Synthesis of Lead-Free CaTiO3 Oxide Perovskite Film through Solution Combustion Method and Its Thickness-Dependent Hysteresis Behaviors within 100 mV Operation
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
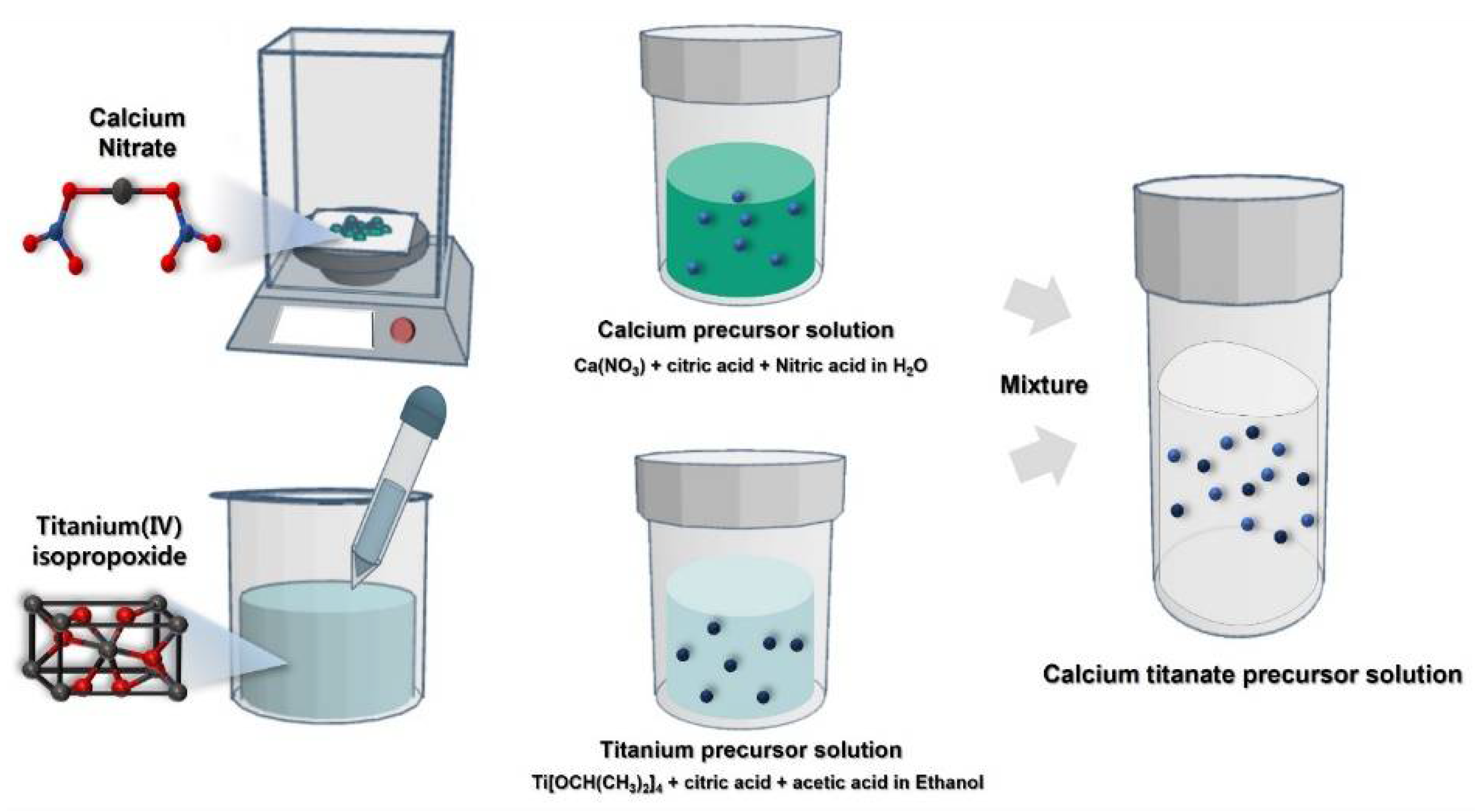
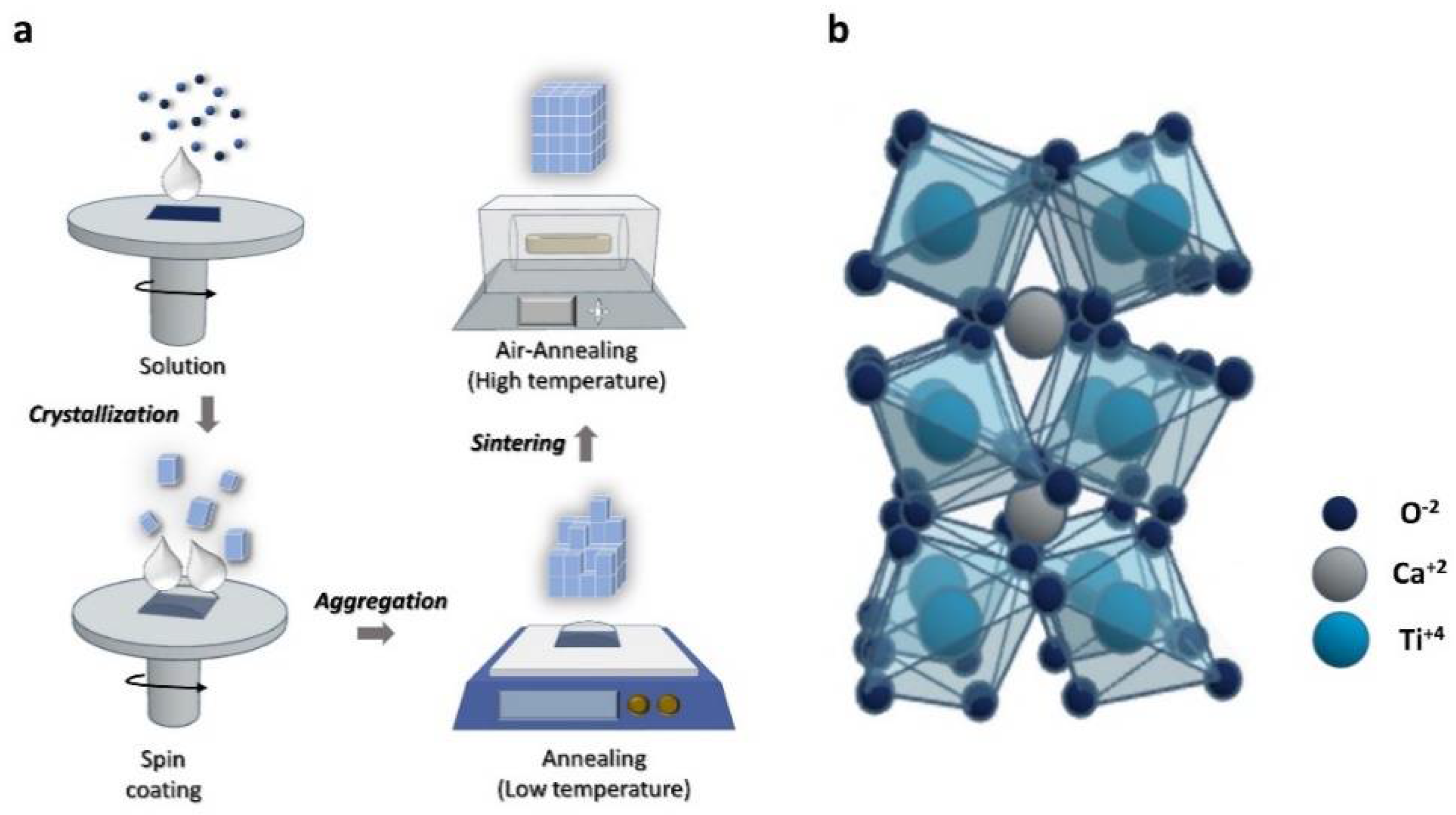
2.2. Precursor Solution Preparation and Device Fabrication
2.3. Characterization and Device Measurement
3. Results and Discussion
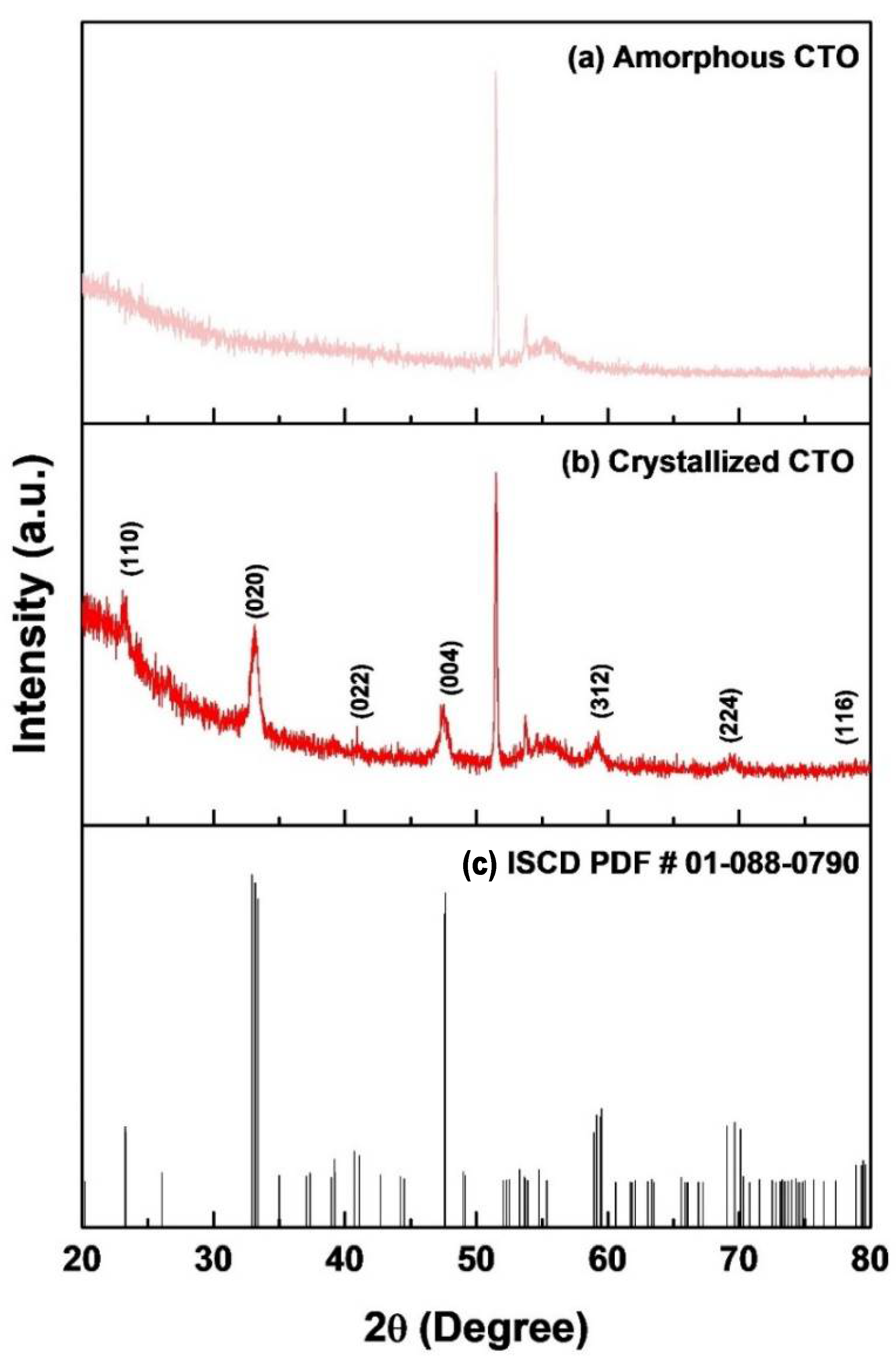
3.1. Synthesis Process and Film Characterization
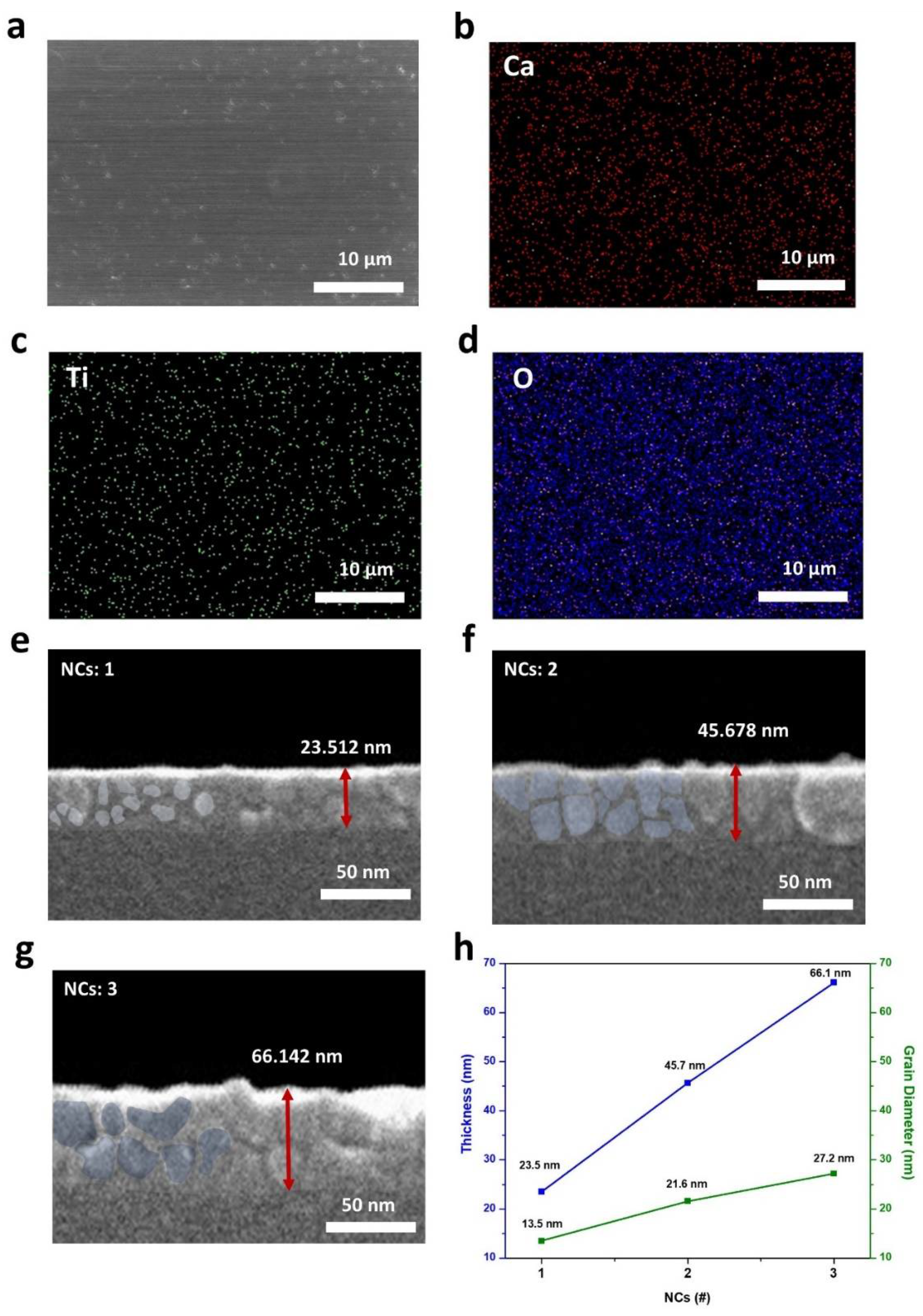
3.2. Electrical Characterization of Perovskite Memristor Dependent on CTO Thickness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahrokhi, S.; Gao, W.; Wang, Y.; Anandan, P.R.; Rahaman, Z.; Singh, S.; Wang, D.; Cazorla, C.; Yuan, G.; Liu, J.; et al. Emergence of Ferroelectricity in Halide Perovskites. Small Methods 2020, 4, 2000149. [Google Scholar] [CrossRef]
- Cai, B.; Chen, X.; Xie, M.; Zhang, S.; Liu, X.; Yang, J.; Zhou, W.; Guo, S.; Zeng, H. A class of Pb-free double perovskite halide semiconductors with intrinsic ferromagnetism, large spin splitting and high Curie temperature. Mater. Horiz. 2018, 5, 961–968. [Google Scholar] [CrossRef]
- Schoop, L.M.; Müchler, L.; Felser, C.; Cava, R. Lone pair effect, structural distortions, and potential for superconductivity in Tl perovskites. Inorg. Chem. 2013, 52, 5479–5483. [Google Scholar] [CrossRef]
- Balachandran, P.V.; Rondinelli, J.M. Interplay of octahedral rotations and breathing distortions in charge-ordering perovskite oxides. Phys. Rev. B 2013, 88, 054101. [Google Scholar] [CrossRef]
- Zhao, W.; Su, R.; Huang, Y.; Wu, J.; Fong, C.F.; Feng, J.; Xiong, Q. Transient circular dichroism and exciton spin dynamics in all-inorganic halide perovskites. Nat. Commun. 2020, 11, 5665. [Google Scholar] [CrossRef]
- Peng, H.; Liu, Y.-H.; Huang, X.-Q.; Liu, Q.; Yu, Z.-H.; Wang, Z.-X.; Liao, W.-Q. Homochiral one-dimensional ABX 3 lead halide perovskites with high-T c quadratic nonlinear optical and dielectric switchings. Mater. Chem. Front. 2021. [CrossRef]
- Niu, T.; Lu, J.; Munir, R.; Li, J.; Barrit, D.; Zhang, X.; Hu, H.; Yang, Z.; Amassian, A.; Zhao, K.; et al. Stable High-Performance Perovskite Solar Cells via Grain Boundary Passivation. Adv. Mater. 2018, 30, e1706576. [Google Scholar] [CrossRef]
- Cho, H.; Jeong, S.-H.; Park, M.-H.; Kim, Y.-H.; Wolf, C.; Lee, C.-L.; Heo, J.H.; Sadhanala, A.; Myoung, N.; Yoo, S. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science 2015, 350, 1222–1225. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Yu, W.; Li, F.; Yu, L.; Niazi, M.R.; Zou, Y.; Corzo, D.; Basu, A.; Ma, C.; Dey, S.; Tietze, M.L. Single crystal hybrid perovskite field-effect transistors. Nat. Commun. 2018, 9, 5354. [Google Scholar] [CrossRef]
- Gu, C.; Lee, J.-S. Flexible hybrid organic–inorganic perovskite memory. ACS Nano 2016, 10, 5413–5418. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. High-Quality Ruddlesden–Popper Perovskite Film Formation for High-Performance Perovskite Solar Cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef]
- Dang, P.; Zhang, Q.; Liu, D.; Li, G.; Lian, H.; Shang, M.; Lin, J. Hetero-valent substitution strategy toward orange-red luminescence in Bi3+ doped layered perovskite oxide phosphors for high color rendering index white light-emitting diodes. Chem. Eng. J. 2021, 420, 127640. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. A bilateral cyano molecule serving as an effective additive enables high-efficiency and stable perovskite solar cells. J. Energy Chem. 2021, 62, 243–251. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous Power Conversion Efficiency and Stability Enhancement of Cs 2 AgBiBr 6 Lead-Free Inorganic Perovskite Solar Cell through Adopting a Multifunctional Dye Interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]
- Wang, W.; Xu, M.; Xu, X.; Zhou, W.; Shao, Z. Perovskite oxide based electrodes for high-performance photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2020, 59, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Ruddlesden–Popper Perovskite Oxides for Photocatalysis-Based Water Splitting and Wastewater Treatment. Energy Fuels 2020, 34, 9208–9221. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Recent Advances in Cs2AgBiBr6-Based Halide Double Perovskites as Lead-Free and Inorganic Light Absorbers for Perovskite Solar Cells. Energy Fuels 2020, 34, 10513–10528. [Google Scholar] [CrossRef]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; van Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054. [Google Scholar] [CrossRef]
- Babayigit, A.; Thanh, D.D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb-and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef] [PubMed]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Tan, S.; Duan, Y.; Wang, Z.-K.; Yang, Y. Opportunities and challenges of lead-free perovskite optoelectronic devices. Trends Chem. 2019, 1, 368–379. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Krishnan, V. Perovskite oxide based materials for energy and environment-oriented photocatalysis. ACS Catal. 2020, 10, 10253–10315. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, J.-M. Resistance switching memory in perovskite oxides. Ann. Phys. 2015, 358, 206–224. [Google Scholar] [CrossRef]
- Asami, K.; Saito, K.; Ohtsu, N.; Nagata, S.; Hanawa, T. Titanium-implanted CaTiO3 films and their changes in Hanks’ solution. Surf. Interface Anal. 2003, 35, 483–488. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Zhou, Y.; Zhao, C.; Yuan, J.; Wu, Z.; Wu, S.; Wang, S. In situ formation of bioactive calcium titanate coatings on titanium screws for medical implants. RSC Adv. 2016, 6, 53182–53187. [Google Scholar] [CrossRef]
- Eduardo, A.C.; de Figueiredo, A.T.; Li, M.S.; Longo, E. Structural disorder-dependent upconversion in Er3+/Yb3+-doped calcium titanate. Ceram. Int. 2014, 40, 15981–15984. [Google Scholar] [CrossRef]
- Qi, J.Q.; Chang, J.X.; Zhang, R.Q.; Zhang, Q.Q.; De Liu, B.; Chen, J.; Han, X.M. Processing, point defects and photoluminescence of Pr-CaTiO3 phosphors. Ceram. Int. 2018, 44, 14342–14347. [Google Scholar] [CrossRef]
- Anzai, A.; Fujiwara, K.; Yamamoto, A.; Yoshida, H. Platinum-loaded lanthanum-doped calcium titanate photocatalysts prepared by a flux method for photocatalytic steam reforming of methane. Catal. Today 2020, 352, 1–9. [Google Scholar] [CrossRef]
- Yoshida, H.; Zhang, L.; Sato, M.; Morikawa, T.; Kajino, T.; Sekito, T.; Matsumoto, S.; Hirata, H. Calcium titanate photocatalyst prepared by a flux method for reduction of carbon dioxide with water. Catal. Today 2015, 251, 132–139. [Google Scholar] [CrossRef]
- Krause, A.; Weber, W.M.; Pohl, D.; Rellinghaus, B.; Kersch, A.; Mikolajick, T. Investigation of band gap and permittivity of the perovskite CaTiO3in ultrathin layers. J. Phys. D Appl. Phys. 2015, 48, 415304. [Google Scholar] [CrossRef]
- Cockayne, E.; Burton, B.P. Phonons and static dielectric constant in CaTiO 3 from first principles. Phys. Rev. B 2000, 62, 3735. [Google Scholar] [CrossRef]
- Carlos, E.; Martins, R.; Fortunato, E.; Branquinho, R. Frontispiece: Solution Combustion Synthesis: Towards a Sustainable Approach for Metal Oxides. Chem. –A Eur. J. 2020, 26. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Huang, W.; Zhang, X.; Wang, G.; Leonardi, M.J.; Huang, Y.; Lu, Z.; Marks, T.J.; Facchetti, A. Nitroacetylacetone as a cofuel for the combustion synthesis of high-performance indium–gallium–zinc oxide transistors. Chem. Mater. 2018, 30, 3323–3329. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kanatzidis, M.G.; Facchetti, A.; Marks, T.J. Low-temperature fabrication of high-performance metal oxide thin-film electronics via combustion processing. Nat. Mater. 2011, 10, 382–388. [Google Scholar] [CrossRef]
- Ivanov, K.; Alekseeva, O.; Kraev, A.; Agafonov, A. Template-free synthesis and properties of mesoporous calcium titanate. Prot. Met. Phys. Chem. Surf. 2019, 55, 667–670. [Google Scholar] [CrossRef]
- Portia, S.A.U.; Rajkumar, S.; Elanthamilan, E.; Merlin, J.P.; Ramamoorthy, K. Effect of annealing temperature on structural, optical and visible light photocatalytic performance of CaTiO3 catalysts synthesized by simple sol-gel technique. Inorg. Chem. Commun. 2020, 119, 108051. [Google Scholar] [CrossRef]
- Srinivasan, G.; Gopalakrishnan, N.; Yu, Y.; Kesavamoorthy, R.; Kumar, J. Influence of post-deposition annealing on the structural and optical properties of ZnO thin films prepared by sol–gel and spin-coating method. Superlattices Microstruct. 2008, 43, 112–119. [Google Scholar] [CrossRef]
- Aswathy, N.; Varghese, J.; Vinodkumar, R. Effect of annealing temperature on the structural, optical, magnetic and electrochemical properties of NiO thin films prepared by sol–gel spin coating. J. Mater. Sci. Mater. Electron. 2020, 31, 16634–16648. [Google Scholar] [CrossRef]
- Park, T.; Choi, H.W.; Hur, J. ZnO/conducting polymer bilayer via sequential spin-coating for enhanced UV sensing. Korean J. Chem. Eng. 2020, 37, 1616–1622. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.-W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Zhao, X.; Zhang, H.; Jiang, J. A hydrothermal route to the synthesis of CaTiO3 nanocuboids using P25 as the titanium source. J. Electron. Mater. 2018, 47, 3045–3050. [Google Scholar] [CrossRef]
- Wanarattikan, P.; Jitthammapirom, P.; Sakdanuphab, R.; Sakulkalavek, A. Effect of grain size and film thickness on the thermoelectric properties of flexible Sb2Te3 thin films. Adv. Mater. Sci. Eng. 2019, 2019, 6954918. [Google Scholar] [CrossRef]
- Strelcov, E.; Kim, Y.; Jesse, S.; Cao, Y.; Ivanov, I.N.; Kravchenko, I.I.; Wang, C.-H.; Teng, Y.-C.; Chen, L.-Q.; Chu, Y.H. Probing local ionic dynamics in functional oxides at the nanoscale. Nano Lett. 2013, 13, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guo, Y.; Zhang, G.; Liu, J.M. High-performance programmable memory devices based on co-doped BaTiO3. Adv. Mater. 2011, 23, 1351–1355. [Google Scholar] [CrossRef] [PubMed]

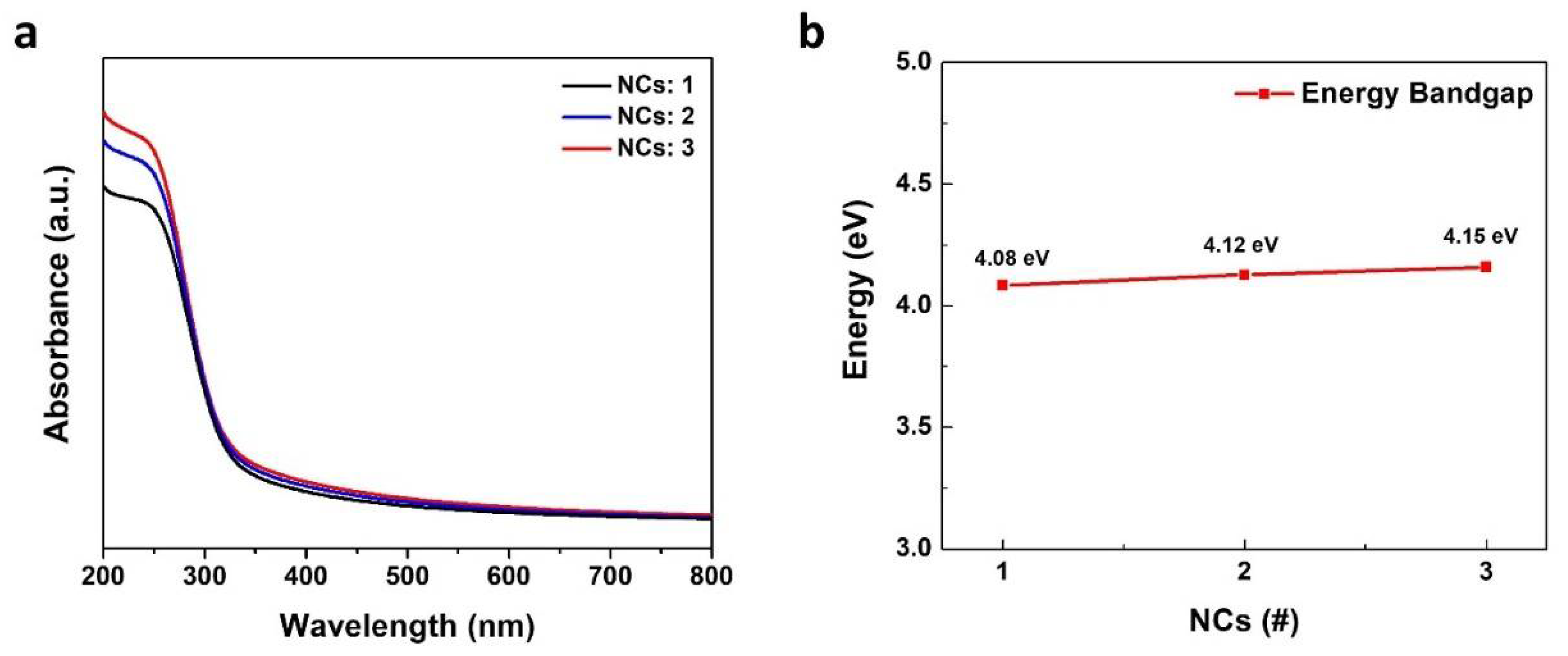
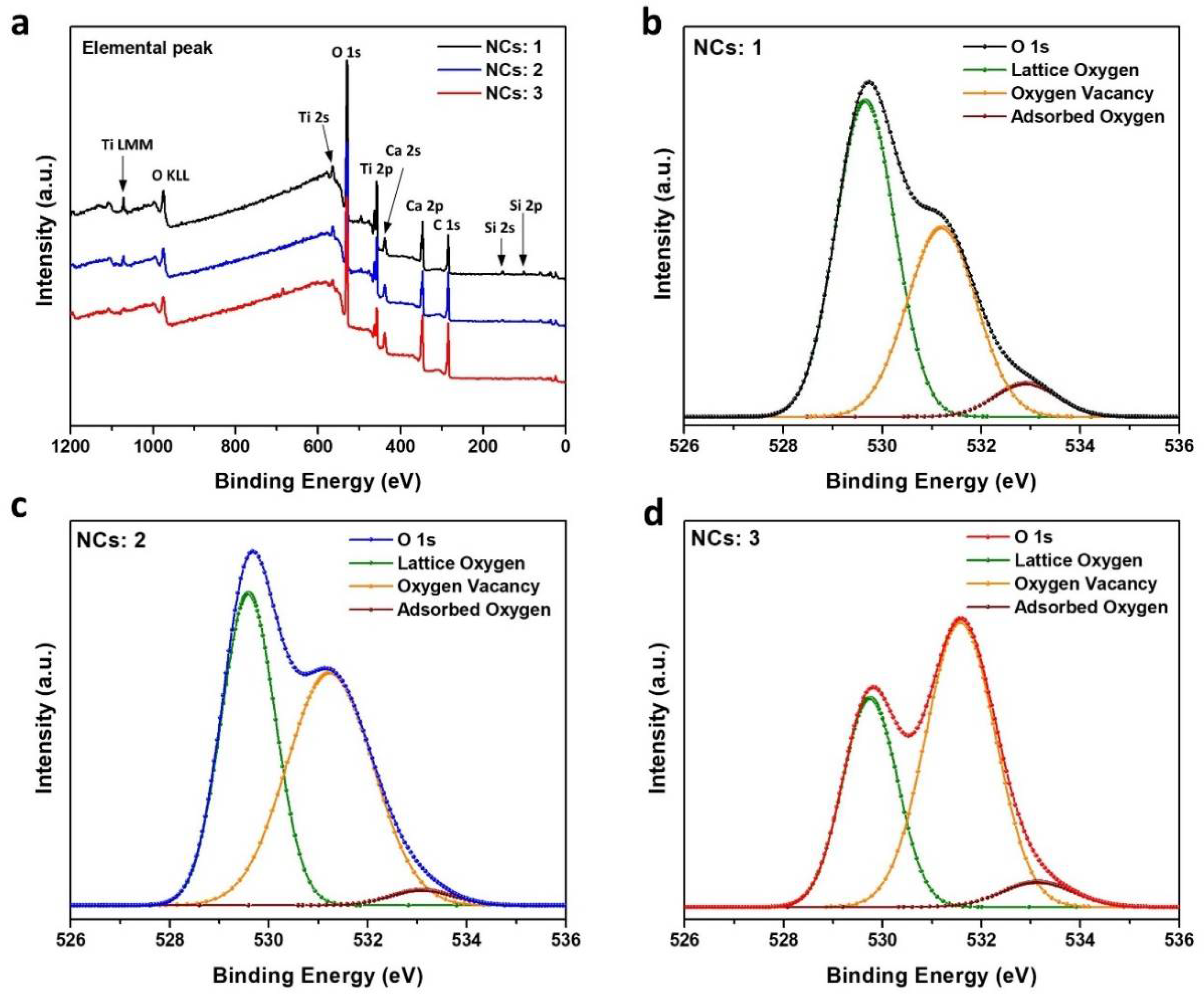

| Relative Ratio of O 1s Peak Areas | |||
|---|---|---|---|
| CTO Film | Lattice | Vacancy | Chemisorb |
| NCs: 1 | 0.55 | 0.38 | 0.06 |
| NCs: 2 | 0.43 | 0.51 | 0.05 |
| NCs: 3 | 0.34 | 0.60 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kwak, S.; Park, T.; Son, B.; Yun, H.J.; Hur, J.; Yoo, H. Synthesis of Lead-Free CaTiO3 Oxide Perovskite Film through Solution Combustion Method and Its Thickness-Dependent Hysteresis Behaviors within 100 mV Operation. Molecules 2021, 26, 5446. https://doi.org/10.3390/molecules26185446
Lee S, Kwak S, Park T, Son B, Yun HJ, Hur J, Yoo H. Synthesis of Lead-Free CaTiO3 Oxide Perovskite Film through Solution Combustion Method and Its Thickness-Dependent Hysteresis Behaviors within 100 mV Operation. Molecules. 2021; 26(18):5446. https://doi.org/10.3390/molecules26185446
Chicago/Turabian StyleLee, Subin, Soyeon Kwak, Taehyun Park, Byoungchul Son, Hyung Joong Yun, Jaehyun Hur, and Hocheon Yoo. 2021. "Synthesis of Lead-Free CaTiO3 Oxide Perovskite Film through Solution Combustion Method and Its Thickness-Dependent Hysteresis Behaviors within 100 mV Operation" Molecules 26, no. 18: 5446. https://doi.org/10.3390/molecules26185446
APA StyleLee, S., Kwak, S., Park, T., Son, B., Yun, H. J., Hur, J., & Yoo, H. (2021). Synthesis of Lead-Free CaTiO3 Oxide Perovskite Film through Solution Combustion Method and Its Thickness-Dependent Hysteresis Behaviors within 100 mV Operation. Molecules, 26(18), 5446. https://doi.org/10.3390/molecules26185446








