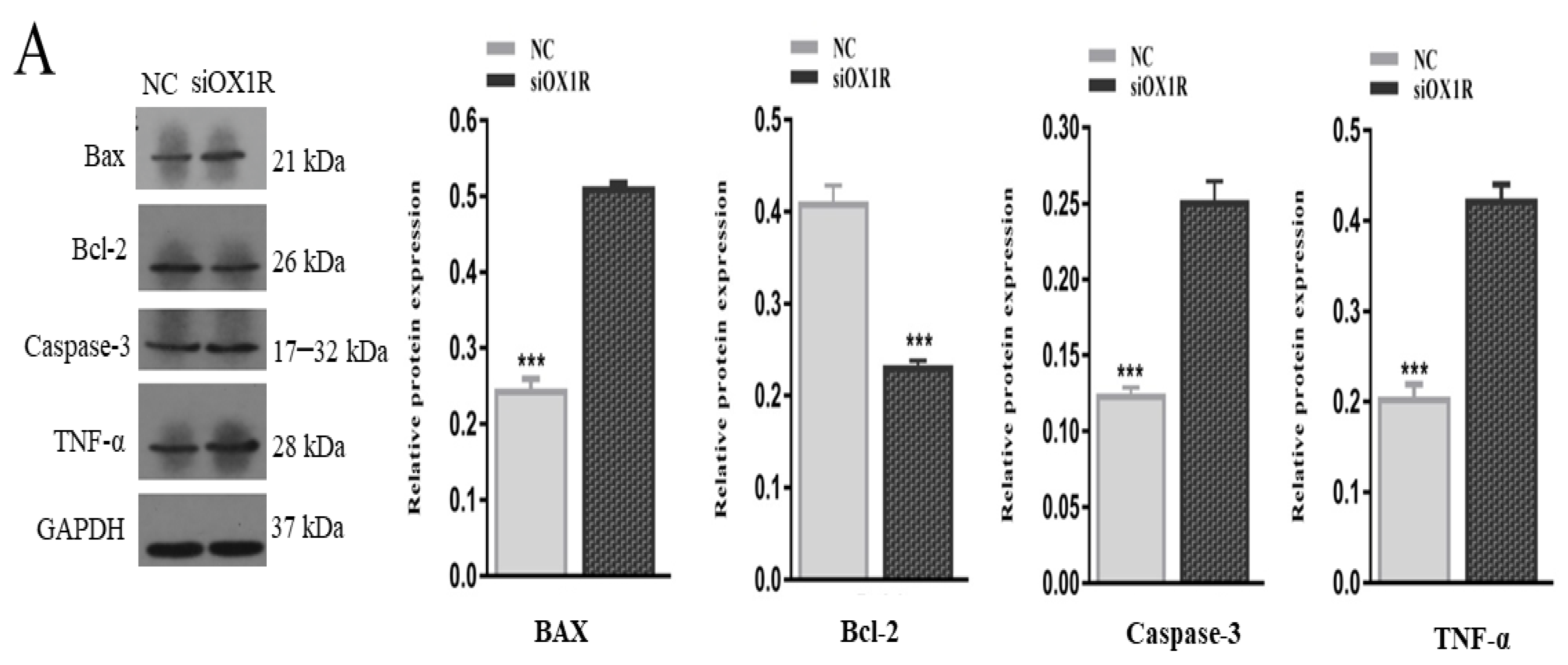
3.4. Knockdown of OX1R Altered the Expression of Apoptosis-Related Genes in Mouse GCs
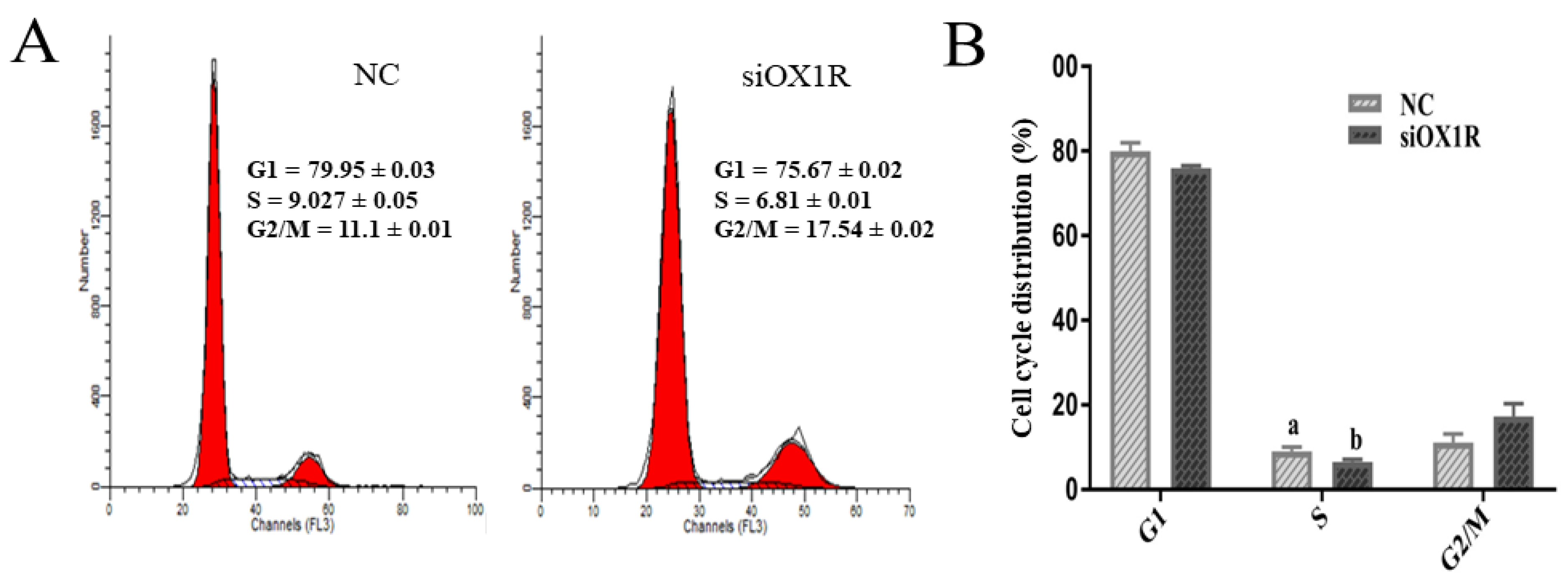
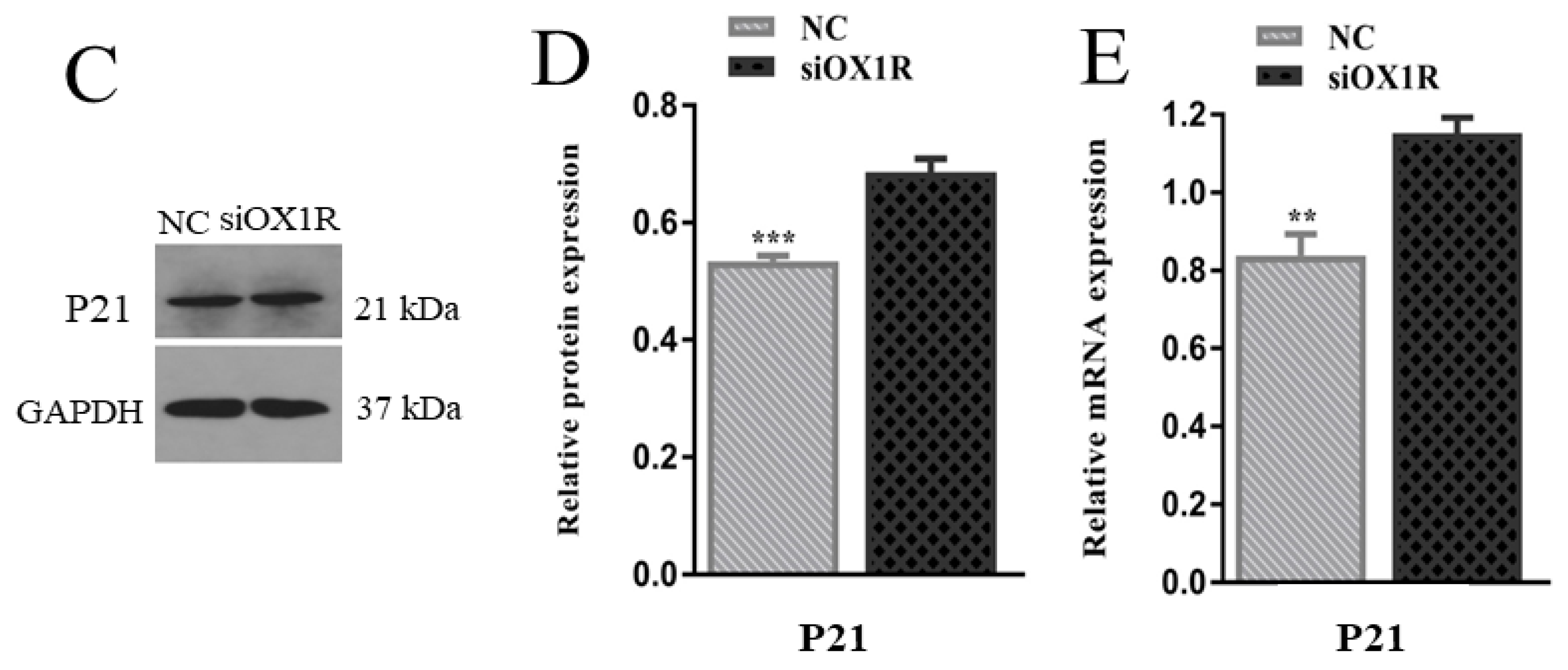
3.5. Downregulation of OX1R Induced S-Phase Arrest of the Cell Cycle in Mouse GCs
3.6. Knockdown of OX1R Inhibited Proliferation of Mouse GCs In Vitro
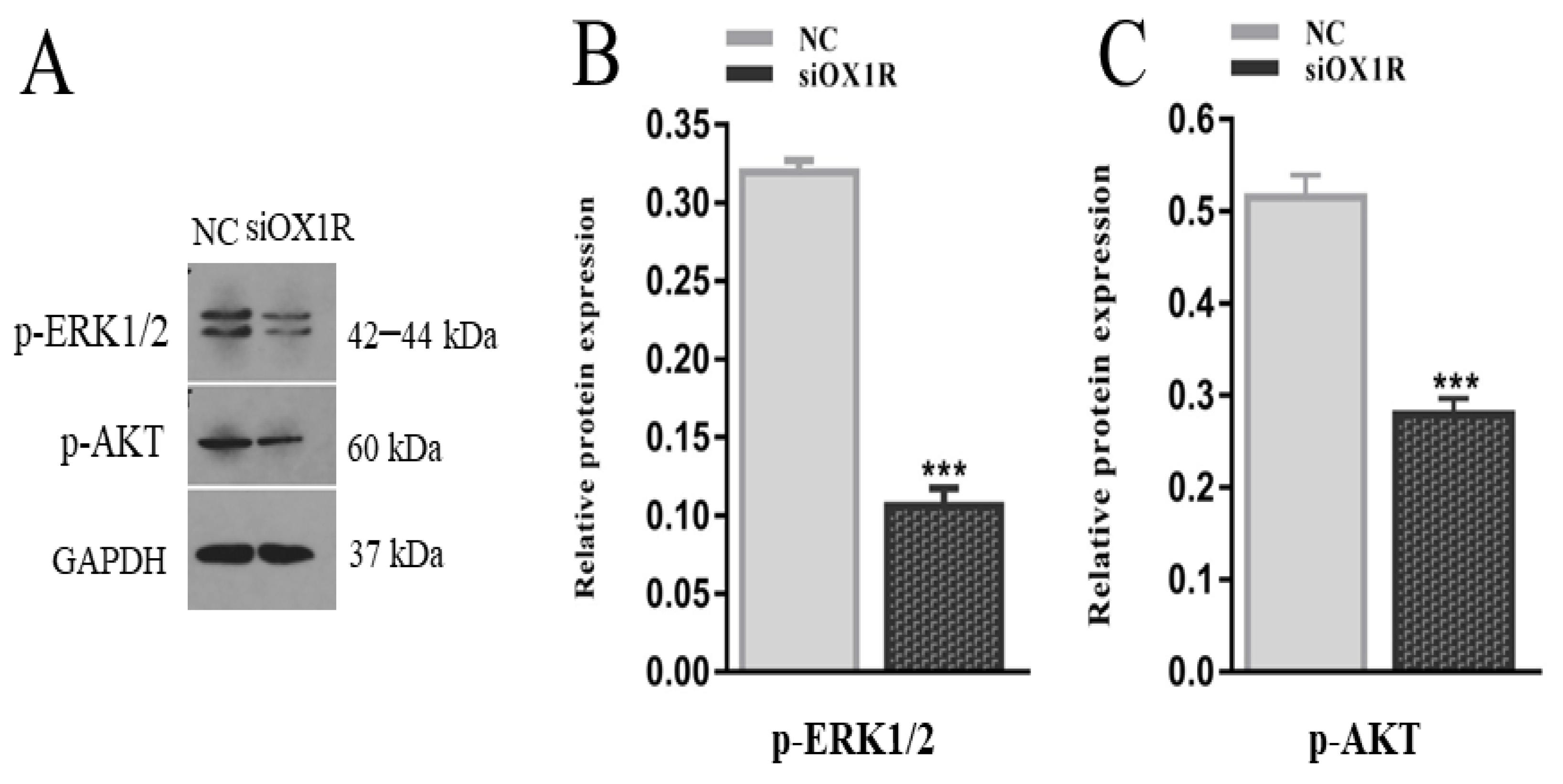
3.7. Knockdown of OX1R Regulates Apoptosis and Proliferation through the AKT/ERK1/2 Signaling Pathway
3.8. Knockdown of OXA Altered the mRNA Expression of Oocyte-Related Factors in Mouse GCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reis, F.M.; Cobellis, L.; Luisi, S.; Driul, L.; Florio, P.; Faletti, A.; Petraglia, F. Paracrine/autocrine control of female reproduction. Gynecol. Endocrinol. 2000, 14, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Salustri, A.; Camaioni, A.; D’Alessandris, C. Endocrine and paracrine regulation of cumulus expansion. Zygote 1996, 4, 313–315. [Google Scholar] [CrossRef]
- Emori, C.; Sugiura, K. Role of oocyte-derived paracrine factors in follicular development. Anim. Sci. J. 2014, 85, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Pendola, F.L.; Eppig, J.J. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: Energy metabolism. Dev. Biol. 2005, 279, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Liu, W.; Xiao, Y.; Zhang, H.; Yang, L. The effects of melatonin on bovine uniparental embryos development in vitro and the hormone secretion of COCs. PeerJ 2017, 5, e3485. [Google Scholar] [CrossRef]
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.N.; Ali, I.; Singh, A.K.; Shrivastav, T.G.; Chaube, S.K. Apoptosis in mammalian oocytes: A review. Apoptosis 2015, 20, 1019–1025. [Google Scholar] [CrossRef]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. Induction of apoptotic cell death via accumulation of autophagosomes in rat granulosa cells. Fertil. Steril. 2011, 95, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.-B.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.F.; Gautvik, V.T.; Bartlett, F.S., III; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Liguori, G.; Tafuri, S.; Miyoshi, C.; Yanagisawa, M.; Squillacioti, C.; De Pasquale, V.; Mirabella, N.; Vittoria, A.; Costagliola, A. Localization of orexin B and orexin-2 receptor in the rat epididymis. Acta Histochem. 2018, 120, 292–297. [Google Scholar] [CrossRef]
- Sakurai, T. The role of orexin in motivated behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef]
- Xu, T.R.; Yang, Y.; Ward, R.; Gao, L.; Liu, Y. Orexin receptors: Multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 2013, 25, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.V.; Purhonen, A.K.; Mäkelä, K.A.; Herzig, K.H. Functions of orexins in peripheral tissues. Acta Physiol. 2008, 192, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Silveyra, P.; Cataldi, N.I.; Lux-Lantos, V.A.; Libertun, C. Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta Physiol. 2010, 198, 355–360. [Google Scholar] [CrossRef]
- Silveyra, P.; Catalano, P.N.; Lux-Lantos, V.; Libertun, C. Impact of proestrous milieu on expression of orexin receptors and preproorexin in rat hypothalamus and hypophysis: Actions of Cetrorelix and Nembutal. Am. J. Physiol. Metab. 2007, 292, E820–E828. [Google Scholar] [CrossRef]
- Cataldi, N.I.; Lux-Lantos, V.A.R.; Libertun, C. Effects of orexins A and B on expression of orexin receptors and progesterone release in luteal and granulosa ovarian cells. Regul. Pept. 2012, 178, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kiezun, M.; Smolinska, N.; Dobrzyn, K.; Szeszko, K.; Rytelewska, E.; Kaminski, T. The effect of orexin A on CYP17A1 and CYP19A3 expression and on oestradiol, oestrone and testosterone secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 2016, 90, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Kiezun, M.; Dobrzyn, K.; Bors, K.; Wyrebek, J.; Kaminska, B.; Kaminski, T.; Smolinska, N. The effect of orexin a on the StAR, CYP11A1 and HSD3B1 gene expression, as well as progesterone and androstenedione secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology 2019, 143, 179–190. [Google Scholar] [CrossRef]
- Gao, Y.; Wen, H.; Wang, C.; Li, Q. SMAD7 antagonizes key TGFβ superfamily signaling in mouse granulosa cells in vitro. Reproduction 2013, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Butterick, T.A.; Nixon, J.P.; Billington, C.J.; Kotz, C.M. Orexin A decreases lipid peroxidation and apoptosis in a novel hypothalamic cell model. Neurosci. Lett. 2012, 524, 30–34. [Google Scholar] [CrossRef]
- Hoyer, D.; Jacobson, L.H. Orexin in sleep, addiction and more: Is the perfect insomnia drug at hand? Neuropeptides 2013, 47, 477–488. [Google Scholar] [CrossRef]
- Inutsuka, A.; Yamanaka, A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front. Endocrinol. 2013, 4, 18. [Google Scholar] [CrossRef]
- Kodadek, T.; Cai, D. Chemistry and biology of orexin signaling. Mol. Biosyst. 2010, 6, 1366–1375. [Google Scholar] [CrossRef]
- Iijima, K.; Jiang, J.Y.; Shimizu, T.; Sasada, H.; Sato, E. Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J. Reprod. Dev. 2005, 51, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.M.; Cowan, R.G.; Harman, R.M.; Hu, C.L.; Porter, D.A. Ovarian follicular growth and atresia: The relationship between cell proliferation and survival. J. Anim. Sci. 2004, 82, E40–E52. [Google Scholar] [CrossRef] [PubMed]
- Sargent, K.M.; Lu, N.; Clopton, D.T.; Pohlmeier, W.E.; Brauer, V.M.; Ferrara, N.; Silversides, D.W.; Cupp, A.S. Loss of vascular endothelial growth factor a (VEGFA) isoforms in granulosa cells using pDmrt-1-cre or Amhr2-Cre reduces fertility by arresting follicular development and by reducing litter size in female mice. PLoS ONE 2015, 10, e0116332. [Google Scholar] [CrossRef] [PubMed]
- Antti, K.; Aaron, J.W.H. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997, 59, 349–363. [Google Scholar]
- Jiang, J.; Cheung, C.K.M.; Wang, Y.; Tsang, B.K. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front. Biosci. 2003, 1, 222–237. [Google Scholar]
- Arosh, J.A.; Banu, S.K.; Chapdelaine, P.; Madore, E.; Sirois, J.; Fortier, A.M. Prostaglandin biosynthesis, transport, and signaling in corpus luteum: A basis for autoregulation of luteal function. Endocrinology 2004, 145, 2551–2560. [Google Scholar] [CrossRef]
- Ju, S.J.; Zhao, Y.; Chang, X.; Guo, L. Orexin a protects cells from apoptosis by regulating FoxO1 and mTORC1 through the OX1R/PI3K/AKT signaling pathway in hepatocytes. Int. J. Mol. Med. 2014, 34, 153–159. [Google Scholar] [CrossRef]
- Espino, J.; Bejarano, I.; Ortiz, A.; Lozano, G.M.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. Melatonin as a potential tool against oxidative damage and apoptosis in ejaculated human spermatozoa. Fertil. Steril. 2010, 94, 1915–1917. [Google Scholar] [CrossRef]
- Kim, M.-R.; Tilly, J.L. Current concepts in Bcl-2 family member regulation of female germ cell development and survival. Biochim. Biophys. Acta (BBA)—Bioenerg. 2004, 1644, 205–210. [Google Scholar] [CrossRef][Green Version]
- Radogna, F.; Albertini, M.C.; de Nicola, M.; Diederich, M.; Bejarano, I.; Ghibelli, L. Melatonin promotes Bax sequestration to mitochondria reducing cell susceptibility to apoptosis via the lipoxygenase metabolite 5-hydroxyeicosatetraenoic acid. Mitochondrion 2015, 21, 113–121. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Cai, K.; Hua, G.; Ahmad, S.; Liang, A.; Han, L.; Wu, C.; Yang, F.; Yang, L. Action Mechanism of Inhibin α-Subunit on the Development of Sertoli Cells and First Wave of Spermatogenesis in Mice. PLoS ONE 2011, 6, e25585. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wu, C.; Riaz, H.; Bai, L.; Chen, J.; Zhen, Y.; Guo, A.; Yang, L. Characterization of the Mechanism of Inhibin α-Subunit Gene in Mouse Anterior Pituitary Cells by RNA Interference. PLoS ONE 2013, 8, e74596. [Google Scholar] [CrossRef]
- Hussein, M.R. Apoptosis in the ovary: Molecular mechanisms. Hum. Reprod. Update 2005, 11, 162–178. [Google Scholar] [CrossRef]
- Wu, J.; Emery, B.R.; Carrell, T.D. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol. Reprod. 2001, 64, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Iatropoulos, M.J.; Williams, G.M. Proliferation markers. Exp. Toxicol. Pathol. 1996, 48, 175–181. [Google Scholar] [CrossRef]
- Suo, L.; Chang, X.; Zhao, Y. The orexin-A-regulated Akt/mTOR pathway promotes cell proliferation through inhibiting apoptosis in pancreatic cancer cells. Front. Endocrinol. (Lausanne) 2018, 9, 647. [Google Scholar] [CrossRef]
- Skotheim, J.M.; di Talia, S.; Siggia, E.D.; Cross, F.R. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature 2008, 454, 291–296. [Google Scholar] [CrossRef]
- Chang, F.; Steelman, L.S.; Lee, J.T.; Shelton, J.G.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.A.; McCubrey, J. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: Potential targeting for therapeutic intervention. Leukemia 2003, 17, 1263–1293. [Google Scholar] [CrossRef]
- Lefloch, R.; Pouysségur, J.; Lenormand, P. Single and Combined Silencing of ERK1 and ERK2 Reveals Their Positive Contribution to Growth Signaling Depending on Their Expression Levels. Mol. Cell. Biol. 2008, 28, 511–527. [Google Scholar] [CrossRef]
- Ryan, K.E.; Casey, S.M.; Canthy, M.J.; Crowe, M.A.; Martin, F.; Evans, A.C.O. Akt and Erk signal transduction pathways are early markers of differentiation in dominant and subordinate ovarian follicles in cattle. Reproduction 2007, 133, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Skrzypski, M.; Kaczmarek, P.; Le, T.; Wojciechowicz, T.; Pruszynska-Oszmalek, E.; Szczepankiewicz, D.; Sassek, M.; Arafat, A.; Wiedenmann, B.; Nowak, K.W.; et al. Effects of orexin A on proliferation, survival, apoptosis and differentiation of 3T3-L1 preadipocytes into mature adipocytes. FEBS Lett. 2012, 586, 4157–4164. [Google Scholar] [CrossRef] [PubMed]
- Paulini, F.; Melo, E.O. The Role of Oocyte-Secreted Factors GDF9 and BMP15 in Follicular Development and Oogenesis. Reprod. Domest. Anim. 2010, 46, 354–361. [Google Scholar] [CrossRef]
- Diaz, F.J.; Wigglesworth, K.; Eppig, J.J. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J. Cell Sci. 2007, 120, 1330–1340. [Google Scholar] [CrossRef]
- Yoshino, O.; McMahon, H.E.; Sharma, S.; Shimasaki, S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc. Natl. Acad. Sci. USA 2006, 103, 10678–10683. [Google Scholar] [CrossRef] [PubMed]






| siRNAs | Sense | Antisense |
|---|---|---|
| NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| siOX1R-A | GGAGGAAGACGGCUAAGAUTT | AUCUUAGCCGUCUUCCUCCTT |
| siOX1R-B | GGCUUUGUGCAAGGUCAUUTT | AAUGACCUUGCACAAAGCCTT |
| siOX1R-C | GCCACCCACUGUUGUUCAATT | UUGAACAACAGUGGGUGGCTT |
| Gene Symbol | Primer Sequences | Temp. (℃) | Product (bp) |
|---|---|---|---|
| OXA-F | GCGCAGAGCTAGAGCCACAT | 59 | 192 |
| OXA-R | TGCTAAAGCGGTGGTAGTTACG | ||
| OX1R-F | GCTAGTGTACGCCAACAGTG | 53 | 216 |
| OX1R-R | GACAGCACGGTAGTGACGG | ||
| P21-F | AACTGACTGCTCCCCTGTCTA | 57 | 108 |
| P21-R | CTCTATGGTTACCGCCTCCTC | ||
| PCNA-F | GTGGATAAAGAAGAGGAGGCG | 58 | 111 |
| PCNA-R | TGTAGGAGACAGTGGAGTGGC | ||
| Bax-F | GCCTCCTCTCCTACTTCGG | 55 | 187 |
| Bax-R | AAAAATGCCTTTCCCCTTC | ||
| Bcl-2-F | TCTCTCGTCGCTACCGTCG | 58 | 123 |
| Bcl-2-R | CCCAGTTCACCCCATCCCT | ||
| GDF9-F | CGGCTCCATCGCTTACAAA | 54 | 187 |
| GDF9-R | CTTCCCCCGCTCACACAGT | ||
| BMP15-F | GAAAATGGTGAGGCTGGTAA | 59 | 152 |
| BMP15-R | GATGAAGTTGATGGCGGTAA | ||
| β-Actin-F | CACGATGGAGGGGCCGGACTCATC | 55 | 241 |
| β-Actin-R | TAAAGACCTCTATGCCAACACAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safdar, M.; Liang, A.; Rajput, S.A.; Abbas, N.; Zubair, M.; Shaukat, A.; Rehman, A.u.; Jamil, H.; Guo, Y.; Ullah, F.; et al. Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway. Molecules 2021, 26, 5635. https://doi.org/10.3390/molecules26185635
Safdar M, Liang A, Rajput SA, Abbas N, Zubair M, Shaukat A, Rehman Au, Jamil H, Guo Y, Ullah F, et al. Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway. Molecules. 2021; 26(18):5635. https://doi.org/10.3390/molecules26185635
Chicago/Turabian StyleSafdar, Muhammad, Aixin Liang, Shahid Ali Rajput, Nasir Abbas, Muhammad Zubair, Aftab Shaukat, Aziz ur Rehman, Huma Jamil, Yan Guo, Farman Ullah, and et al. 2021. "Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway" Molecules 26, no. 18: 5635. https://doi.org/10.3390/molecules26185635
APA StyleSafdar, M., Liang, A., Rajput, S. A., Abbas, N., Zubair, M., Shaukat, A., Rehman, A. u., Jamil, H., Guo, Y., Ullah, F., & Yang, L. (2021). Orexin-A Regulates Follicular Growth, Proliferation, Cell Cycle and Apoptosis in Mouse Primary Granulosa Cells via the AKT/ERK Signaling Pathway. Molecules, 26(18), 5635. https://doi.org/10.3390/molecules26185635







