Tualang Honey: A Decade of Neurological Research
Abstract
1. Introduction
2. Results and Discussion
2.1. Nootropic Effects of Tualang Honey
2.2. Antinociceptive Effects of Tualang Honey
2.3. Stress-Relieving Effects of Tualang Honey
2.4. Antidepressive Effects of Tualang Honey
2.5. Anxiolytic Effects of Tualang Honey
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dan, P.; Omar, S.; Wan Ismail, W.I. Psychochemical Analysis of Several Natural Malaysian Honeys and Adulterated Honey. In Proceedings of the IOP Conference Series, Materials Science and Engineering, Volume 440, International Fundamentum Science Symposium 2018, Terengganu, Malaysia, 25–26 June 2018. [Google Scholar] [CrossRef]
- Norjihada Izzah Ismail, N.I.; Abdul Kadir, M.R.; Mahmood, N.H.; Singh, O.P.; Iqbal, N.; Zulkifli, R.M. Apini and Meliponini foraging activities influence the phenolic content of different types of Malaysian honey. J. Apic. Res. 2016, 55, 137–150. [Google Scholar] [CrossRef]
- Devanesan, S.; Premila, K.S.; Shailaja, K.K. Influence of climate change on rubber honey production. Nat. Rubber Res. 2011, 24, 170–173. [Google Scholar]
- Moniruzzaman, M.; Chowdhury, M.A.; Rahman, M.A.; Sulaiman, S.A.; Gan, S.H. Determination of mineral, trace element, and pesticide levels in honey samples originating from different regions of Malaysia compared to manuka honey. BioMed Res. Int. 2014, 2014, 10. Available online: https://pubmed.ncbi.nlm.nih.gov/24982869/ (accessed on 24 July 2021). [CrossRef]
- Ghazali, F.C. Morphological characterization study of Malaysian honey-A VPSEM, EDX randomised attempt. Ann. Microsc. 2009, 9, 93–102. [Google Scholar]
- Visavadia, B.G.; Honeysett, J.; Danford, M.H. Manuka honey dressing: An effective treatment for chronic wound infections. Br. J. Oral. Maxillofac. Surg. 2008, 46, 55–56. Available online: https://pubmed.ncbi.nlm.nih.gov/17113690/ (accessed on 24 July 2021). [CrossRef]
- Chua, L.S.; Adnan, N.A. Biochemical and nutritional components of selected honey samples. Acta Sci. Pol. Technol. Aliment 2014, 13, 169–179. Available online: https://pubmed.ncbi.nlm.nih.gov/24876312/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Chew, C.Y.; Chua, L.S.; Soontorngun, N.; Lee, C.T. Discovering potential bioactive compounds from Tualang honey. Agric. Nat. Resour. 2018, 52, 361–365. [Google Scholar] [CrossRef]
- Nurul, S.M.; Gan, S.; Halim, A.; Shah, N.S.M.; Sukari, H. Analysis of volatile compounds of Malaysian Tualang (Koompassia excelsa) honey using gas chromatography mass spectrometry. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 180–188. Available online: https://pubmed.ncbi.nlm.nih.gov/24146441/ (accessed on 24 July 2021). [CrossRef]
- Khalil, M.I.; Alam, N.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011, 76, C921–C928. Available online: https://pubmed.ncbi.nlm.nih.gov/22417491/ (accessed on 24 July 2021). [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.; Sirajudeen, K.N. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. Available online: https://pubmed.ncbi.nlm.nih.gov/21530807/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. MJMS 2013, 20, 6–13. [Google Scholar]
- Abou El-Soud, N.H. Honey between traditional uses and recent medicine. Maced. J. Med. Sci. 2012, 5, 205–214. [Google Scholar]
- Jones, R. Honey and healing through the ages. J. ApiProd. ApiMed. Sci. 2009, 1, 1–5. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharm. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Ahmad, F.; Khalid, J.; Yong, Y.K. Malaysian Tualang Honey and Its Potential Anti-Cancer Properties: A Review. Sains Malays. 2018, 47, 2705–2711. [Google Scholar]
- Mohd Kamal, D.A.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalifa, S.A.; Abd El-Wahed, A.; Gao, R.; Guo, Z.; Tahir, H.E.; Zhao, C.; Du, M.; Farag, M.A.; Musharraf, S.G.; et al. Honeybee products: An updated review of neurological actions. Trends Food Sci. Technol. 2020, 101, 17–27. [Google Scholar] [CrossRef]
- Othman, Z.; Zakaria, R.; Hussain, N.H.N.; Hassan, A.; Shafin, N.; Al-Rahbi, B.; Ahmad, A.H. Potential Role of Honey in Learning and Memory. Med. Sci. 2015, 3, 3–15. [Google Scholar] [CrossRef]
- Othman, Z.; Shafin, N.; Zakaria, R.; Hussain, N.H.N.; Mohammad, W.M.Z.W. Improvement in immediate memory after 16 weeks of tualang honey (Agro Mas) supplement in healthy postmenopausal women. Menopause 2011, 18, 1219–1224. Available online: https://pubmed.ncbi.nlm.nih.gov/21926932/ (accessed on 24 July 2021). [CrossRef]
- Shafin, N.; Othman, Z.; Zakaria, R.; Nik Hussain, N.H. Tualang honey supplementation reduces blood oxidative stress levels/activities in postmenopausal women. ISRN Oxidative Med. 2014, 364836, 4. [Google Scholar] [CrossRef]
- Shafin, N.; Zakaria, R.; Othman, Z.; Nik, N.H. Improved blood oxidative status is not associated with better memory performance in postmenopausal women receiving Tualang honey supplementation. J. Biochem. Pharmacol. Res. 2014, 2, 110–116. [Google Scholar]
- Yahaya, R.; Zahary, M.N.; Othman, Z.; Ismail, R.; Him, N.A.S.N.; Abd Aziz, A.; Dahlan, R.; Jusoh, A.F. Tualang honey supplementation as cognitive enhancer in patients with schizophrenia. Heliyon 2020, 6, e03948. Available online: https://pubmed.ncbi.nlm.nih.gov/32426546/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Zaid, S.S.; Sulaiman, S.A.; Sirajudeen, K.N.; Othman, N.H. The effects of Tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats–animal model for menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. Available online: https://pubmed.ncbi.nlm.nih.gov/20347861/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Han, D.-H.; Denison, M.S.; Tachibana, H.; Yamada, K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2002, 66, 1479–1487. Available online: https://pubmed.ncbi.nlm.nih.gov/12224631/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Abbruzzese, G.; Morón-Oset, J.; Díaz-Castroverde, S.; García-Font, N.; Roncero, C.; López-Muñoz, F.; Marco Contelles, J.L.; Oset-Gasque, M.J. Neuroprotection by Phytoestrogens in the Model of Deprivation and Resupply of Oxygen and Glucose In Vitro: The Contribution of Autophagy and Related Signaling Mechanisms. Antioxidants 2020, 9, 545. Available online: https://pubmed.ncbi.nlm.nih.gov/32580379/ (accessed on 24 July 2021). [CrossRef]
- Soni, M.; Rahardjo, T.B.W.; Soekardi, R.; Sulistyowati, Y.; Yesufu-Udechuku, A.; Irsan, A.; Hogervorst, E. Phytoestrogens and cognitive function: A review. Maturitas 2014, 77, 209–220. Available online: https://pubmed.ncbi.nlm.nih.gov/24486046/ (accessed on 24 July 2021). [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann. N. Y. Acad. Sci. 2017, 1403, 150–163. Available online: https://pubmed.ncbi.nlm.nih.gov/28945939/ (accessed on 24 July 2021). [CrossRef]
- Hussain, A.; Tabrez, E.S.; Muhammad, A.; Peela, J.R. The Mechanisms of Dietary Phytoestrogen as a Potential Treatment and Prevention Agent against Alzheimer’s Disease. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 321–327. Available online: https://pubmed.ncbi.nlm.nih.gov/30311580/ (accessed on 24 July 2021). [CrossRef]
- Rancan, L.; Puig, A.; Balibrea, J.M.; Paredes, S.D.; García, C.; Jiménez, L.; Fernández-Tresguerres, J.A.; Vara, E. Protective effects of 17-β-oestradiol and phytoestrogen on age-induced oxidative stress and inhibition of surfactant synthesis in rat type II pneumocytes. Int. J. Food Sci. Nutr. 2020, 72, 26–36. Available online: https://pubmed.ncbi.nlm.nih.gov/32314935/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Zaid, S.S.M.; Ruslee, S.S.; Mokhtar, M.H. Protective Roles of Honey in Reproductive Health: A Review. Molecules 2021, 26, 3322. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ismail, Z.I.M.; Muthuraju, S. Tualang honey supplement improves memory performance and hippocampal morphology in stressed ovariectomized rats. Acta Histochem. 2014, 116, 79–88. Available online: https://pubmed.ncbi.nlm.nih.gov/23810156/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Zakaria, R.; Al Rahbi, B.; Ahmad, A.H.; Said, R.M.; Othman, Z.; Azman, K.F.; Aziz, C.B.A. Menopause Rodent Models: Suitability for Cognitive Aging Research. Int. Med. J. 2019, 26, 1–3. [Google Scholar]
- Kim, J.J.; Song, E.Y.; Kim, J.J.; Song, E.Y.; Kosten, T.A. Stress effects in the hippocampus: Synaptic plasticity and memory. Stress 2006, 9, 1–11. Available online: https://pubmed.ncbi.nlm.nih.gov/16753928/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Muthuraju, S.; Wan Mohammad, W.M.Z. Mood and memory function in ovariectomised rats exposed to social instability stress. BioMed Res. Int. 2013, 2013, 493643. [Google Scholar] [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. The effects of Tualang honey supplement on medial prefrontal cortex morphology and cholinergic system in stressed ovariectomised rats. Int. J. Appl. Res. Nat. Prod. 2014, 7, 28–36. [Google Scholar]
- Azman, K.F.; Zakaria, R.; AbdAziz, C.; Othman, Z.; Al-Rahbi, B. Tualang honey improves memory performance and decreases depressive-like behavior in rats exposed to loud noise stress. Noise Health 2015, 17, 83–89. Available online: https://pubmed.ncbi.nlm.nih.gov/25774610/ (accessed on 24 July 2021). [CrossRef]
- Azman, K.F.; Zakaria, R.; Abdul Aziz, C.B.; Othman, Z. Tualang honey attenuates noise stress-induced memory deficits in aged rats. Oxidative Med. Cell. Longev. 2016, 2016, 1549158. Available online: https://pubmed.ncbi.nlm.nih.gov/27119005/ (accessed on 24 July 2021). [CrossRef]
- Azman, K.F.; Zakaria, R.; Othman, Z.; Abdul Aziz, C.B. Neuroprotective effects of Tualang honey against oxidative stress and memory decline in young and aged rats exposed to noise stress. J. Taibah Univ. Sci. 2018, 12, 273–284. [Google Scholar] [CrossRef]
- Rosenzweig, E.S.; Barnes, C.A. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003, 69, 143–179. Available online: https://pubmed.ncbi.nlm.nih.gov/12758108/ (accessed on 24 July 2021). [CrossRef]
- Spencer, J. The impact of flavonoids on memory: Physiological and molecular considerations. Chem. Soc. Rev. 2009, 38, 1152–1161. Available online: https://pubmed.ncbi.nlm.nih.gov/19421586/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Kamarulzaidi, M.A.; Yusoff, M.Z.M.; Mohamed, A.M.; Adli, D.H. Tualang honey consumption enhanced hippocampal pyramidal count and spatial memory performance of adult male rats. Sains Malays. 2016, 45, 215–220. [Google Scholar]
- Mohd Yusoff, N.L.; Kamarulzaidi, M.A.; Tiong, S.Y.X.; Hasan Adli, D.S. Morphometric Study of Hippocampal CA1 Pyramidal Neurons after Tualang Honey Administration. Malays. J. Microsc. 2018, 14, 80–87. [Google Scholar]
- Freas, C.A.; Roth II, T.C.; LaDage, L.D.; Pravosudov, V.V. Hippocampal neuron soma size is associated with population differences in winter climate severity in food-caching chickadees. Funct. Ecol. 2013, 27, 1341–1349. [Google Scholar] [CrossRef]
- Qaid, E.Y.A.; Zakaria, R.; Yusof, N.A.M.; Sulaiman, S.F.; Shafin, N.; Othman, Z.; Ahmad, A.H.; Abd Aziz, C.B.; Muthuraju, S. Tualang Honey Ameliorates Hypoxia-induced Memory Deficits by Reducing Neuronal Damage in the Hippocampus of Adult Male Sprague Dawley Rats. Turk. J. Pharm. Sci. 2020, 17, 555–564. [Google Scholar] [CrossRef]
- Yaacob, W.M.; Long, I.; Zakaria, R.; Othman, Z. Tualang Honey and its Methanolic Fraction Improve LPS-induced Learning and Memory Impairment in Male Rats: Comparison with Memantine. Curr. Nutr. Food Sci. 2020, 16, 333–342. [Google Scholar] [CrossRef]
- Maiti, P.; Muthuraju, S.; Ilavazhagan, G.; Singh, S.B. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav. Brain Res. 2008, 189, 233–243. Available online: https://pubmed.ncbi.nlm.nih.gov/18321600/ (accessed on 24 July 2021). [CrossRef]
- Muthuraju, S.; Maiti, P.; Pati, S.; Solanki, P.; Sharma, A.K.; Singh, S.B.; Prasad, D.; Ilavazhagan, G. Role of cholinergic markers on memory function of rats exposed to hypobaric hypoxia. Eur. J. Pharmacol. 2011, 672, 96–105. Available online: https://pubmed.ncbi.nlm.nih.gov/21924263/ (accessed on 24 July 2021). [CrossRef]
- Wan Yaacob, W.M.H.; Long, I.; Zakaria, R. Tualang honey and its methanolic fraction ameliorate lipopolysaccharide-induced oxidative stress, amyloid deposition and neuronal loss of the rat hippocampus. Adv. Tradit. Med. 2021, 21, 121–129. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflamm. 2008, 5, 37. Available online: https://pubmed.ncbi.nlm.nih.gov/18759972/ (accessed on 24 July 2021). [CrossRef]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Front. Aging Neurosci. 2018, 10, 42. Available online: https://pubmed.ncbi.nlm.nih.gov/29520228/ (accessed on 24 July 2021). [CrossRef]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; Xu, J.; et al. Gallic Acid Disrupts Aβ1-42 Aggregation and Rescues Cognitive Decline of APP/PS1 Transgenic Mouse. Neurobiol. Dis. 2018, 124, 67–80. Available online: https://pubmed.ncbi.nlm.nih.gov/30447302/ (accessed on 24 July 2021). [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. Available online: https://pubmed.ncbi.nlm.nih.gov/23966081/ (accessed on 24 July 2021). [CrossRef]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. Available online: https://pubmed.ncbi.nlm.nih.gov/17296232/ (accessed on 24 July 2021). [CrossRef]
- Saxena, A.; Phyu, H.; Al-Ani, I.; Oothuman, P. Improved spatial learning and memory performance following Tualang honey treatment during cerebral hypoperfusion-induced neurodegeneration. J. Transl. Sci. 2016, 2, 264–271. [Google Scholar] [CrossRef][Green Version]
- Tang, S.P.; Kuttulebbai Nainamohamed Salam, S.; Jaafar, H.; Gan, S.H.; Muzaimi, M.; Sulaiman, S.A. Tualang Honey Protects the Rat Midbrain and Lung against Repeated Paraquat Exposure. Oxidative Med. Cell. Longev. 2017, 2017, 4605782. Available online: https://pubmed.ncbi.nlm.nih.gov/28127418/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. Available online: https://pubmed.ncbi.nlm.nih.gov/22229125/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Oprica, M.; Eriksson, C.; Schultzberg, M. Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system. J. Cell. Mol. Med. 2003, 7, 127–140. Available online: https://pubmed.ncbi.nlm.nih.gov/12927051/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Sairazi, N.S.M.; Sirajudeen, K.; Asari, M.A.; Mummedy, S.; Muzaimi, M.; Sulaiman, S.A. Effect of tualang honey against KA-induced oxidative stress and neurodegeneration in the cortex of rats. BMC Complement. Altern. Med. 2017, 17, 31. Available online: https://pubmed.ncbi.nlm.nih.gov/28068984/ (accessed on 24 July 2021).
- Sairazi, N.; Sirajudeen, K.; Muzaimi, M.; Swamy, M.; Asari, M.A.; Sulaiman, S.A. Tualang honey attenuates kainic acid-induced oxidative stress in rat cerebellum and brainstem. Int. J. Pharm. Pharm. Sci. 2017, 9, 155–162. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.; Muzaimi, M.; Mummedy, S.; Asari, M.A.; Sulaiman, S.A. Tualang honey reduced neuroinflammation and caspase-3 activity in rat brain after kainic acid-induced status epilepticus. Evid.-Based Complement. Altern. Med. 2018, 2018, 7287820. Available online: https://pubmed.ncbi.nlm.nih.gov/30108663/ (accessed on 24 July 2021). [CrossRef]
- Huang, H.-L.; Lin, C.-C.; Jeng, K.-C.G.; Yao, P.-W.; Chuang, L.-T.; Kuo, S.-L.; Hou, C.-W. Fresh green tea and gallic acid ameliorate oxidative stress in kainic acid-induced status epilepticus. J. Agric. Food Chem. 2012, 60, 2328–2336. Available online: https://pubmed.ncbi.nlm.nih.gov/22324774/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Ban, J.Y.; Nguyen, H.T.T.; Lee, H.-J.; Cho, S.O.; Ju, H.S.; Kim, J.Y.; Bae, K.; Song, K.-S.; Seong, Y.H. Neuroprotective properties of gallic acid from Sanguisorbae Radix on amyloid β protein (25–35)-Induced toxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2008, 31, 149–153. Available online: https://pubmed.ncbi.nlm.nih.gov/18175960/ (accessed on 24 July 2021). [CrossRef]
- Abdullah, B.; Lazim, N.M.; Salim, R. The effectiveness of Tualang honey in reducing post-tonsillectomy pain. Turk. J. Ear Nose Throat 2015, 25, 137–143. Available online: https://pubmed.ncbi.nlm.nih.gov/26050853/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Imran, F.-H.; Dorai, A.A.; Halim, A.S.; Sulaiman, W.A.W. Tualang honey hydrogel in the treatment of split-skin graft donor sites. J. ApiProd. ApiMed. Sci. 2011, 3, 33–37. [Google Scholar] [CrossRef]
- Khalid, K.M.; Ramli, N.; Nasir, A.; Van Rostenberghe, H.; Taib, F.; Ibrahim, N.R. A Randomized Controlled Trial Comparing the Effects of Honey versus Sucrose as Analgesia during Venipuncture in the Newborns. Int. Med. J. 2019, 26, 460–463. [Google Scholar]
- Abd Aziz, C.B.; Ismail, C.A.N.; Iberahim, M.I.; Mohamed, M.; Kamaruljan, S. Effects of Different Doses of Tualang Honey on Pain Behavior in Rats with Formalin-Induced Inflammation. J. Physiol. 2014, 27, 39–43. [Google Scholar]
- Abd Aziz, C.B.; Ismail, C.A.N.; Hussin, C.M.C.; Mohamed, M. The Antinociceptive Effects of Tualang Honey in Male Sprague-Dawley Rats: A Preliminary Study. J. Tradit. Complement. Med. 2014, 4, 298–302. Available online: https://pubmed.ncbi.nlm.nih.gov/25379476/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Ismail, C.A.N.; Hussin, C.M.C.; Mohamed, M.; Abd Aziz, C.B. Preemptive Effects of Administration of Tualang Honey on Inflammatory Responses in Adult Male Rats. J. Pharm. Nutr. Sci. 2017, 7, 6–12. [Google Scholar] [CrossRef]
- Hasim, H.; Suhaimi, S.Q.A.; Aziz, C.B.A.; Yaw, T.W.; Hassan, S.K. Comparison of antinociceptive and antioxidative effects of Tualang honey and Vitamin C in a rat model of inflammatory pain. Indian J. Nat. Prod. Resour. 2020, 11, 52–59. [Google Scholar]
- Abd Aziz, C.B.; Suhaimi, S.Q.A.; Hasim, H.; Ahmad, A.H.; Long, I.; Zakaria, R. Effects of Tualang honey in modulating nociceptive responses at the spinal cord in offspring of prenatally stressed rats. J. Integr. Med. 2019, 17, 66–70. Available online: https://pubmed.ncbi.nlm.nih.gov/30591413/ (accessed on 24 July 2021). [CrossRef]
- Kwon, K.H.; Murakami, A.; Ohigashi, H. Suppressive effects of natural and synthetic agents on dextran sulfate sodium-induced interleukin-1β release from murine peritoneal macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 436–439. [Google Scholar] [CrossRef]
- Abd Aziz, C.B.; Ahmad, R.; Mohamed, M.; Yusof, W.N.W. The effects of Tualang honey intake during prenatal stress on pain responses in the rat offsprings. Eur. J. Integr. Med. 2013, 5, 326–331. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Chen, W.; Zhao, Y.; Li, H.; Qing, C.; Jia, N.; Bai, Z.; Liu, J. Prenatal stress causes gender-dependent neuronal loss and oxidative stress in rat hippocampus. J. Neurosci. Res. 2004, 78, 837–844. Available online: https://pubmed.ncbi.nlm.nih.gov/15499594/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Butkevich, I.; Barr, G.; Mikhailenko, V. Effect of prenatal stress on serotonergic neurons in dorsal raphe nucleus and on pain behavior during neonatal period. Ross. Fiziol. Zhurnal Im. IM Sechenova 2015, 101, 758–768. Available online: https://pubmed.ncbi.nlm.nih.gov/26591049/ (accessed on 24 July 2021).
- Tavassoli, E.; Saboory, E.; Teshfam, M.; Rasmi, Y.; Roshan-Milani, S.; Ilkhanizadeh, B.; Hesari, A.K. Effect of prenatal stress on density of NMDA receptors in rat brain. Int. J. Dev. Neurosci. 2013, 31, 790–795. Available online: https://pubmed.ncbi.nlm.nih.gov/24120877/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Asari, M.A.; Zulkaflee, M.H.; Sirajudeen, K.; Yusof, N.A.M.; Sairazi, N.S.M. Tualang honey and DHA-rich fish oil reduce the production of pro-inflammatory cytokines in the rat brain following exposure to chronic stress. J. Taibah Univ. Med. Sci. 2019, 14, 317–323. Available online: https://pubmed.ncbi.nlm.nih.gov/31488962/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Haron, M.N.; Rahman, W.F.W.A.; Sulaiman, S.A.; Mohamed, M. Tualang honey ameliorates restraint stress-induced impaired pregnancy outcomes in rats. Eur. J. Integr. Med. 2014, 6, 657–663. [Google Scholar] [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Enhancement of BDNF concentration and restoration of the hypothalamic-pituitary-adrenal axis accompany reduced depressive-like behaviour in stressed ovariectomised rats treated with either Tualang honey or estrogen. Sci. World J. 2014, 2014, 310821. Available online: https://pubmed.ncbi.nlm.nih.gov/24550703/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Singh, M.; Meyer, E.M.; Simpkins, J.W. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology 1995, 136, 2320–2324. Available online: https://pubmed.ncbi.nlm.nih.gov/7720680/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Lagunas, N.; Calmarza-Font, I.; Diz-Chaves, Y.; Garcia-Segura, L.M. Long-term ovariectomy enhances anxiety and depressive-like behaviors in mice submitted to chronic unpredictable stress. Horm. Behav. 2010, 58, 786–791. Available online: https://pubmed.ncbi.nlm.nih.gov/20691693/ (accessed on 24 July 2021). [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R.; Aziz, C.B.A.; Othman, Z. Tualang Honey Exerts Antidepressant-like Effects and Antioxidant Properties in Stress-exposed Rats. Malays. J. Appl. Sci. 2019, 4, 15–25. [Google Scholar]
- Engelmann, M.; Ebner, K.; Landgraf, R.; Wotjak, C.T. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm. Behav. 2006, 50, 496–501. Available online: https://pubmed.ncbi.nlm.nih.gov/16875693/ (accessed on 24 July 2021). [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Protective effects of Tualang honey against oxidative stress and anxiety-like behaviour in stressed ovariectomized rats. Int. Sch. Res. Not. 2014, 2014, 521065. Available online: https://pubmed.ncbi.nlm.nih.gov/27379299/ (accessed on 24 July 2021). [CrossRef]
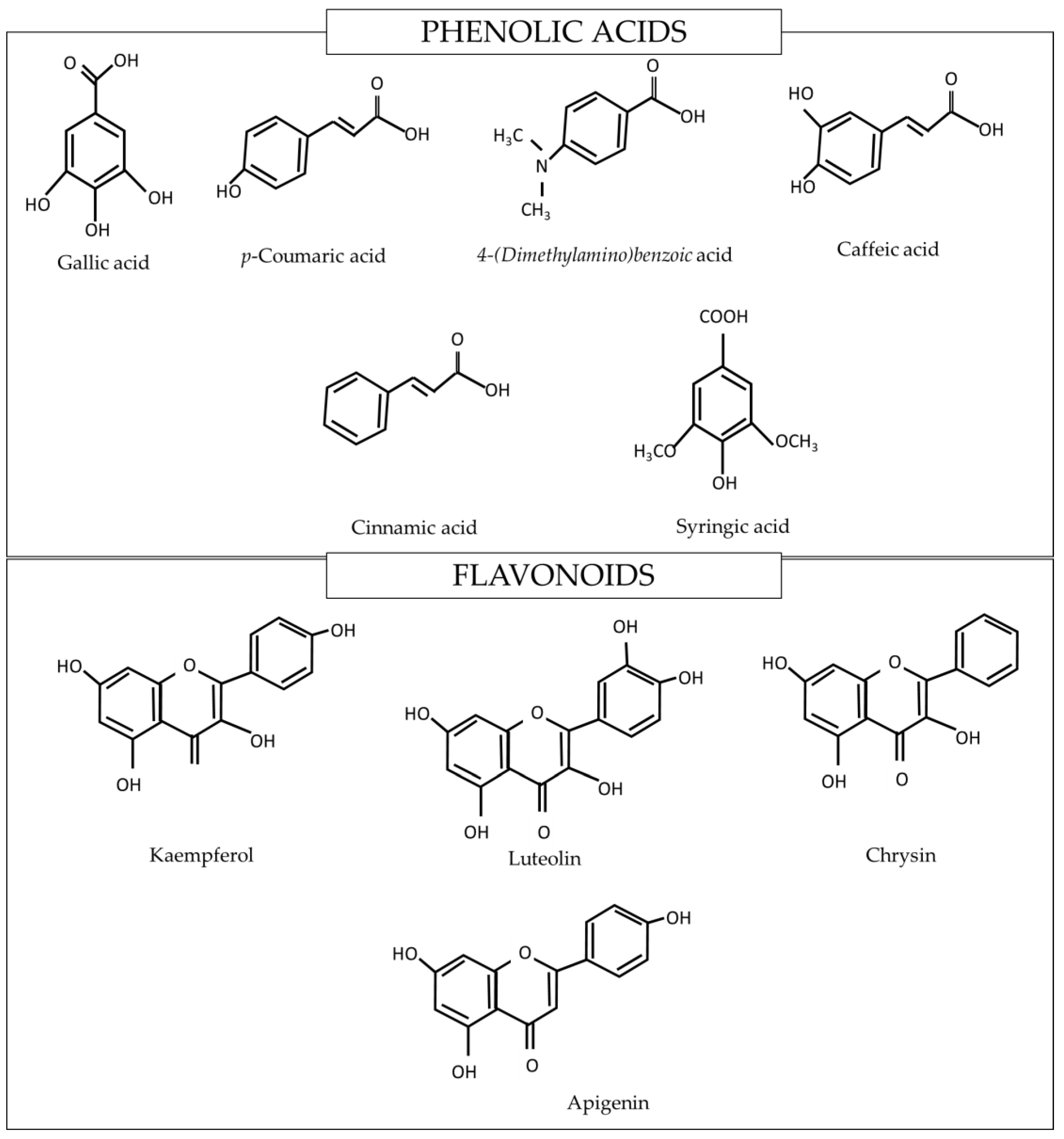

| Physiochemical Properties | Tualang Honey | Manuka Honey |
|---|---|---|
| Appearance | Dark brown | Light to dark brown |
| Specific gravity | 1.34 | 1.39 |
| pH | 3.6–4.0 | 3.2–4.2 |
| Moisture content | 23.30% | 18.70% |
| Total reducing sugars | 67.50% | 76.00% |
| Fructose | 29.60% | 40.00% |
| Glucose | 30.00% | 36.20% |
| Sucrose | 0.60% | 2.80% |
| Maltose | 7.90% | 1.20% |
| Potassium | 0.51% | 1.00% |
| Calcium | 0.18% | 1.00% |
| Magnesium | 0.11% | 1.00% |
| Sodium | 0.26% | 0.0008% |
| Carbon | 41.58% | - |
| Oxygen | 57.67% | - |
| Study Model | Subject | Dose, Method of Administration and Duration of Tualang Honey Supplementation | Findings | Reference |
|---|---|---|---|---|
| Humans | Postmenopausal women (n = 102) | 20 g/day, oral, 16 weeks | Improved verbal learning and immediate memory performance in honey-treated participants comparable with oestrogen and progestin therapy | [21,22,23] |
| Schizophrenia patients (n = 80) | 20 g/day, oral, 8 weeks | Improvement in total learning score across domains in immediate memory using MVAVLT in honey-treated schizophrenic patients | [24] | |
| Animal models | Ovariectomised Sprague Dawley Rats (n = 10 per group) | 200 mg/kg/bwt, oral, 18 days | Improved short term and long-term memory in Tualang honey-treated comparable to oestrogen-treated in ovariectomised rats exposed to social instability stress | [34] |
| Young and aged male Sprague Dawley Rats (n = 12 per group) | 200 mg/kg/bwt, oral, 28–35 days | Improved short- and long-term memory function in aged rats exposed to loud noise stress treated with Tualang honey compared to untreated rats | [39] | |
| Young and adult male Sprague Dawley rats (n = 12 per group) | 70% honey concentration, forced feeding, 12 weeks | Improved spatial memory performance in honey-treated rats compared to untreated rats | [44] | |
| Adult male Sprague Dawley Rats (n = 12 per group) | 200 mg/kg/bwt, oral, 14 days | Tualang honey pre-treatment showed protective effects against hypoxia-induced memory deficits compared to untreated rats | [47] | |
| Adult male Sprague Dawley Rats (n = 18 per group) | 200 mg/kg/bwt, (methanolic fraction MTH 150mg/kg), IP, 14 days | Tualang honey and MTH improved spatial and recognition memory in LPS-induced memory deficits comparable to memantine | [51] | |
| Chronic cerebral hypoperfusion male Sprague Dawley Rats (n = 10 per group) | 1.2 g/kg, oral, 10 weeks | Improved spatial memory performance in honey-treated cerebral hypoperfusion rats compared to untreated rats | [57] | |
| Adult male Sprague Dawley Rats (n = 18 per group) | TH pre-treatment (1.0 g/kg bwt) five times every 12 h | Improvement in locomotor activity in kainic acid-induced rats pre-treated with TH compared to without TH | [61] |
| Study Models | Subject | Dose, Method of Administration and Duration of Tualang Honey Supplementation | Findings | Reference |
|---|---|---|---|---|
| Humans | Patients (3–18 y/o) underwent tonsillectomy (n = 38 each group) | Topical 2–3 mL Tualang Honey (applied on both tonsillar bed by a 3 mL syringe) + 4 mL Tualang honey three times daily for 7 days | Early postoperative pain was relieved slightly faster in Tualang honey and antibiotic group compared to the antibiotic only group | [66] |
| Patients (13–65 y/o) underwent skin grafting (n = 35) | Honey hydrogel (Tualang honey was added to a mixture of 15% polyvinyl pyrrolidone (Kollidon 90), 1% protein-free agar (Oxoid) solution and 1% polyethylene glycol) | Tualang honey hydrogel may be effective in the treatment of split-skin graft donor sites with minimal pain, discomfort and pruritus. | [67] | |
| Neonates more than 37 weeks gestation, birth weight more than 2.5 kg, (n = 78) | 2 mL of Tualang honey, oral, blinded sampling, pre-packed in 3 mL syringe, administered directly onto dorsum of infants tongue over 30 secs duration of procedure (during venepuncture) | Tualang honey was effective in relieving venepuncture pain compared to 24% sucrose | [68] | |
| Animal models | Adult male Sprague Dawley rats (n = 24) | 0.2, 1.2 or 2.4 g/kg, oral, 5 and 10 days | Preemptive administration of Tualang honey 1.2 g/kg for 5 days and 1.2, as well as 2.4 g/kg for 10 days, had a reduction in the pain behaviour score comparable to prednisolone in formalin-induced rats | [69,70,71,72] |
| Male rat offsprings (n = 24) | 1.2 g/kg, oral, 3 weeks | Tualang honey treated group had a significant reduction in the formalin test score in phase 1 and phase 2 compared to the stressed only group. | [73,75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, K.F.; Aziz, C.B.A.; Zakaria, R.; Ahmad, A.H.; Shafin, N.; Ismail, C.A.N. Tualang Honey: A Decade of Neurological Research. Molecules 2021, 26, 5424. https://doi.org/10.3390/molecules26175424
Azman KF, Aziz CBA, Zakaria R, Ahmad AH, Shafin N, Ismail CAN. Tualang Honey: A Decade of Neurological Research. Molecules. 2021; 26(17):5424. https://doi.org/10.3390/molecules26175424
Chicago/Turabian StyleAzman, Khairunnuur Fairuz, Che Badariah Abd Aziz, Rahimah Zakaria, Asma Hayati Ahmad, Nazlahshaniza Shafin, and Che Aishah Nazariah Ismail. 2021. "Tualang Honey: A Decade of Neurological Research" Molecules 26, no. 17: 5424. https://doi.org/10.3390/molecules26175424
APA StyleAzman, K. F., Aziz, C. B. A., Zakaria, R., Ahmad, A. H., Shafin, N., & Ismail, C. A. N. (2021). Tualang Honey: A Decade of Neurological Research. Molecules, 26(17), 5424. https://doi.org/10.3390/molecules26175424






