Walking around Ribosomal Small Subunit: A Possible “Tourist Map” for Electron Holes
Abstract
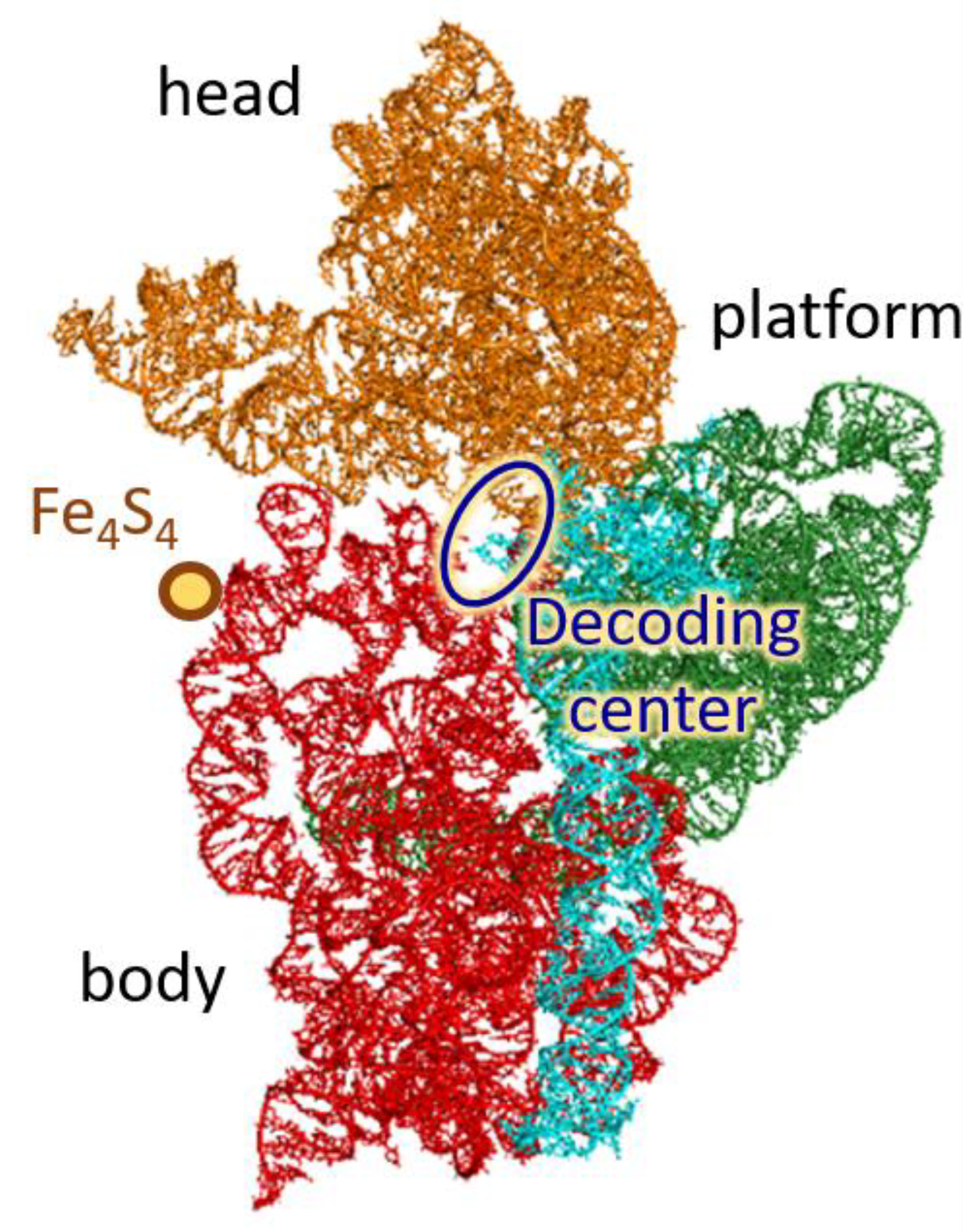
1. Introduction
2. Methods
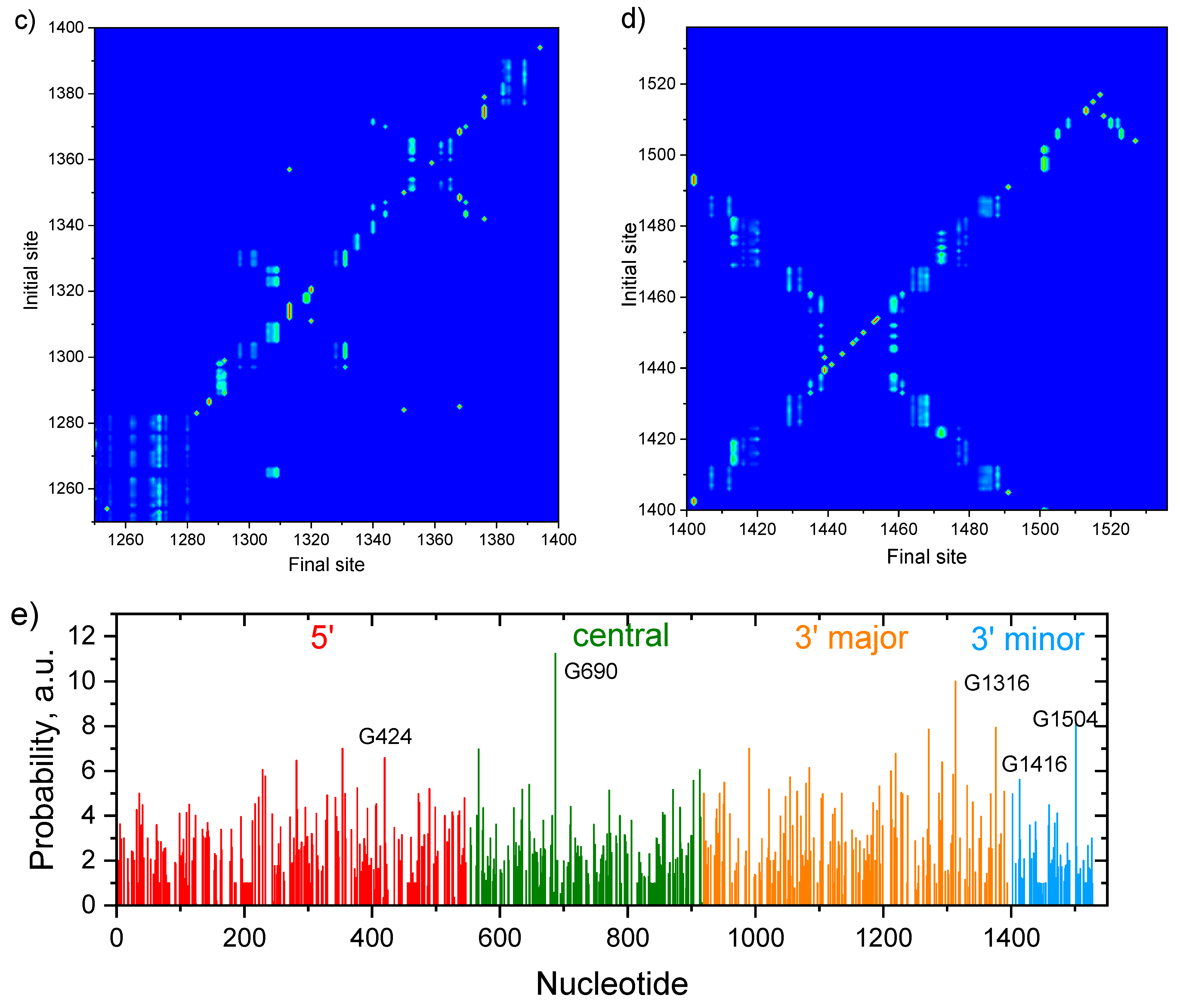
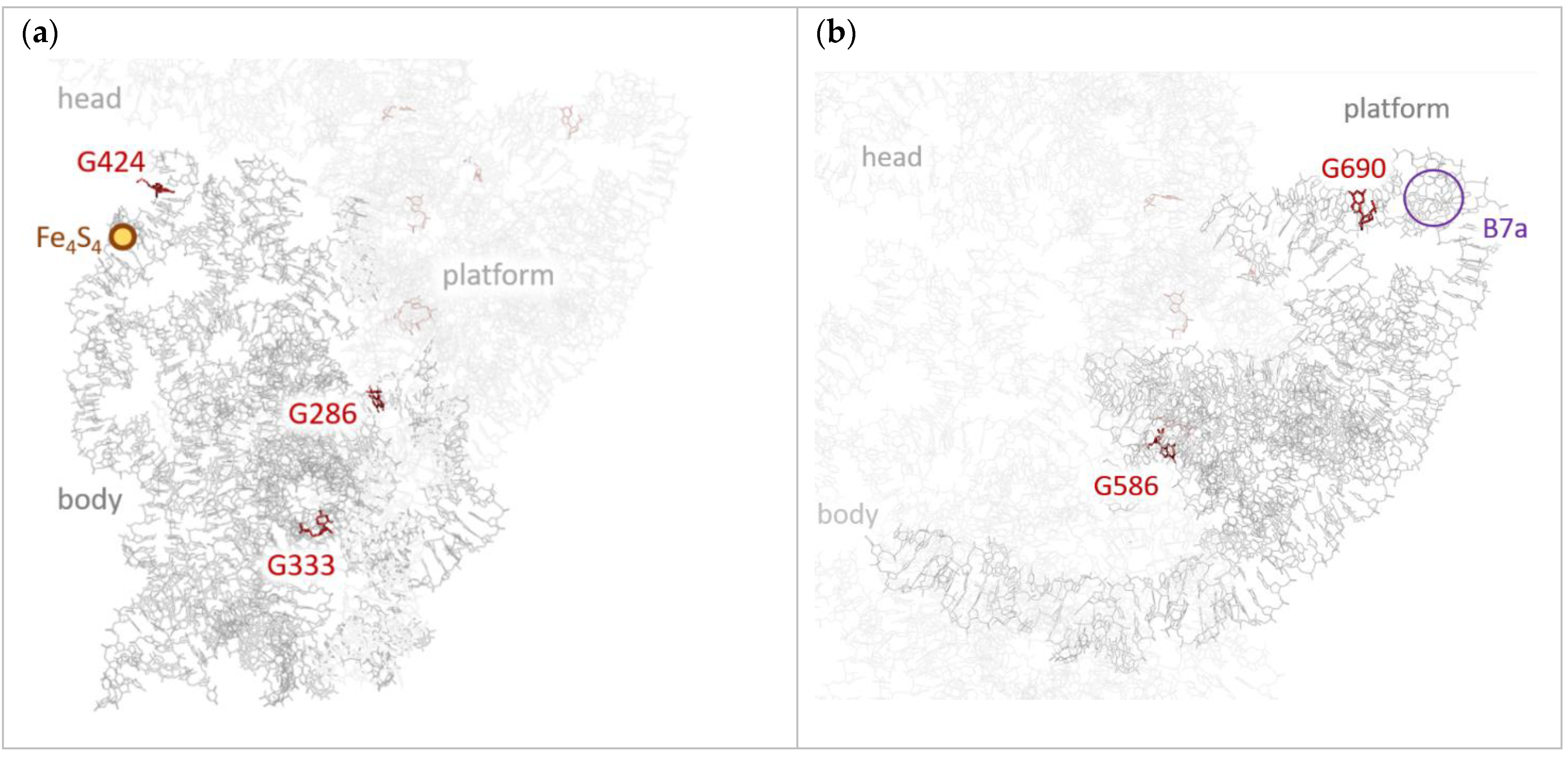
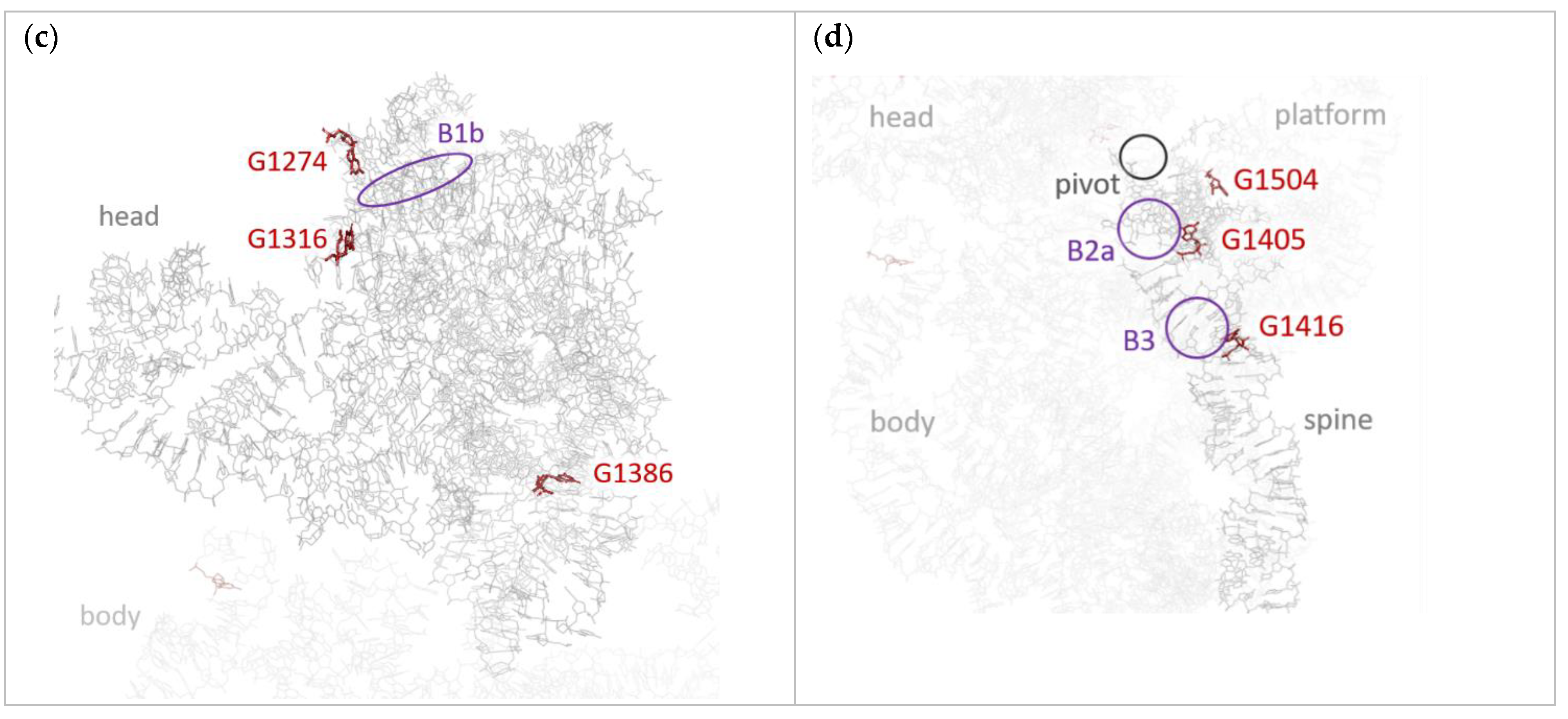
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Schuwirth, B.S.; Borovinskaya, M.A.; Hau, C.W.; Zhang, W.; Vila-Sanjurjo, A.; Holton, J.M.; Cate, J.H.D. Structures of the bacterial ribosome at 3.5 Å resolution. Science 2005, 310, 827–834. [Google Scholar] [CrossRef]
- Belardinelli, R.; Sharma, H.; Caliskan, N.; Cunha, C.E.; Peske, F.; Wintermeyer, W.; Rodninal, M.V. Choreography of molecular movements during ribosome progression along mRNA. Nat. Struct. Mol. Biol. 2016, 23, 342–348. [Google Scholar] [CrossRef]
- Ling, C.; Ermolenko, D.N. Structural insights into ribosome translocation. WIREs RNA 2016, 7, 620–636. [Google Scholar] [CrossRef]
- Li, W.; Agirrezabala, X.; Lei, J.; Bouakaz, L.; Brunelle, J.L.; Ortiz-Meoz, R.F.; Green, R.; Sanyal, S.; Ehrenberg, M.; Frank, J. Recognition of aminoacyl-tRNA: A common molecular mechanism revealed by cryo-EM. EMBO J. 2008, 27, 3322–3331. [Google Scholar] [CrossRef]
- Noller, H.F.; Lancaster, L.; Mohan, S.; Zhou, J. Ribosome structural dynamics in translocation: Yet another functional role for ribosomal RNA. Q. Rev. Biophys. 2017, 50, e12. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Fischer, N.; Maracci, C.; Stark, H. Ribosome dynamics during decoding. Philos. Trans. R. Soc. B 2017, 372, 20160182. [Google Scholar] [CrossRef] [PubMed]
- Paci, M.; Fox, G.E. Centers of motion associated with EF-Tu binding to the ribosome. RNA Biol. 2016, 13, 524–530. [Google Scholar] [CrossRef][Green Version]
- Finkelstein, A.V.; Razin, S.V.; Spirin, A.S. Intersubunit mobility of the ribosome. Mol. Biol. 2018, 52, 799–811. [Google Scholar] [CrossRef]
- Mohan, S.; Noller, H.F. Recurring RNA structural motifs underlie the mechanics of L1 stalk movement. Nat. Commun. 2017, 8, 14285. [Google Scholar] [CrossRef]
- Gindulyte, A.; Bashan, A.; Agmon, I.; Massa, L.; Yonath, A.; Karle, J. The transition state for formation of the peptide bond in the ribosome. Proc. Natl. Acad. Sci. USA 2006, 103, 13327–13332. [Google Scholar] [CrossRef]
- Poirot, O.; Timsit, Y. Neuron-like networks between ribosomal proteins within the ribosome. Sci. Rep. 2016, 6, 26485. [Google Scholar] [CrossRef] [PubMed]
- Kürkçüoğlu, Ö. Exploring allosteric communication in multiple states of the bacterial ribosome using residue network analysis. Turk. J. Biol. 2018, 42, 392–404. [Google Scholar] [CrossRef]
- Makarova, T.M.; Bogdanov, A.A. Allosteric regulation of the ribosomal A site revealed by molecular dynamics simulations. Biochimie 2019, 167, 179–186. [Google Scholar] [CrossRef]
- Sosorev, A.; Kharlanov, O. Organic nanoelectronics inside us: Charge transport and localization in RNA could orchestrate ribosome operation. Phys. Chem. Chem. Phys. 2021, 23, 7037–7047. [Google Scholar] [CrossRef]
- Rooman, M.; Cauët, E.; Liévin, J.; Wintjens, R. Conformations consistent with charge migration observed in DNA and RNA X-ray structures. J. Biomol. Struct. Dyn. 2011, 28, 949–954. [Google Scholar] [CrossRef][Green Version]
- Blumberger, J. Recent advances in the theory and molecular simulation of biological electron transfer reactions. Chem. Rev. 2015, 115, 11191–11238. [Google Scholar] [CrossRef] [PubMed]
- Corbella, M.; Voityuk, A.A.; Curutchet, C. How abasic sites impact hole transfer dynamics in GC-rich DNA sequences. Phys. Chem. Chem. Phys. 2018, 20, 23123–23131. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Holt, M.E.; Thompson, M.K.; Salay, L.E.; Ethlinger, A.C.; Chazin, W.J.; Barton, J.K. The [4Fe4S] cluster of human DNA primase functions as a redox switch using DNA charge transport. Science 2017, 355, eaag1789. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.O.; Tsai, C.-L.; Ishida, J.P.; Tainer, J.A. Emerging critical roles of Fe-S clusters in DNA replication and repair. BBA Mol. Cell Res. 2015, 1853, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Bag, S.; Maiti, P.K. Remarkable similarity of force induced dsRNA onformational changes to stretched dsDNA and their detection using electrical measurements. Phys. Chem. Chem. Phys. 2018, 20, 28920–28928. [Google Scholar] [CrossRef] [PubMed]
- Eley, D.D.; Spivey, D.I. Semiconductivity of organic substances. Part 9.—Nucleic acid in the dry state. Trans. Faraday Soc. 1962, 58, 411–415. [Google Scholar] [CrossRef]
- McDonnell, K.J.; Chemler, J.A.; Bartels, P.L.; O’Brien, E.; Marvin, M.L.; Ortega, J.; Stern, R.H.; Raskin, L.; Li, G.-M.; Sherman, D.H.; et al. A human MUTYH variant linking colonic polyposis to redox degradation of the [4Fe4S]2⁺ cluster. Nat. Chem. 2018, 10, 873–880. [Google Scholar] [CrossRef]
- Wimberly, B.T.; Brodersen, D.E.; Clemons, W.M.; Morgan-Warren, R.J.; Carter, A.P.; Vonrhein, C.; Hartschk, T.; Ramakrishnan, V. Structure of the 30S ribosomal subunit. Nature 2000, 407, 327–339. [Google Scholar] [CrossRef]
- Rozov, A.; Khusainov, I.; Omari, K.E.; Duman, R.; Mykhaylyk, V.; Yusupov, M.; Westhof, E.; Wagner, A.; Yusupova, G. Importance of potassium ions for ribosome structure and function revealed by long-wavelength X-ray diffraction. Nat. Commun. 2019, 10, 2519. [Google Scholar] [CrossRef]
- Ortmann, F.; Hannewald, K.; Bechstedt, F. Charge Transport in Guanine-Based Materials. J. Phys. Chem. B 2009, 113, 7367–7371. [Google Scholar] [CrossRef]
- Marcus, R.A. On the theory of oxidation-reduction reactions involving electron transfer I. J. Chem. Phys. 1956, 24, 966–978. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Gordon, M.S.; Schmidt, M.W. Advances in electronic structure theory: GAMESS a decade later. In Theory and Applications of Computational Chemistry: The First Forty Years; Dykstra, C.E., Frenking, G., Kim, K.S., Scuseria, G.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1167–1189. [Google Scholar]
- Zhang, G.; Musgrave, C.B. Comparison of DFT Methods for molecular orbital eigenvalue calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef]
- Sosorev, A.Y.; Nuraliev, M.K.; Feldman, E.V.; Maslennikov, D.R.; Borshchev, O.V.; Skorotetcky, M.S.; Surin, N.M.; Kazantsev, M.S.; Ponomarenko, S.A.; Paraschuk, D.Y. Impact of terminal substituents on the electronic, vibrational and optical properties of thiophene–phenylene co-oligomers. Phys. Chem. Chem. Phys. 2019, 21, 11578–11588. [Google Scholar] [CrossRef]
- Sosorev, A.Y.; Trukhanov, V.A.; Maslennikov, D.R.; Borshchev, O.V.; Polyakov, R.A.; Skorotetcky, M.S.; Surin, N.M.; Kazantsev, M.S.; Dominskiy, D.I.; Tafeenko, V.A.; et al. Fluorinated thiophene-phenylene co-oligomers for optoelectronic devices. ACS Appl. Mater. Interfaces 2020, 12, 9507–9519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Coropceanu, V.; Brédas, J.-L. The WSPC Reference on Organic Electronics: Organic Semiconductors; Brédas, J.-L., Marder, S.R., Eds.; World Scientific: Singapore, 2016. [Google Scholar]
- Sosorev, A.Y. Role of intermolecular charge delocalization and its dimensionality in efficient band-like electron transport in crystalline 2,5-difluoro-7,7,8,8-tetracyanoquinodimethane (F2-TCNQ). Phys. Chem. Chem. Phys. 2017, 19, 25478–25486. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, B.; Kirkpatrick, J.; Andrienko, D. Density-functional based determination of intermolecular charge transfer properties for large-scale morphologies. Phys. Chem. Chem. Phys. 2010, 12, 11103–11113. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, J. An approximate method for calculating transfer integrals based on the ZINDO Hamiltonian. Int. J. Quantum Chem. 2008, 108, 51–56. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kobayashi, N.; Hosoi, S.; Koshitani, N.; Murakami, D.; Shirasawa, R.; Kudo, Y.; Hobara, D.; Tokita, Y.; Itabashi, M. Hopping and band mobilities of pentacene, rubrene, and 2,7-dioctyl[1]benzothieno[3,2-b][1]benzothiophene (C8-BTBT) from first principle calculations. J. Chem. Phys. 2013, 139, 014707. [Google Scholar] [CrossRef]
- Sosorev, A.Y. Modeling of hole transport within the ribosomal small subunit. Russ. J. Bioorg. Chem. 2021. accepted. [Google Scholar]
- Kratochvílová, I.; Todorciuc, T.; Král, K.; Němec, H.; Bunček, M.; Šebera, J.; Záliš, S.; Vokáčová, Z.; Sychrovský, V.; Bednárová, L.; et al. Charge transport in DNA oligonucleotides with various base-pairing patterns. J. Phys. Chem. B 2010, 114, 5196–5205. [Google Scholar] [CrossRef]
- Takada, T.; Kawai, K.; Fujitsuka, M.; Majima, T. Direct observation of hole transfer through double-helical DNA over 100 Å. Proc. Natl. Acad. Sci. USA 2004, 101, 14002–14006. [Google Scholar] [CrossRef]
- Sosorev, A.Y. Simple charge transport model for efficient search of high-mobility organic semiconductor crystals. Mater. Des. 2020, 192, 108730. [Google Scholar] [CrossRef]
- Ostroverkhova, O. Organic optoelectronic materials: Mechanisms and applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Köhler, A.; Bässler, H. Electronic Processes in Organic Semiconductors: An Introduction; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Agirrezaballa, X.; Frank, J. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q. Rev. Biophys. 2009, 42, 159–200. [Google Scholar] [CrossRef]
- Liu, Q.; Fredrick, K. Intersubunit Bridges of the Bacterial Ribosome. J. Mol. Biol. 2016, 428, 2146–2164. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, R.; Keinan, S.; Balaeff, A.; Beratan, D.N. Nucleic acid charge transfer: Black, white and gray. Coord. Chem. Rev. 2011, 255, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.T.; Illas, F.; Mas-Torrent, M. Dependence of charge transfer reorganization energy on carrier localization in organic molecular crystals. Phys. Chem. Chem. Phys. 2008, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rooman, M.; Wintjens, R. Sequence and conformation effects on ionization potential and charge distribution of homo-nucleobase stacks using M06-2X hybrid density functional theory calculations. J. Biomol. Struct. Dyn. 2014, 32, 532–545. [Google Scholar] [CrossRef]
- Barnett, R.N.; Cleveland, C.L.; Joy, A.; Landman, U.; Schuster, G.B. Charge migration in DNA: Ion-gated transport. Science 2001, 294, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Lafont, B.A.P.; Sil, D.; Li, Y.; Bollinger, J.M.; Krebs, C.; Pierson, T.C.; Linehan, W.M.; Rouault, T.A. Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets. Science 2021, 373, 236–241. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosorev, A.Y. Walking around Ribosomal Small Subunit: A Possible “Tourist Map” for Electron Holes. Molecules 2021, 26, 5479. https://doi.org/10.3390/molecules26185479
Sosorev AY. Walking around Ribosomal Small Subunit: A Possible “Tourist Map” for Electron Holes. Molecules. 2021; 26(18):5479. https://doi.org/10.3390/molecules26185479
Chicago/Turabian StyleSosorev, Andrey Yu. 2021. "Walking around Ribosomal Small Subunit: A Possible “Tourist Map” for Electron Holes" Molecules 26, no. 18: 5479. https://doi.org/10.3390/molecules26185479
APA StyleSosorev, A. Y. (2021). Walking around Ribosomal Small Subunit: A Possible “Tourist Map” for Electron Holes. Molecules, 26(18), 5479. https://doi.org/10.3390/molecules26185479





