Screening and Characterization of Two Extracellular Polysaccharide-Producing Bacteria from the Biocrust of the Mu Us Desert
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Screening of Two Strains and the Characteristics of Strain YL24-3
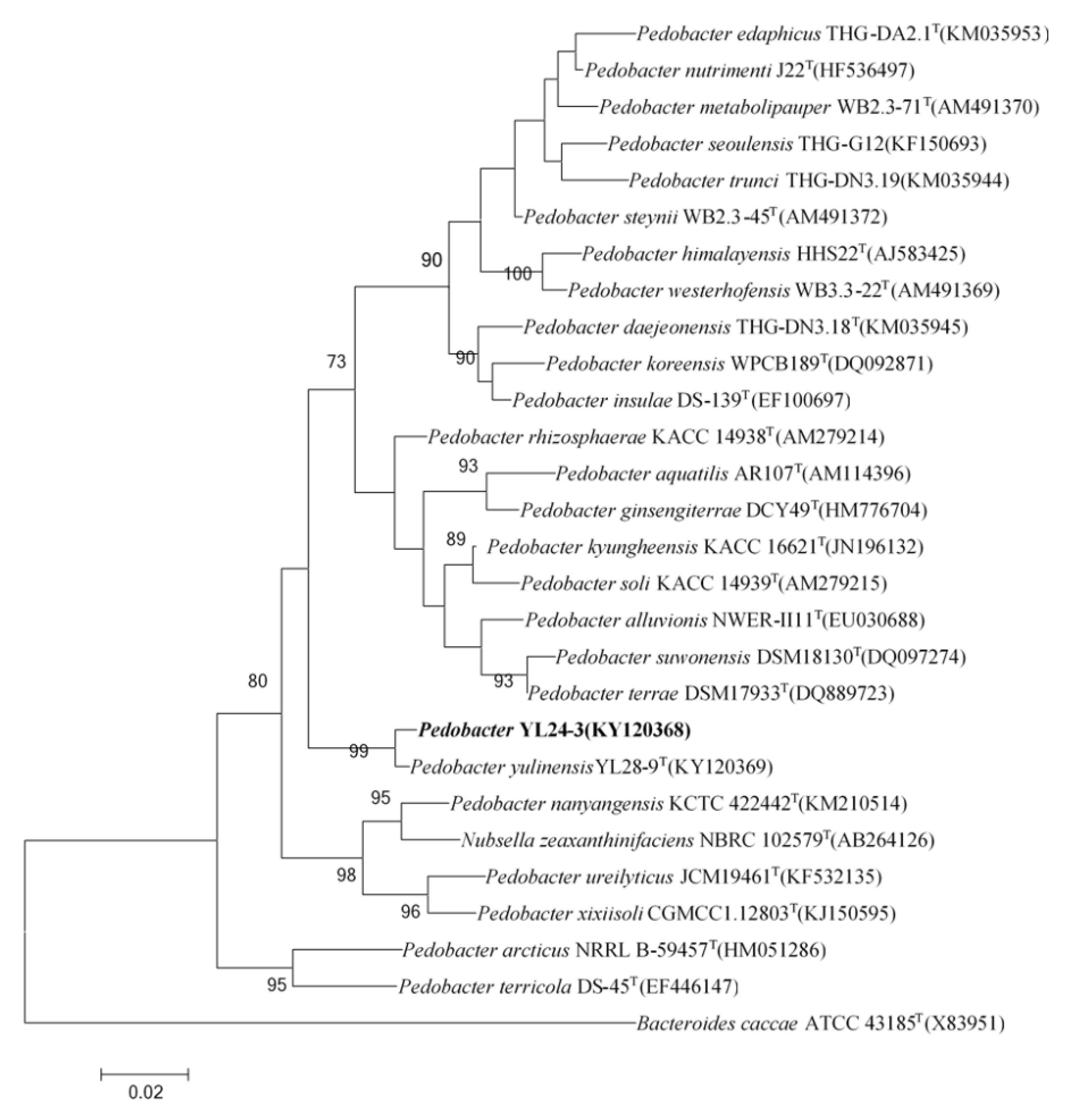
2.1.1. The Phylogenetic Relationship of the Pedobacter Strain YL24-3
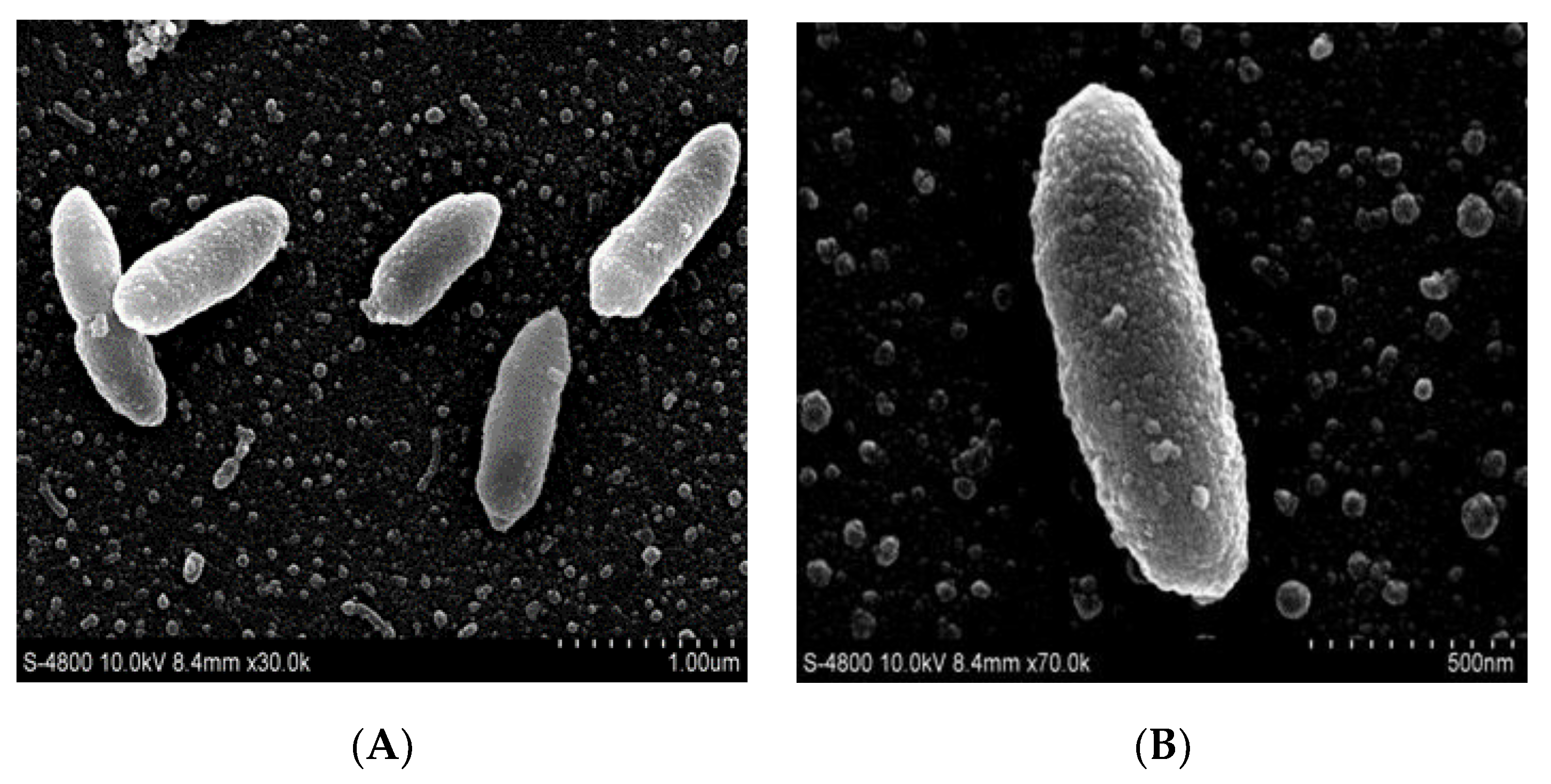
2.1.2. Morphological and Physiological Characteristics
2.1.3. The Physiological and Biochemical Characteristics
2.1.4. The Results of Polar Lipid and Respiratory Quinone Analyses
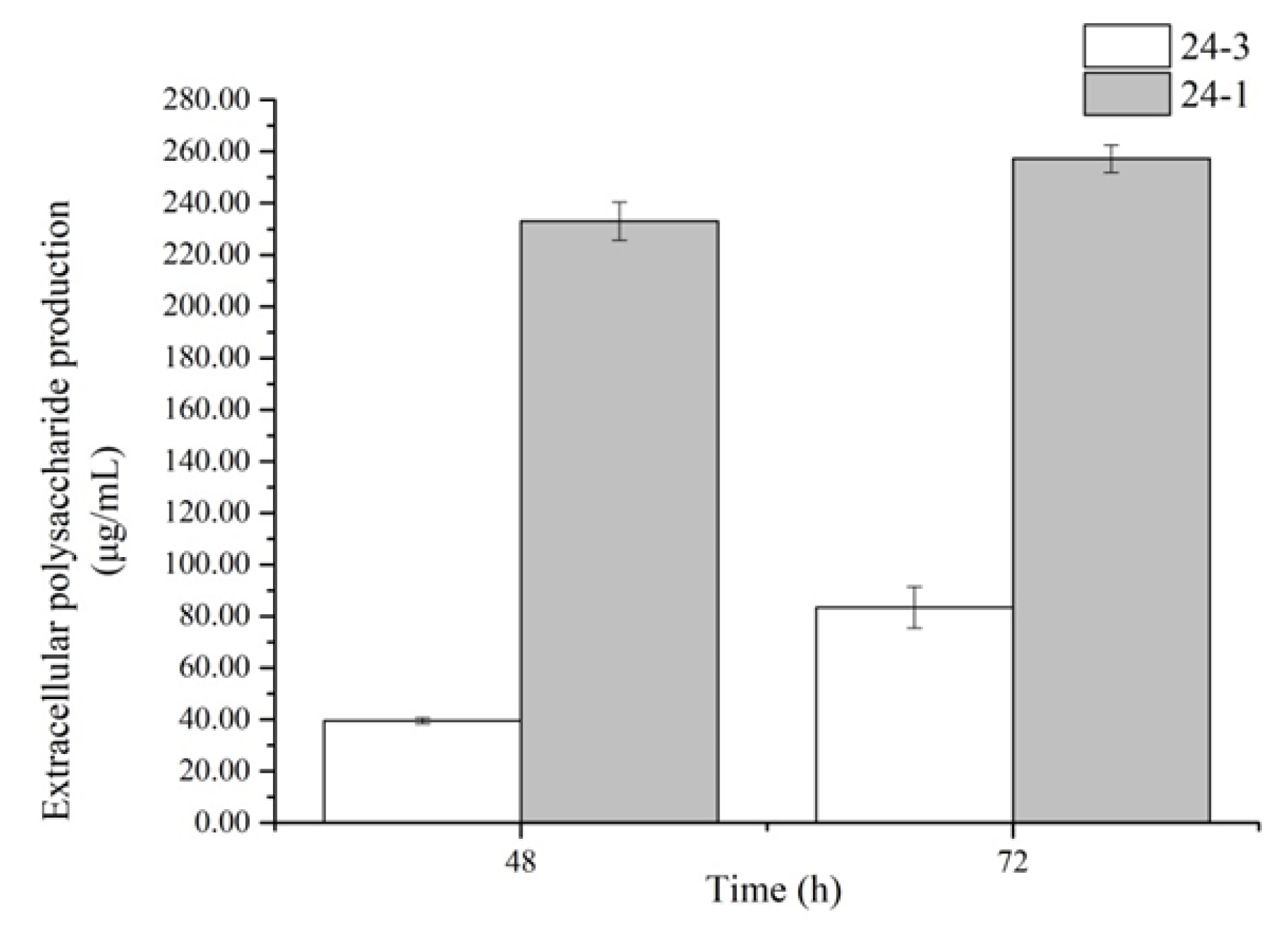
2.2. The Contents of Extracellular Polysaccharides Produced by the Strains
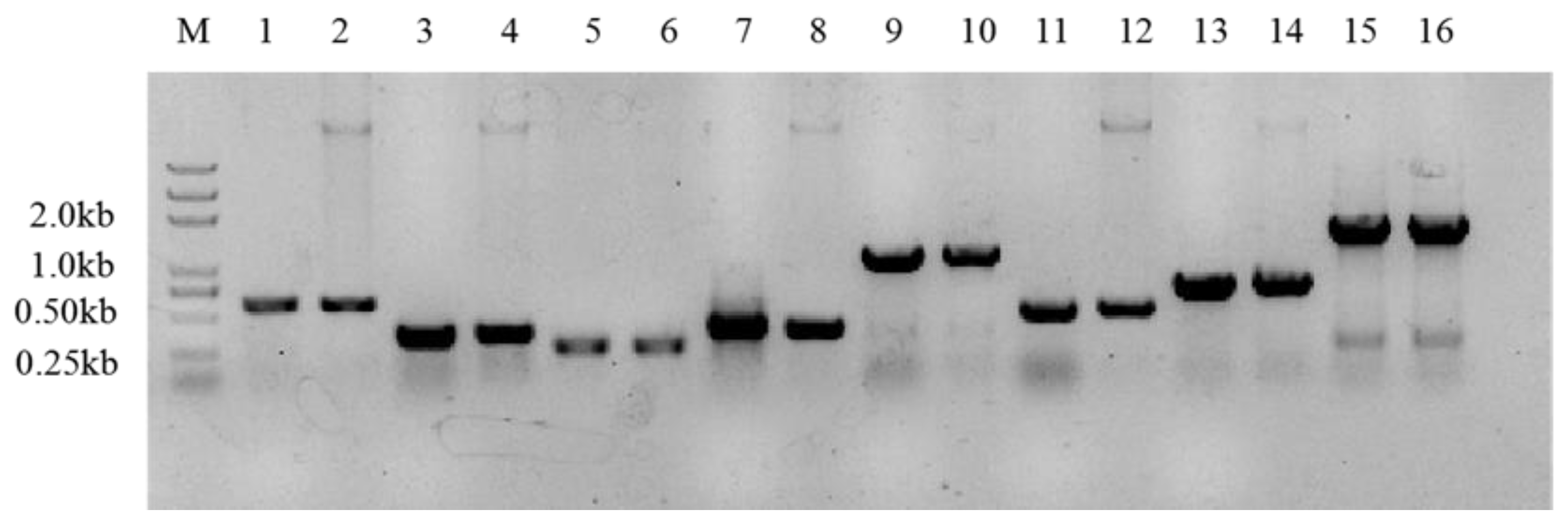
2.3. Exopolysaccharide-Related Genes of the Bacteria
3. Materials and Methods
3.1. Bacterial Strain Screening
3.2. The Characteristics of Pedobacter Strain YL24-3
3.2.1. Determination of 16S rRNA Gene Sequencing and Phylogenetic Analysis
3.2.2. Determination of G + C Content
3.2.3. Morphological and Physiological Characteristics
3.2.4. Analysis of Physiological and Biochemical Characteristics
3.2.5. Polar Lipid and Respiratory Quinone Analysis
3.3. Determination of the Contents of Extracellular Polysaccharides Produced by Bacteria YL24-1 and YL24-3
3.4. Amplification of the Extracellular Polysaccharide-Producing Genes of the Strains
3.5. The Structural Genes of Extracellular Polysaccharide-Producing Bacteria YL24-1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chiquoine, L.P.; Abella, S.R.; Bowker, M.A. Rapidly restoring biological soil crusts and ecosystem functions in a severely disturbed desert ecosystem. Ecol. Appl. 2016, 26, 1260–1272. [Google Scholar] [CrossRef] [Green Version]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol. Rev. 2013, 37, 36–54. [Google Scholar] [CrossRef] [Green Version]
- Kidron, G.J.; Xiao, B.; Benenson, I. Data variability or paradigm shift? Slow versus fast recovery of biological soil crusts-a review. Sci. Total Environ. 2020, 721, 137683. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Hu, J. The Antioxidant Activities of Natural Polysaccharides. Curr. Drug Targets. 2017, 18, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Castillo-Monroy, A.P.; Bowker, M.A.; Maestre, F.T.; Rodríguez-Echeverría, S.; Martinez, I.; Barraza-Zepeda, C.E.; Escolar, C. Relationships between biological soil crusts, bacterial diversity and abundance, and ecosystem functioning: Insights from a semi-arid Mediterranean environment. J. Veg. Sci. 2011, 22, 165–174. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Upadhyay, L.S.B. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int. J. Biol. Macromol. 2020, 157, 577–583. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfield, C. Bacterial extracellular polysaccharides. Can. J. Microbiol. 1988, 34, 415–420. [Google Scholar] [CrossRef]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of Bacterial Capsular Polysaccharides and Exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [CrossRef]
- Whiteley, C.G.; Lee, D.J. Bacterial diguanylate cyclases: Structure, function and mechanism in exopolysaccharide biofilm development. Biotechnol. Adv. 2015, 33, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.A.; Coplin, D.L. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 1992, 46, 307–346. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M. Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int. J. Mol. Sci. 2011, 12, 7898–7933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, H.-J.; Leigh, J.A. Two genes that regulate exopolysaccharide production in Rhizobium meliloti. J. Bacteriol. 1990, 172, 5254–5259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.-H.; Wang, B.-D.; Zhang, L.-M.; Zhang, J.-G.; Chen, S.-L. Pedobacter yulinensis sp. nov., isolated from sandy soil, and emended description of the genus Pedobacter. Int. J. Syst. Evol. Microbiol. 2018, 68, 2523–2529. [Google Scholar] [CrossRef]
- Steyn, P.L.; Segers, P.; Vancanneyt, M.; Sandra, P.; Kersters, K.; Joubert, J.J. Classification of heparinolytic bacteria into a new genus, Pedobacter, comprising four species: Pedobacter heparinus comb. nov., Pedobacter piscium comb. nov., Pedobacter africanus sp. nov. And Pedobacter saltans sp. nov. proposal of the family Sphingobacteriaceae fam. nov. Int. J. Syst. Bacteriol. 1998, 48, 165–177. [Google Scholar]
- Vanparys, B.; Heylen, K.; Lebbe, L.; De Vos, P. Pedobacter caeni sp. nov., a novel species isolated from a nitrifying inoculum. Int. J. Syst. Evol. Microbiol. 2005, 55, 1315–1318. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.Y.; Choi, D.H.; Cho, B.C. Pedobacter roseus sp. nov., isolated from a hypertrophic pond, and emended description of the genus Pedobacter. Int. J. Syst. Evol. Microbiol. 2006, 56, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Gallego, V.; García, M.T.; Ventosa, A. Pedobacter aquatilissp. nov., isolated from drinking water, and emended description of the genus Pedobacter. Int. J. Syst. Evol. Microbiol. 2006, 56, 1853–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.U.; Kim, Y.J.; Shin, D.H.; Weon, H.Y.; Kwon, S.W.; Seong, C.N.; Ka, J.O. Pedobacter namyangjuensis sp. nov. Isolated from Soil and Reclassification of Nubsella zeaxanthinifaciens Asker et al. 2008 as Pedobacter zeaxanthinifaciens comb. nov. J. Microbiol. 2013, 51, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Spröer, C.; Schumann, P.; Schinner, F. Pedobacter cryoconitis sp. nov., a facultative psychrophile from alpine glacier cryoconite. Int. J. Syst. Evol. Microbiol. 2003, 53, 1291–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaji, S.; Chaturvedi, P.; Reddy, G.S.N.; Suresh, K. Pedobacter himalayensis sp. nov., from the Hamta glacier located in the Himalayan mountain ranges of India. Int. J. Syst. Evol. Microbiol. 2005, 55, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-E.; Shin, J.Y.; Park, S.Y.; Mavlonov, G.T.; Yi, E.J.; Lee, E.H.; Lee, J.M.; Yi, T.H. Pedobacter kyungheensis sp. nov., with ginsenoside converting activity. J. Gen. Appl. Microbiol. 2012, 58, 309–316. [Google Scholar] [PubMed] [Green Version]
- Kwon, S.W.; Son, J.A.; Kim, S.J.; Kim, Y.S.; Park, I.C.; Bok, J.I.; Weon, H.Y. Pedobacter rhizosphaerae sp. nov. and Pedobacter soli sp. nov., isolated from rhizosphere soil of Chinese cabbage (Brassica campestris). Int. J. Syst. Evol. Microbiol. 2011, 61, 2874–2879. [Google Scholar] [CrossRef] [PubMed]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and reanalysis of domain-specific 16S primers. J. Microbiol. Meth. 2003, 55, 541–555. [Google Scholar] [CrossRef] [Green Version]
- Rainey, F.A.; Rainey, N.W.; Kroppenstedt, R.M.; Stackebrandt, E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: Proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 1996, 46, 1088–1092. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetic analysis version 7.0 for bigger data- sets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Mesbah, M.N.; Whitman, W.B.; Mesbah, M. Determination of the G+C Content of Prokaryotes. Method Microbiol. 2011, 38, 299–324. [Google Scholar]
- Zhang, L.; Wang, Y.; Dai, J.; Tang, Y.-L.; Yang, Q.; Luo, X.-S.; Fang, C.-X. Bacillus korlensis sp. nov., a moderately halotolerant bacterium isolated from a sand soil sample in China. Int. J. Syst. Evol. Microbiol. 2009, 59, 1787–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamlage, B. Methods for General and Molecular Bacteriology. Am. Soc. Microbiol. 1996, 40, 103. [Google Scholar]
- Hwang, K.S.; Kim, H.U.; Charusanti, P.; Palsson, B.Ø.; Lee, S.Y. Systems biology and biotechnology of Streptomyces, species for the production of secondary metabolites. Biotechnol. Adv. 2014, 32, 255–268. [Google Scholar] [CrossRef]
- Minnikin, D.E.; O’Donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Meth. 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, E.; Freisleben, H.J.; Krisnamurti, D.G.B.; Estuningtyas, A.; Mulyanto, C.; Ridwan, R. Fractionation and purification of membrane lipids from the archaeon Thermoplasma acidophilum DSM 1728/10217. Sep. Purif. Technol. 2013, 153, 119–126. [Google Scholar] [CrossRef]
- Collins, M.D.; Goodfellow, M.; Minnikin, D.E. Fatty acid, isoprenoid quinone and polar lipid composition in the classification of Curtobacterium and related taxa. J. Gen. Microbiol. 1980, 118, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, K.-Y.; Li, M.-H.; Chen, C.-Q.; Yang, X.; Li, Q.-Q.; Cheng, J.-L.; Zhang, L.; Shen, X.-H. Paenibacillus qinlingensis sp. nov., an indole-3-acetic acid-producing bacterium isolated from roots of Sinopodophyllum hexandrum (Royle) Ying. Int. J. Syst. Evol. Microbiol. 2017, 67, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Quesada, E.; Bejar, V.; Calvo, C. Exopolysaccharide production by Volcaniella eurihalina. Cell Mol. Life SCI 1993, 49, 1037–1041. [Google Scholar] [CrossRef]
- Quesada, E.; Moral, A.; Béjar, V. Comparative methods for isolation of Volcaniella eurihalina exopolysaccharide. Biotechnol. Lett. 1994, 8, 701–706. [Google Scholar] [CrossRef]
- Ding, R.; Wu, X.-C.; Qian, C.-D.; Teng, Y.; Li, O.; Zhan, Z.-J.; Zhao, Y.-H. Isolation and identification of lipopeptide antibiotics from Paenibacillus elgii B69 with inhibitory activity against methicillin-resistant Staphylococcus aureus. J. Microbiol. 2011, 49, 942–949. [Google Scholar] [CrossRef]
- Zhu, T.; Heo, H.-J.; Row, K.-H. Optimization of crude polysaccharides extraction from Hizikia fusiformis using response surface methodology. Carbohyd. Polym. 2010, 82, 106–110. [Google Scholar] [CrossRef]


| Characteristic | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Colony color | Pink | Orange | Pinkish-yellow | Yellow | Yellow |
| Motile | − | − | + | + | − |
| Catalase | + | + | − | + | + |
| Oxidase | + | − | − | + | + |
| Maximum growth temperature (°C) | 37 | 42 | 40 | 37 | 37 |
| pH range for growth | 6.0–9.0 | 6.0–9.0 | 5.0–10.0 | 6.0–9.0 | 5.0–9.0 |
| Growth in 3% (w/v) NaCl | − | + | + | − | W |
| Hydrolysis of | |||||
| DNA | W | + | − | + | + |
| Casein | − | − | − | + | − |
| Starch | − | W | + | W | − |
| Tween-20 | W | + | + | − | − |
| Tween-80 | − | + | + | − | − |
| Nitrate reduction | − | − | − | − | − |
| Arginine dihydrolase | − | + | + | − | − |
| Glucose acidification | − | + | − | − | − |
| Urease | − | + | + | − | − |
| Assimilation of | |||||
| d-Mannose | + | − | + | + | + |
| Malic acid | − | − | − | + | + |
| Enzyme activities | |||||
| Esterase (C4) | − | − | W | W | W |
| Esterase lipase (C8) | + | + | W | W | W |
| Cystine arylamidase | + | − | W | W | W |
| Trypsin | − | − | + | + | + |
| α-Chymotrypsin | − | − | W | + | − |
| α-Galactosidase | + | W | W | − | − |
| β-Galactosidase | − | + | + | − | − |
| α-Mannosidase | + | − | − | + | + |
| α-Fucosidase | − | + | − | − | − |
| Acid production from | |||||
| d-Arabinose | + | − | − | − | W |
| l-Arabinose | W | − | − | W | − |
| d-Galactose | + | W | W | − | + |
| d-Glucose | + | + | + | W | + |
| Fructose | W | − | + | − | W |
| Methyl α-d-glucoside | + | W | − | − | − |
| Salicin | W | W | W | + | + |
| Melibiose | W | W | − | − | W |
| Starch | + | W | + | W | − |
| Glycogen | − | − | + | W | − |
| l-Fucose | W | − | + | − | W |
| DNA G + C content (mol.%) | 47.8 | 50.4 | 38.4 | 38.6 | 36.1 |
| The Type and Concentration of Antibiotic | Tested Strain | ||
|---|---|---|---|
| YL24-3 | YL28-9T | ||
| Ampicillin (Amp) | 30 | + | + |
| 50 | + | + | |
| 100 | − | − | |
| Streptomycin (Str) | 30 | + | + |
| 50 | + | + | |
| 100 | − | + | |
| Chloramphenicol (Chl) | 20 | − | − |
| 30 | − | − | |
| 50 | − | − | |
| Gentamycin (Gen) | 30 | + | + |
| 50 | + | + | |
| 100 | − | − | |
| Erythromycin (Ery) | 150 | ++ | ++ |
| 200 | + | + | |
| 300 | − | − | |
| Spectinomycin (Spe) | 50 | + | + |
| 100 | + | + | |
| 150 | + | − | |
| Kanamycin (Kan) | 30 | − | + |
| 50 | − | + | |
| 100 | − | − | |
| Apramycin (Apr) | 30 | + | + |
| 50 | + | + | |
| 100 | + | − | |
| Penicillin (Pen) | 30 | + | + |
| 50 | + | + | |
| 100 | − | − | |
| Hygromycin (Hyg) | 50 | ++ | ++ |
| 100 | + | + | |
| 200 | − | − | |
| Nystatin (Nyt) | 5 | ++ | ++ |
| 10 | + | + | |
| 30 | − | − | |
| Control (H2O) | 0 | ++ | ++ |
| Metal Ion | YL24-3 | YL28-9T | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| Cu2+ | 19 | 20 | 30.6 | 36 | 36 | 25.5 | 28.5 | 26 | 42.6 | 40.6 |
| Zn2+ | 22.6 | 29 | 28 | 36.6 | 49 | 21 | 22.5 | 27.5 | 42 | 51 |
| Pb2+ | 21.6 | 26 | 44 | 40 | 40 | 22 | 21.5 | 30 | 37.6 | 40.6 |
| Mn2+ | 7.6 | 13 | 11.6 | 11.6 | 16 | 8.5 | 10 | 19.5 | 31 | 30 |
| Fe3+ | 10.3 | 18 | 19.6 | 21 | 23 | 10.5 | 16 | 20 | 19 | 23.6 |
| Cd2+ | 58.6 | 60 | 64.6 | 70 | 75.6 | 55.5 | 57 | 53.5 | 69 | 78.6 |
| Cr6+ | 62 | 78 | 103.6 | 102 | 122 | 49.5 | 61 | 82.5 | 83 | 88 |
| Fatty Acids | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Straight-Chain Saturated | |||||
| C14:0 | TR | TR | 3.3 | TR | TR |
| C16:0 | 1.4 | 3.5 | ND | TR | TR |
| C18:0 | ND | 1.6 | ND | ND | TR |
| C19:0 | TR | 1.2 | ND | TR | ND |
| C15:0 | TR | TR | 2 | TR | TR |
| C15:0 | ND | ND | TR | 1.4 | 1.5 |
| C16:0 | TR | TR | 2.7 | 1.1 | 1.5 |
| C17:0 | TR | TR | TR | 1.6 | TR |
| Branched-Chain | |||||
| Iso-C14:0 | ND | 4.8 | 3.3 | TR | TR |
| Iso-C15:0 | 42.5 | 31.7 | 31.8 | 26.2 | 28.9 |
| Iso-C16:0 | TR | TR | TR | 3.6 | TR |
| Iso-C17:0 | TR | TR | ND | TR | TR |
| Iso-C15:0 | 3.9 | 3.6 | 9.9 | 3.7 | 3.5 |
| Iso-C16:0 | TR | ND | 1.9 | 4.6 | 2.6 |
| Iso-C17:0 | 15.3 | 6.7 | 20 | 13.7 | 11.5 |
| Anteiso-C14:0 | ND | 1.16 | ND | ND | ND |
| Anteiso-C15:0 | 2.5 | 3.3 | 6.7 | 1.2 | 1.1 |
| Monounsaturated | |||||
| C15:1ω6c | TR | TR | TR | 1.6 | 3.2 |
| *Summed Feature 1 | 1.1 | 1.1 | 1.1 | TR | TR |
| *Summed Feature 3 | 17.8 | 26.2 | 18.2 | 19.9 | 27.1 |
| *Summed Feature 4 | 1.1 | ND | ND | ND | ND |
| *Summed Feature 9 | 9.7 | 6.5 | 2 | 4.2 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Zhao, S.; Bold, N.; Zhang, J.; Hu, Z.; Hu, X.; Gao, Y.; Chen, S.; Wei, Y. Screening and Characterization of Two Extracellular Polysaccharide-Producing Bacteria from the Biocrust of the Mu Us Desert. Molecules 2021, 26, 5521. https://doi.org/10.3390/molecules26185521
Xue Z, Zhao S, Bold N, Zhang J, Hu Z, Hu X, Gao Y, Chen S, Wei Y. Screening and Characterization of Two Extracellular Polysaccharide-Producing Bacteria from the Biocrust of the Mu Us Desert. Molecules. 2021; 26(18):5521. https://doi.org/10.3390/molecules26185521
Chicago/Turabian StyleXue, Zhanfang, Shuting Zhao, Nomin Bold, Jianguo Zhang, Zhimin Hu, Xiaofeng Hu, Ying Gao, Shaolin Chen, and Yahong Wei. 2021. "Screening and Characterization of Two Extracellular Polysaccharide-Producing Bacteria from the Biocrust of the Mu Us Desert" Molecules 26, no. 18: 5521. https://doi.org/10.3390/molecules26185521
APA StyleXue, Z., Zhao, S., Bold, N., Zhang, J., Hu, Z., Hu, X., Gao, Y., Chen, S., & Wei, Y. (2021). Screening and Characterization of Two Extracellular Polysaccharide-Producing Bacteria from the Biocrust of the Mu Us Desert. Molecules, 26(18), 5521. https://doi.org/10.3390/molecules26185521








