Iodide Analogs of Arsenoplatins—Potential Drug Candidates for Triple Negative Breast Cancers
Abstract
:1. Introduction
2. Results and Discussion
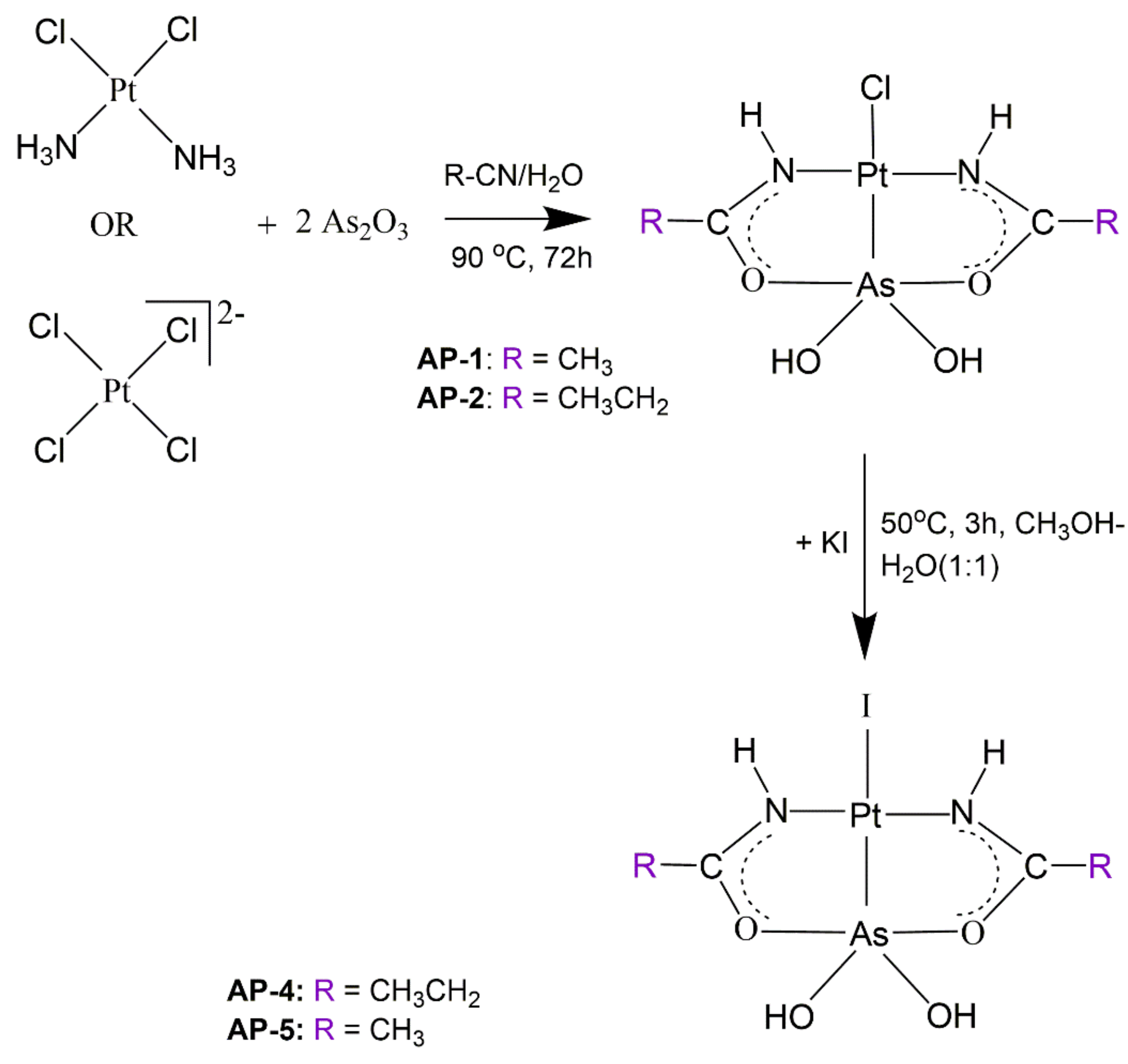
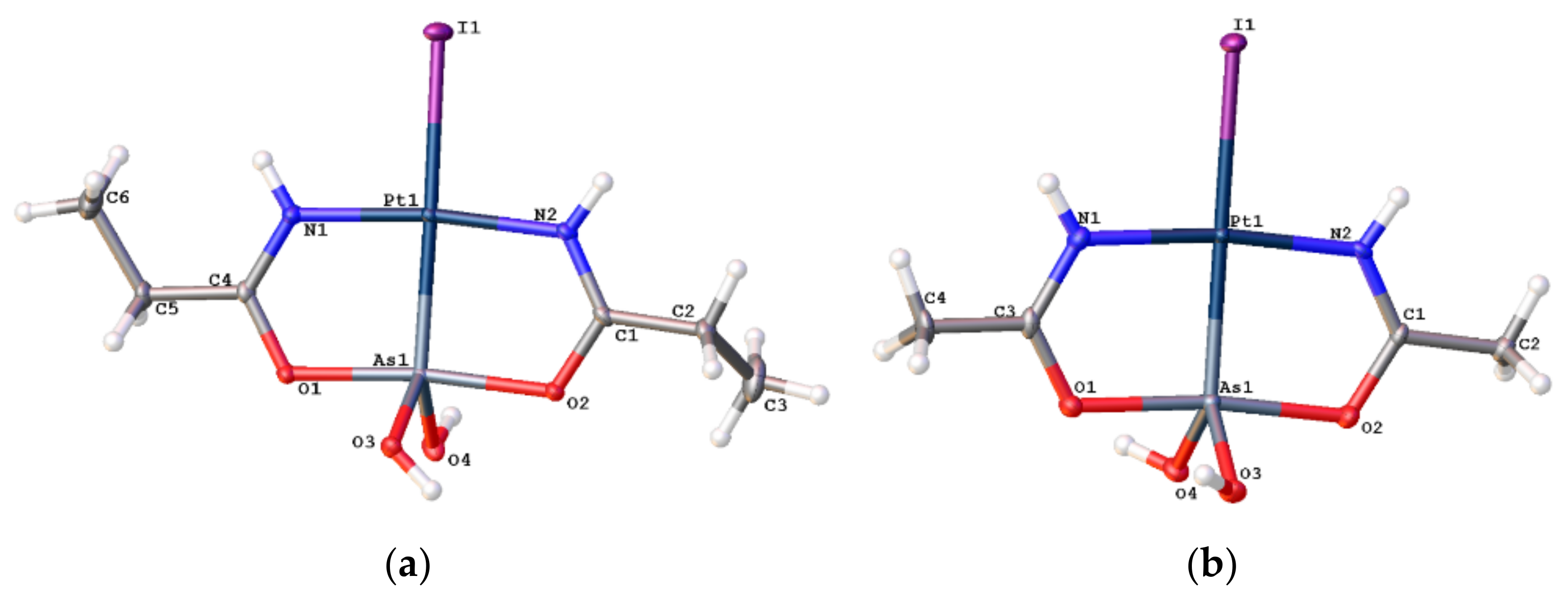
2.1. Syntheses and X-ray Structures
2.2. Infrared Spectroscopy
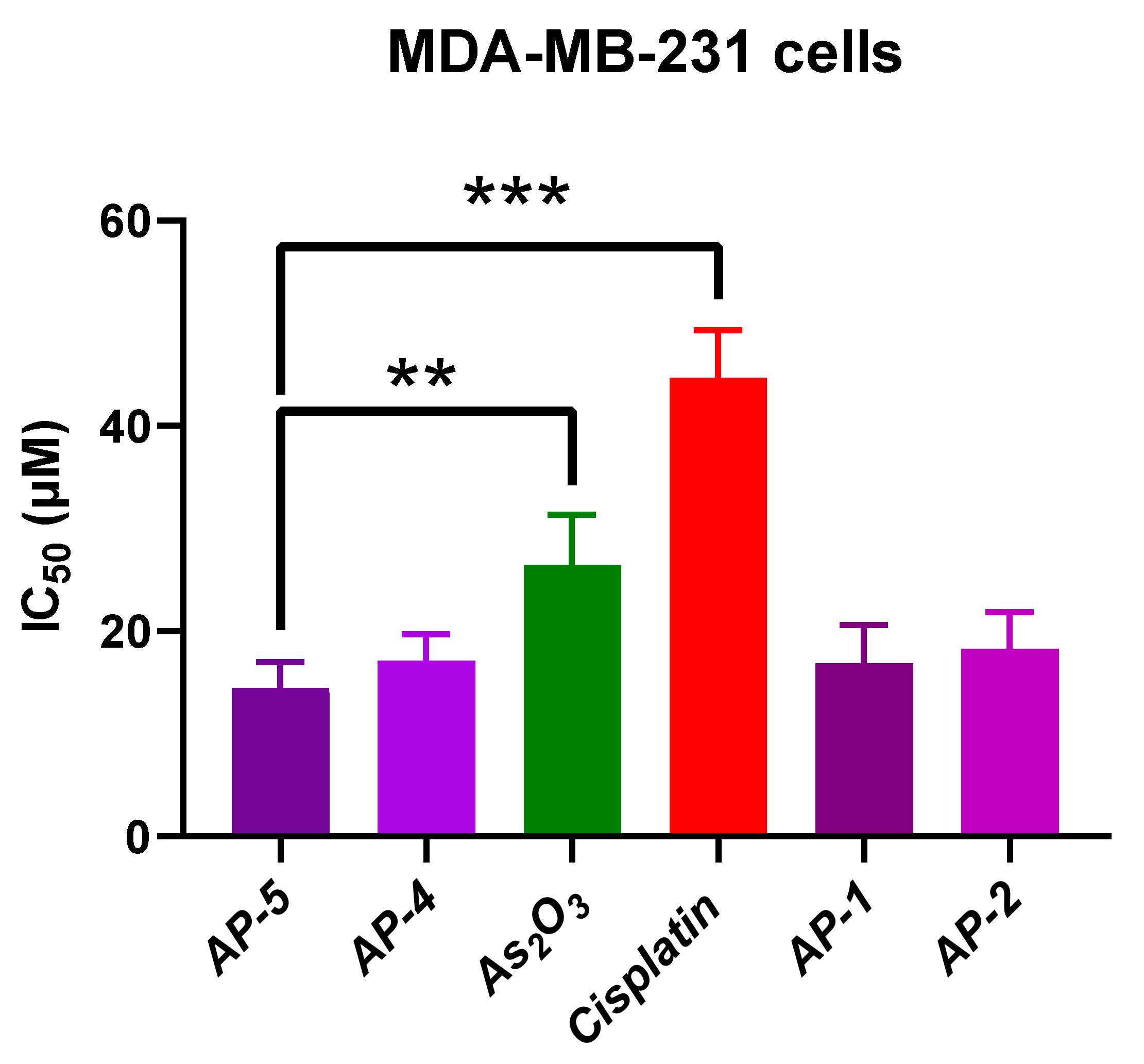
2.3. Cytotoxic Activity
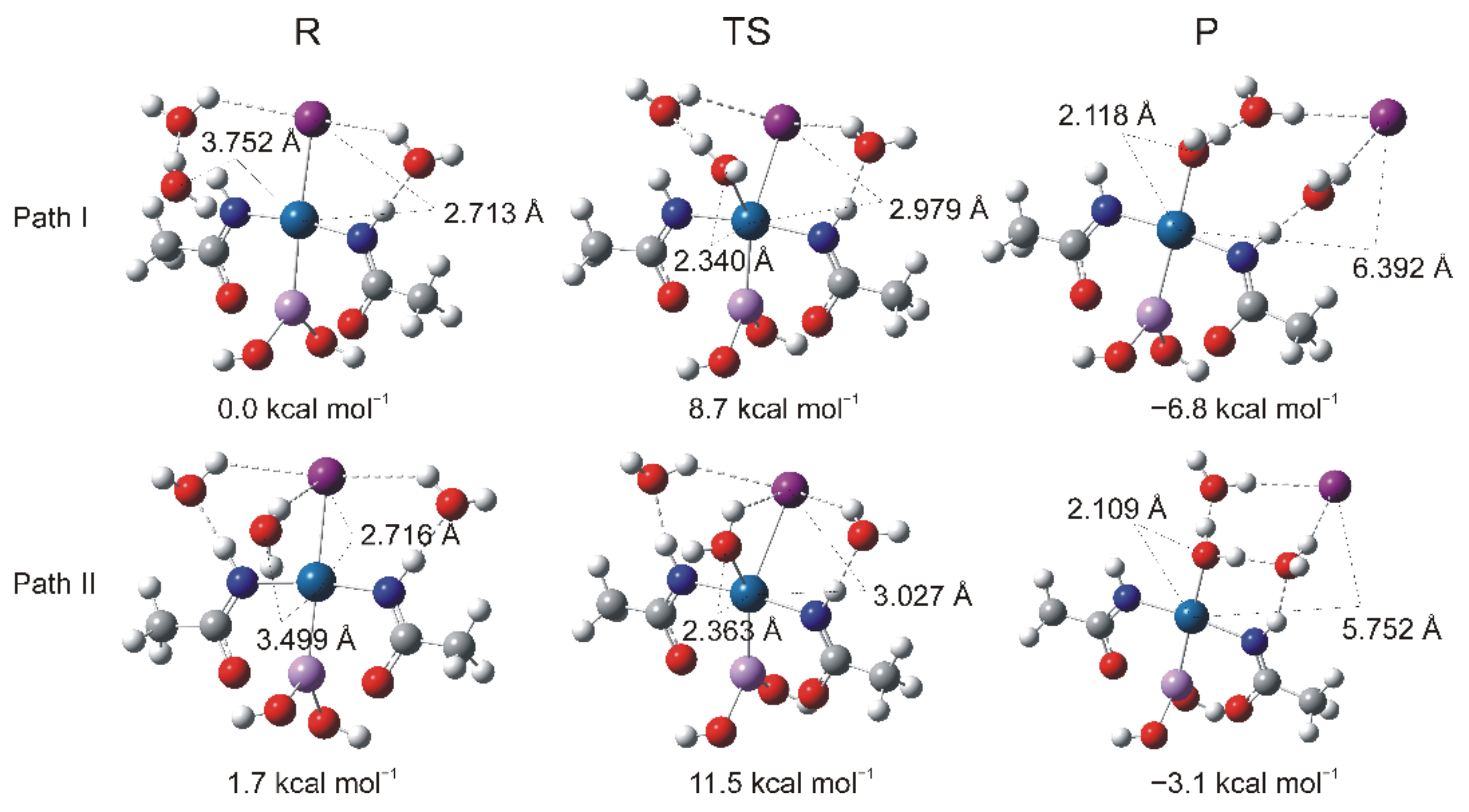
2.4. DFT Calculations
The Energetics of the Hydrolysis Reaction
3. Materials and Methods
3.1. General Experimental Considerations
3.2. Syntheses of Arsenoplatin Compounds
3.3. Crystallographic Measurements
3.4. Biological Studies
3.4.1. Cell Lines and Cell Culture
3.4.2. Cell Viability Assay
3.5. DFT Calculations
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lee, A.; Djamgoz, M.B.A. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018, 62, 110–122. [Google Scholar] [CrossRef]
- Arundhathi, J.R.D.; Mathur, S.R.; Gogia, A.; Deo, S.V.S.; Mohapatra, P.; Prasad, C.P. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol. Biol. Rep. 2021, 48, 4733–4745. [Google Scholar] [CrossRef]
- Mittal, P.; Singh, S.; Singh, A.; Singh, I.K. Current advances in drug delivery systems for treatment of Triple negative breast cancer (TNBC). Chem. Biol. Lett. 2020, 7, 1–12. [Google Scholar]
- Bai, X.; Ni, J.N.; Beretov, J.; Graham, P.; Li, Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles’ heel? Cancer Lett. 2021, 497, 100–111. [Google Scholar] [CrossRef]
- Omarini, C.; Guaitoli, G.; Pipitone, S.; Moscetti, L.; Cortesi, L.; Cascinu, S.; Piacentini, F. Neoadjuvant treatments in triple-negative breast cancer patients: Where we are now and where we are going. Cancer Manag. Res. 2018, 10, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Haynes, B.; Gajan, A.; Nangia-Makker, P.; Shekhar, M.P. RAD6B is a major mediator of triple negative breast cancer cisplatin resistance: Regulation of translesion synthesis/Fanconi anemia crosstalk and BRCA1 independence. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165561. [Google Scholar] [CrossRef]
- De Talhouet, S.; Peron, J.; Vuilleumier, A.; Friedlaender, A.; Viassolo, A.A.; Bodmer, A.; Treilleux, I.; Lang, N.; Tille, J.-C.; Chappuis, P.O.; et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci. Rep. 2020, 10, 7073. [Google Scholar] [CrossRef]
- Telli, M.L.; Hellyer, J.; Audeh, W.; Jensen, K.C.; Bose, S.; Timms, K.M.; Gutin, A.; Abkevich, V.; Peterson, R.N.; Neff, C.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Connolly, R.M.; Roussos Torres, E.T.; Thompson, A. Best Foot Forward: Neoadjuvant Systemic Therapy as Standard of Care in Triple-Negative and HER2-Positive Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chemistry 2021, 27, 8891–8917. [Google Scholar] [CrossRef] [PubMed]
- Balsa, L.M.; Ferraresi-Curotto, V.; Lavecchia, M.J.; Echeverría, G.A.; Piro, O.E.; García-Tojal, J.; Pis-Diez, R.; González-Baró, A.C.; Leon, I.E. Anticancer activity of a new copper(ii) complex with a hydrazone ligand. Structural and spectroscopic characterization, computational simulations and cell mechanistic studies on 2D and 3D breast cancer cell models. Dalton Trans. 2021, 50, 9812–9826. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Schur, R.M.; Lu, Z.-R. Targeted Mesoporous Silica Nanoparticles Delivering Arsenic Trioxide with Environment Sensitive Drug Release for Effective Treatment of Triple Negative Breast Cancer. ACS Biomater. Sci. Eng. 2016, 2, 501–507. [Google Scholar] [CrossRef]
- Xin, X.; Wen, T.; Gong, L.-B.; Deng, M.-M.; Hou, K.-Z.; Xu, L.; Shi, S.; Qu, X.-J.; Liu, Y.-P.; Che, X.-F.; et al. Inhibition of FEN1 Increases Arsenic Trioxide-Induced ROS Accumulation and Cell Death: Novel Therapeutic Potential for Triple Negative Breast Cancer. Front. Oncol. 2020, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- Marzo, T.; La Mendola, D. The Effects on Angiogenesis of Relevant Inorganic Chemotherapeutics. Curr. Top. Med. Chem. 2021, 21, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, Y.; Si, L.; Zhang, Z.; Li, Z. Interaction of EZH2 and P65 is involved in the arsenic trioxide-induced anti-angiogenesis in human triple-negative breast cancer cells. Cell Biol. Toxicol. 2019, 35, 361–371. [Google Scholar] [CrossRef]
- Arreal, L.; Piva, M.; Fernández, S.; Revandkar, A.; Schaub-Clerigué, A.; Villanueva, J.; Zabala-Letona, A.; Pujana, M.; Astobiza, I.; Cortazar, A.R.; et al. Targeting PML in triple negative breast cancer elicits growth suppression and senescence. Cell Death Differ. 2020, 27, 1186–1199. [Google Scholar] [CrossRef]
- Ponente, M.; Campanini, L.; Cuttano, R.; Piunti, A.; Delledonne, G.A.; Coltella, N.; Valsecchi, R.; Villa, A.; Cavallaro, U.; Pattini, L.; et al. PML promotes metastasis of triple-negative breast cancer through transcriptional regulation of HIF1A target genes. JCI Insight 2017, 2, e87380. [Google Scholar] [CrossRef]
- Chen, H.; Pazicni, S.; Krett, N.L.; Ahn, R.W.; Penner-Hahn, J.E.; Rosen, S.T.; O’Halloran, T.V. Coencapsulation of Arsenic- and Platinum-based Drugs for Targeted Cancer Treatment. Angew. Chem. 2009, 121, 9459–9463. [Google Scholar] [CrossRef] [Green Version]
- Miodragović, Ð.; Swindell, E.P.; Sattar Waxali, Z.; Bogachkov, A.; O’Halloran, T.V. Beyond cisplatin: Combination therapy with arsenic trioxide. Inorg. Chim. Acta 2019, 496, 119030. [Google Scholar] [CrossRef] [PubMed]
- Miodragović, Ð.U.; Quentzel, J.A.; Kurutz, J.W.; Stern, C.L.; Ahn, R.W.; Kandela, I.; Mazar, A.; O’Halloran, T.V. Robust Structure and Reactivity of Aqueous Arsenous Acid—Platinum(II) Anticancer Complexes. Angew. Chem. Int. Ed. 2013, 52, 10749–10752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miodragović, Đ.; Merlino, A.; Swindell, E.P.; Bogachkov, A.; Ahn, R.W.; Abuhadba, S.; Ferraro, G.; Marzo, T.; Mazar, A.P.; Messori, L.; et al. Arsenoplatin-1 Is a Dual Pharmacophore Anticancer Agent. J. Am. Chem. Soc. 2019, 141, 6453–6457. [Google Scholar] [CrossRef] [PubMed]
- Cirri, D.; Pillozzi, S.; Gabbiani, C.; Tricomi, J.; Bartoli, G.; Stefanini, M.; Michelucci, E.; Arcangeli, A.; Messori, L.; Marzo, T. PtI2(DACH), the iodido analogue of oxaliplatin as a candidate for colorectal cancer treatment: Chemical and biological features. Dalton Trans. 2017, 46, 3311–3317. [Google Scholar] [CrossRef]
- Marzo, T.; Pillozzi, S.; Hrabina, O.; Kasparkova, J.; Brabec, V.; Arcangeli, A.; Bartoli, G.; Severi, M.; Lunghi, A.; Totti, F.; et al. cis-Pt. I2(NH3)2: A reappraisal. Dalton Trans. 2015, 44, 14896–14905. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, D.; Platella, C.; Riccardi, C.; Merlino, A.; Marzo, T.; Massai, L.; Messori, L.; Montesarchio, D. A first-in-class and a fished out anticancer platinum compound: Cis-[PtCl2(NH3)2] and cis-[PtI2(NH3)2] compared for their reactivity towards DNA model systems. Dalton Trans. 2016, 45, 8587–8600. [Google Scholar] [CrossRef]
- Cleare, M.J.; Hoeschele, J.D. Studies on the antitumor activity of group VIII transition metal complexes. Part, I. Platinum (II) complexes. Bioinorg. Chem. 1973, 2, 187–210. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. Synthetic methods for the preparation of platinum anticancer complexes. Chem. Rev. 2014, 114, 4470–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pažout, R.; Housková, J.; Dušek, M.; Maixner, J.; Kačer, P. Platinum precursor of anticancer drug: A structure fixed by long intermolecular N–H···I and C–H···I hydrogen bonds. Struct. Chem. 2011, 22, 1325–1330. [Google Scholar] [CrossRef]
- Naka, K.; Kato, T.; Watase, S.; Matsukawa, K. Organic Vapor Triggered Repeatable On-Off Crystalline-State Luminescence Switching. Inorg. Chem. 2012, 51, 4420–4422. [Google Scholar] [CrossRef] [PubMed]
- Bouché, M.; Dahm, G.; Maisse-François, A.; Achard, T.; Bellemin-Laponnaz, S. Selective Formation of cis-N-Heterocyclic Carbene-PtII-Pnictogen Complexes and in vitro Evaluation of Their Cytotoxic Activities toward Cancer Cells. Eur. J. Inorg. Chem. 2016, 2016, 2828–2836. [Google Scholar] [CrossRef]
- Miklášová, N.; Fischer-Fodor, E.; Lönnecke, P.; Tomuleasa, C.I.; Virag, P.; Schrepler, M.P.; Mikláš, R.; Dumitrescu, L.S.; Hey-Hawkins, E. Antiproliferative effect of novel platinum(II) and palladium(II) complexes on hepatic tumor stem cells in vitro. Eur. J. Med. Chem. 2012, 49, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.K.; Guerney, P.J.; Goodwin, H.J.; McPartlin, M. Formation of platinum(II) compounds containing isomeric σ-vinylic and σ-allylic six-membered chelate rings by deprotonation of the co-ordinated isopropenyl group: X-ray structure of the σ-vinylic complex di-µ-acetato-bis[o-isopropenylphenyl(diphenyl)arsineplatinum(II)]. J. Chem. Soc. Chem. Commun. 1978, 20, 861–863. [Google Scholar]
- Mitsuo, K.; Kozo, K. Molecular Structure of Acetamide as Studied by Gas Electron Diffraction. Bull. Chem. Soc. Jpn. 1973, 46, 3048–3051. [Google Scholar]
- Bahfenne, S.; Frost, R.L. A Review of the Vibrational Spectroscopic Studies of Arsenite, Antimonite, and Antimonate Minerals. Appl. Spectrosc. Rev. 2010, 45, 101–129. [Google Scholar] [CrossRef] [Green Version]
- Vitnik, V.D.; Vitnik, Ž.J.; Banjac, N.R.; Valentić, V.; Ušćumlić, G.S.; Juranic, I.O. Quantum mechanical and spectroscopic (FT-IR, 13C, 1H NMR and UV) investigations of potent antiepileptic drug 1-(4-chloro-phenyl)-3-phenyl-succinimide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 42–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, M.P.; Uvdal, P. New Scale Factors for Harmonic Vibrational Frequencies Using the B3LYP Density Functional Method with the Triple-ζ Basis Set 6-311+G.(d,p). J. Phys. Chem. A 2005, 109, 2937–2941. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Fernandez, J.R.; Eckhardt, B.L.; Lee, J.; Lim, B.; Pearson, T.; Seitz, R.S.; Hout, D.R.; Schweitzer, B.L.; Nielsen, T.J.; Lawrence, O.R.; et al. Identification of triple-negative breast cancer cell lines classified under the same molecular subtype using different molecular characterization techniques: Implications for translational research. PLoS ONE 2020, 15, e0231953. [Google Scholar] [CrossRef]
- Malavia, N.; Kuche, K.; Ghadi, R.; Jain, S. A bird’s eye view of the advanced approaches and strategies for overshadowing triple negative breast cancer. J. Control. Release 2020, 330, 72–100. [Google Scholar] [CrossRef]
- Vranic, S.; Gatalica, Z.; Wang, Z.Y. Update on the molecular profile of the MDA-MB-453 cell line as a model for apocrine breast carcinoma studies. Oncol. Lett. 2011, 2, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Moore, N.L.; Buchanan, G.; Harris, J.M.; Selth, L.A.; Bianco-Miotto, T.; Hanson, A.R.; Birrell, S.N.; Butler, L.M.; Hickey, T.E.; Tilley, W.D. An androgen receptor mutation in the MDA-MB-453 cell line model of molecular apocrine breast cancer compromises receptor activity. Endocr. Relat. Cancer 2012, 19, 599–613. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Buchanan, G.; Harris, J.M.; Selth, L.A.; Bianco-Miotto, T.; Hanson, A.R.; Birrell, S.N.; Butler, L.M.; Hickey, T.E.; Tilley, W.D. Molecular characterization of breast cancer cell lines through multiple omic approaches. Breast Cancer Res. 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, A.; Rahmanto, A.S.; Johansson, H.; Franco, M.; Viiliäinen, J.; Gazi, M.; Frings, O.; Fredlund, E.; Spruck, C.; Lehtiö, J.; et al. PTEN and DNA-PK determine sensitivity and recovery in response to WEE1 inhibition in human breast cancer. eLife 2020, 9, e57894. [Google Scholar] [CrossRef]
- Qu, Y.; Han, B.; Yu, Y.; Yao, W.; Bose, S.; Karlan, B.Y.; Giuliano, A.E.; Cui, X. Evaluation of MCF10A as a Reliable Model for Normal Human Mammary Epithelial Cells. PLoS ONE 2015, 10, e0131285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Liu, H.; Zhou, H.; Zhu, X.; Zhao, Z.; Chi, X.; Shan, H.; Gao, J. A facile route to core-shell nanoparticulate formation of arsenic trioxide for effective solid tumor treatment. Nanoscale 2016, 8, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Rahaman, H.; Ghosh, S.K.; Sengupta, M. Synthesis and Characterization of Arsenic(III) Oxide Nanoparticles as Potent Inhibitors of MCF 7 Cell Proliferation through Proapoptotic Mechanism. BioNanoScience 2020, 10, 420–429. [Google Scholar] [CrossRef]
- Ahn, R.W.; Barrett, S.L.; Raja, M.R.; Jozefik, J.K.; Spaho, L.; Chen, H.; Bally, M.B.; Mazar, A.P.; Avram, M.J.; Winter, J.N.; et al. Nano-Encapsulation of Arsenic Trioxide Enhances Efficacy against Murine Lymphoma Model while Minimizing Its Impact on Ovarian Reserve In Vitro and In Vivo. PLoS ONE 2013, 8, e58491. [Google Scholar] [CrossRef]
- Fei, W.; Li, C.; Tao, J.; Wendong, X.C.; Yiqing, Y.; Zhang, Y.; Yao, Y.; Song, Q.; Li, F.; Zheng, C. Construction of arsenic-metal complexes loaded nanodrugs for solid tumor therapy: A mini review. Int. J. Pharm. 2020, 583, 119385. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, X.; Wang, P.; Wu, Q.; Qi, X.; Han, X.; Chen, L.; Meng, X.; Xu, K. Delivery of Arsenic Trioxide by Multifunction Nanoparticles To Improve the Treatment of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 8016–8029. [Google Scholar] [CrossRef]
- Ahn, R.W.; Chen, F.; Chen, H.; Stern, S.T.; Clogston, J.D.; Patri, A.K.; Raja, M.R.; Swindell, E.P.; Parimi, V.; Cryns, V.L.; et al. A novel nanoparticulate formulation of arsenic trioxide with enhanced therapeutic efficacy in a murine model of breast cancer. Clin. Cancer Res. 2010, 16, 3607–3617. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, Z.; You, X.Z. Hydrolysis Theory for Cisplatin and Its Analogues Based on Density Functional Studies. J. Am. Chem. Soc. 2001, 123, 9378–9387. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Deubel, D.V. Hydrolysis of the Anticancer Drug Cisplatin: Pitfalls in the Interpretation of Quantum Chemical Calculations. J. Chem. Theory Comput. 2006, 2, 103–106. [Google Scholar] [CrossRef]
- Lucas, M.F.A.; Pavelka, M.; Alberto, M.E.; Russo, N. Neutral and Acidic Hydrolysis Reactions of the Third Generation Anticancer Drug Oxaliplatin. J. Phys. Chem. B 2009, 113, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.; Lucas, M.F.; Russo, N. On the hydrolysis mechanism of the second-generation anticancer drug carboplatin. Chemistry 2007, 13, 10108–10116. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D. Perspective on “Density-functional thermochemistry. III. The role of exact exchange”. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Marino, T.; Parise, A.; Russo, N. The role of arsenic in the hydrolysis and DNA metalation processes in an arsenous acid—platinum(ii) anticancer complex. Phys. Chem. Chem. Phys. 2017, 19, 1328–1334. [Google Scholar] [CrossRef]
- Tolbatov, I.; Coletti, C.; Marrone, A.; Re, N. Reactivity of arsenoplatin complex versus water and thiocyanate: A DFT benchmark study. Theor. Chem. Acc. 2020, 139, 1–11. [Google Scholar] [CrossRef]
- Carloni, P.; Sprik, M.; Andreoni, W. Key Steps of the cis-Platin-DNA Interaction: Density Functional Theory-Based Molecular Dynamics Simulations. J. Phys. Chem. B 2000, 104, 823–835. [Google Scholar] [CrossRef]
- Robertazzi, A.; Platts, A.J. Hydrogen bonding, solvation, and hydrolysis of cisplatin: A theoretical study. J. Comput. Chem. 2004, 25, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Sengupta, P.S.; Mukherjee, A.K. A detailed theoretical DFT study of the hydrolysis mechanism of orally active anticancer drug ZD0473. Chem. Phys. Lett. 2010, 487, 108–115. [Google Scholar] [CrossRef]
- Banerjee, S.; Sengupta, P.S.; Mukherjee, A.K. trans-Platinum anticancer drug AMD443: A detailed theoretical study by DFT–TST method on the hydrolysis mechanism. Chem. Phys. Lett. 2010, 497, 142–148. [Google Scholar] [CrossRef]
- Zhu, C.; Raber, J.; Eriksson, L.A. Hydrolysis Process of the Second Generation Platinum-Based Anticancer Drug cis-Amminedichlorocyclohexylamineplatinum(II). J. Phys. Chem. B 2005, 109, 12195–12205. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; Lucas, M.F.A.; Pavelka, M.; Russo, N. The second-generation anticancer drug Nedaplatin: A theoretical investigation on the hydrolysis mechanism. J. Phys. Chem. B 2009, 113, 14473–14479. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A.J. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Kuznik, N.; Wendt, O.F. The trans effect and trans influence of triphenyl arsine in platinum(ii) complexes. A comparative mechanistic and structural study. J. Chem. Soc. Dalton Trans. 2002, 3074–3078. [Google Scholar] [CrossRef]
- Chval, Z.; Sip, M.; Burda, J.V. The trans effect in square-planar platinum(II) complexes—A density functional study. J. Comput. Chem. 2008, 29, 2370–2381. [Google Scholar] [CrossRef]
- Michera, L.; Nekadová, M.; Burda, J.V. Reactions of cisplatin and glycine in solution with constant pH: A computational study. Phys. Chem. Chem. Phys. 2012, 14, 12571–12579. [Google Scholar] [CrossRef]
- Ponce-Vargas, M.; Klein, J.; Hénon, E. Novel approach to accurately predict bond strength and ligand lability in platinum-based anticancer drugs. Dalton Trans. 2020, 49, 12632–12642. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Huang, H.; Liu, N.; Liao, Y.; Liu, N.; Cai, J.; Xia, X.; Guo, Z.; Li, Y.; Wen, Q.; Yin, Q.; et al. Platinum-containing compound platinum pyrithione suppresses ovarian tumor proliferation through proteasome inhibition. J. Exp. Clin. Cancer Res. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, J.; Millam, T. GaussView; Version 5.0.9; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, T. Natural bond orbital method. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]


| MDA-MB-231 | MDA-MB-453 | MDA-MB-468 | MCF-12A | |
|---|---|---|---|---|
| AP-5 | 14.5 ± 2.52 | 12.70 ± 2.52 | 2.11 ± 1.89 | 15.47 ± 2.87 |
| AP-4 | 17.16 ± 2.55 | 13.23 ± 2.65 | 1.70 ± 0.76 | 25.02 ± 2.14 (**) |
| AP-1 | 16.94 ± 3.70 | 12.02 ± 1.34 | 1.88 ± 0.88 | 23.43 ± 6.62 |
| AP-2 | 18.31 ± 3.59 | 13.75 ± 3.26 | 2.05 ± 0.87 | 23.84 ± 4.12 |
| As(OH)3 | 26.49 ± 4.88 (**) | 15.63 ± 1.70 | 3.90 ± 0.21 (**) | 27.25 ± 3.46 (*) |
| Cisplatin | 44.7 ± 4.63 (***) | 20.87 ± 0.85 (**) | 2.26 ± 0.33 | 19.42 ± 6.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miodragović, Ð.; Qiang, W.; Sattar Waxali, Z.; Vitnik, Ž.; Vitnik, V.; Yang, Y.; Farrell, A.; Martin, M.; Ren, J.; O’Halloran, T.V. Iodide Analogs of Arsenoplatins—Potential Drug Candidates for Triple Negative Breast Cancers. Molecules 2021, 26, 5421. https://doi.org/10.3390/molecules26175421
Miodragović Ð, Qiang W, Sattar Waxali Z, Vitnik Ž, Vitnik V, Yang Y, Farrell A, Martin M, Ren J, O’Halloran TV. Iodide Analogs of Arsenoplatins—Potential Drug Candidates for Triple Negative Breast Cancers. Molecules. 2021; 26(17):5421. https://doi.org/10.3390/molecules26175421
Chicago/Turabian StyleMiodragović, Ðenana, Wenan Qiang, Zohra Sattar Waxali, Željko Vitnik, Vesna Vitnik, Yi Yang, Annie Farrell, Matthew Martin, Justin Ren, and Thomas V. O’Halloran. 2021. "Iodide Analogs of Arsenoplatins—Potential Drug Candidates for Triple Negative Breast Cancers" Molecules 26, no. 17: 5421. https://doi.org/10.3390/molecules26175421
APA StyleMiodragović, Ð., Qiang, W., Sattar Waxali, Z., Vitnik, Ž., Vitnik, V., Yang, Y., Farrell, A., Martin, M., Ren, J., & O’Halloran, T. V. (2021). Iodide Analogs of Arsenoplatins—Potential Drug Candidates for Triple Negative Breast Cancers. Molecules, 26(17), 5421. https://doi.org/10.3390/molecules26175421







