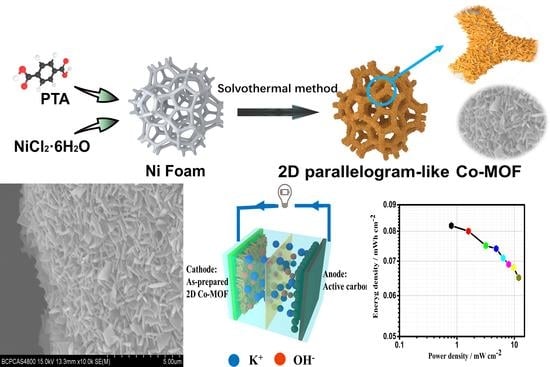
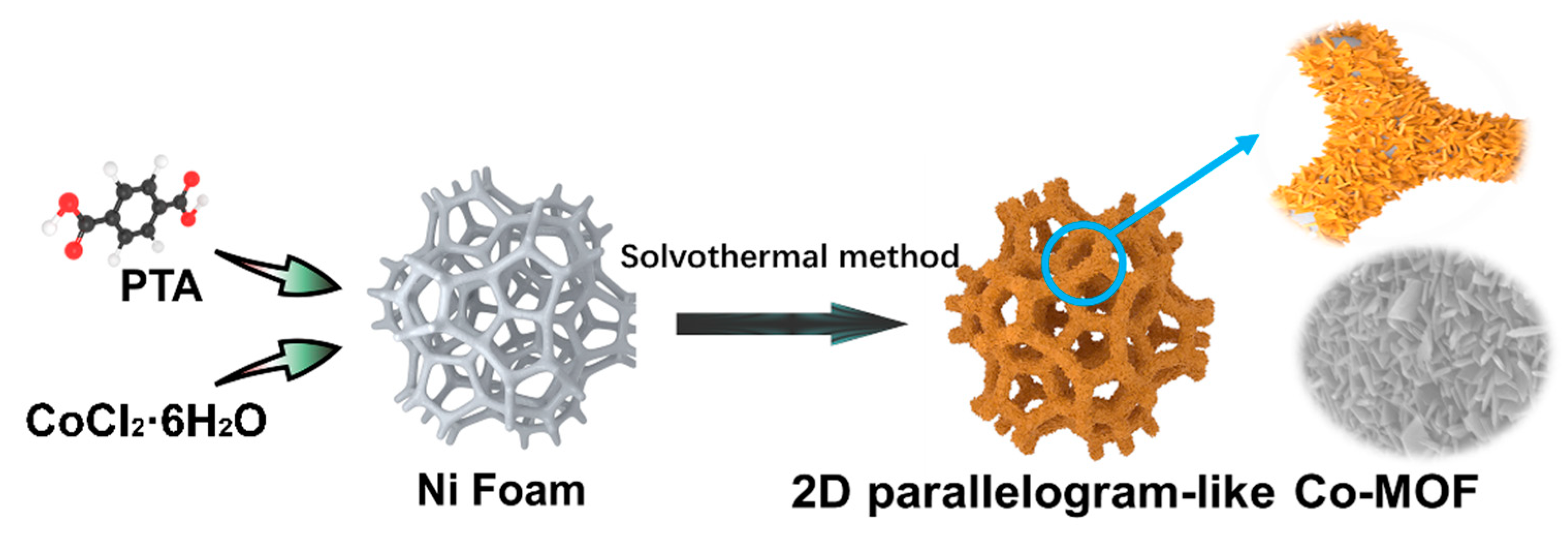
Fabrication of Vertical-Standing Co-MOF Nanoarrays with 2D Parallelogram-like Morphology for Aqueous Asymmetric Electrochemical Capacitors
Abstract
:1. Introduction
2. Results and Discussion
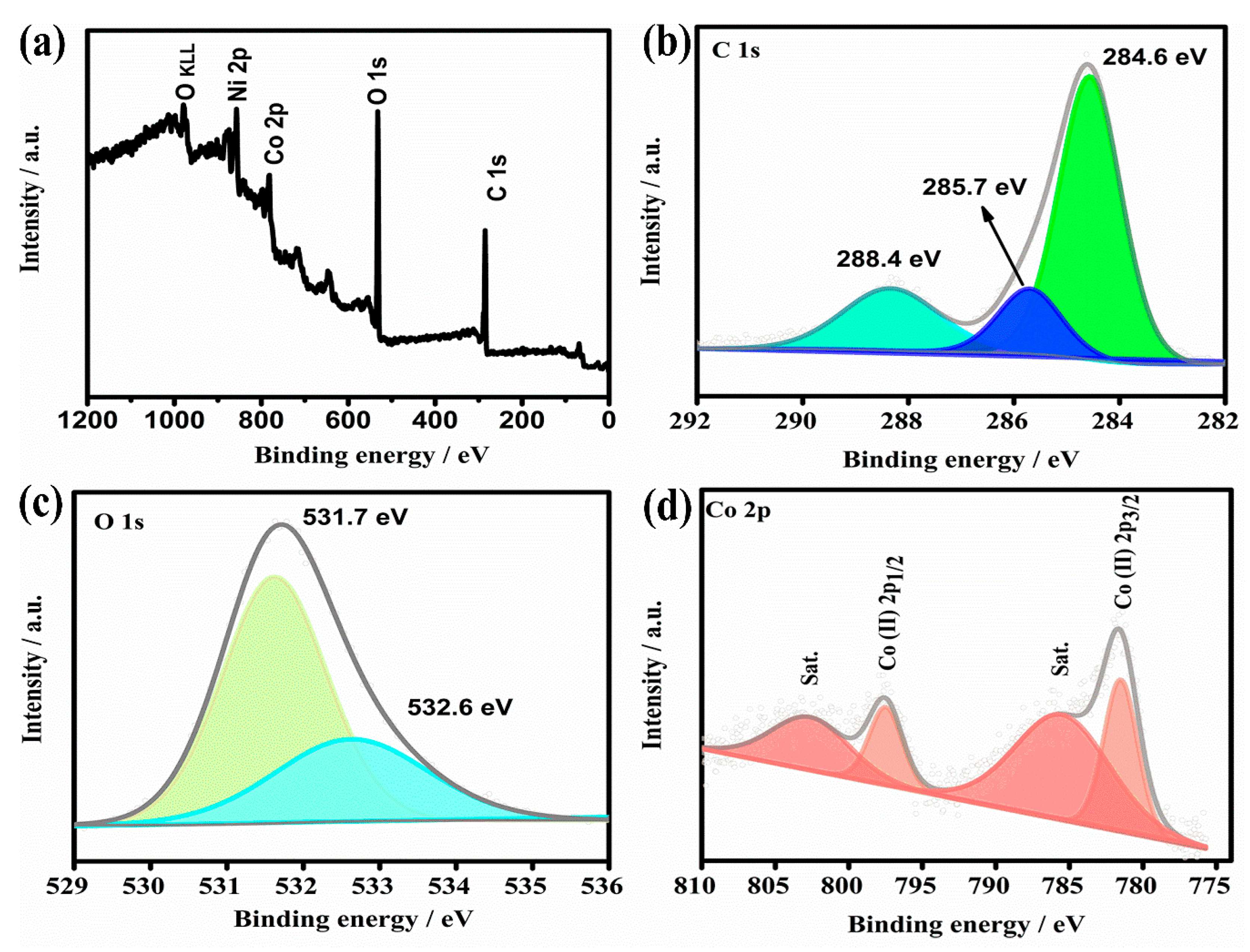
2.1. Structure Characterization of As-Prepared Co-MOF on Ni Foam
2.2. Morphological Characterization of As-Prepared Co-MOF on Ni Foam
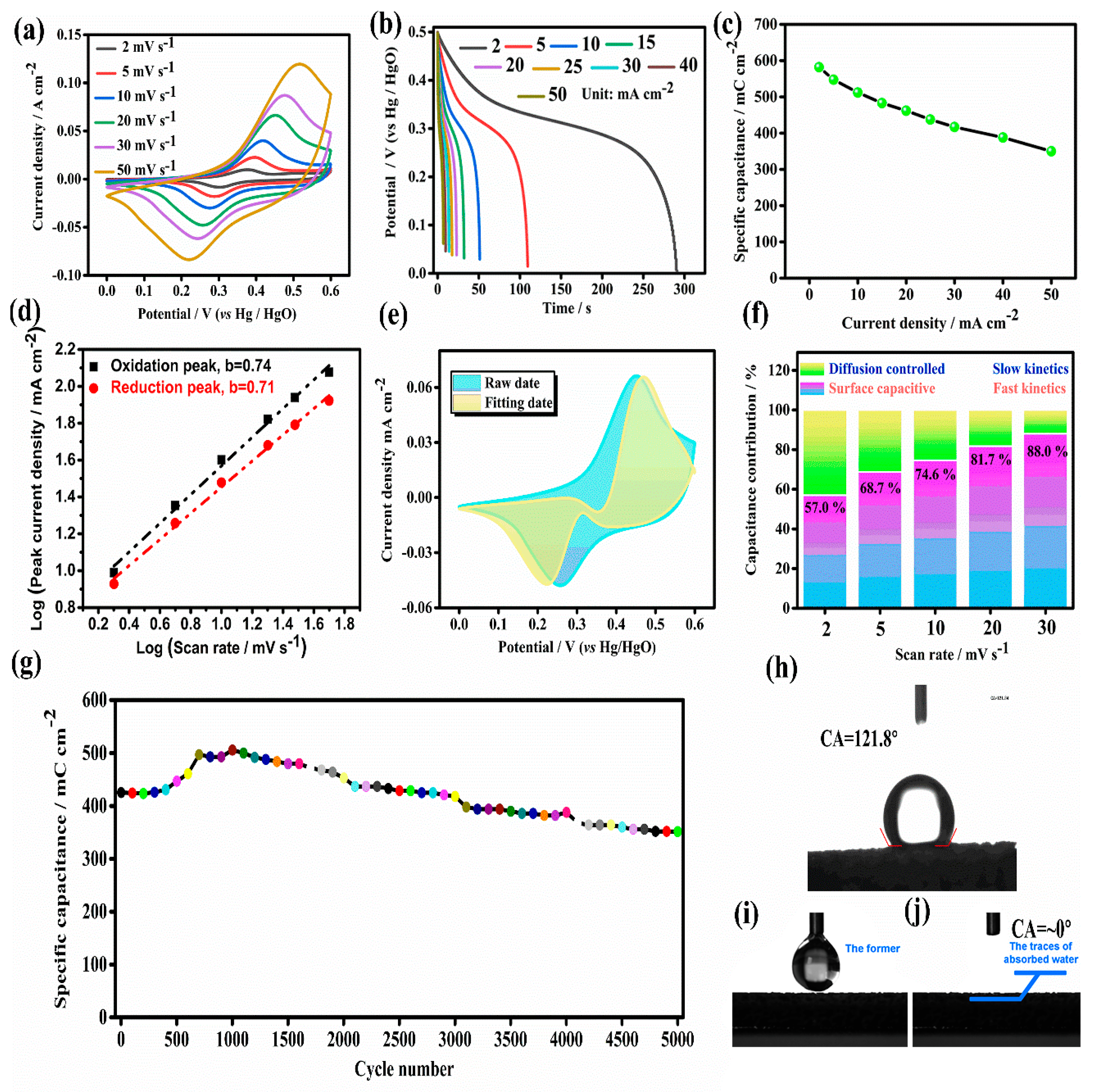
2.3. Electrochemical Capacitive Perforamcne of As-Prepared Co-MOF on Ni Foam
2.4. Electrochemical Perforamcne of AEC Device
3. Materials and Methods
3.1. Materials
3.2. Preparation of Co-MOF on Ni Foam
3.3. Physical Characterization
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, N.; Bai, X.; Pan, D.; Dong, B.; Wei, R.; Naik, N.; Patil, R.; Guo, Z. Recent advances of asymmetric supercapacitors. Adv. Mater. Interfaces 2021, 8, 2001710. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, Y.; Yu, M.; Liu, P. Recent smart methods for achieving high-energy asymmetric supercapacitors. Small 2018, 2, 1700230. [Google Scholar] [CrossRef]
- Sun, J.; Wu, C.; Sun, X.; Hu, H.; Zhi, C.; Hou, L.; Yuan, C. Recent progresses in high-energy-density all pseudocapacitive-electrode-materials-based asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 9443–9464. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.; Mirzaeian, M.; Fletcher, S.; Sillars, F.; Rennie, A.; Shitta-Bey, G.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano. Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Evanko, B.; Boettcher, S.; Yoo, S.; Stucky, G. Redox-enhanced electrochemical capacitors: Status, opportunity, and best practices for performance evaluation. ACS Energy Lett. 2017, 2, 2518–2590. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Ding, B.; Wang, Y.; Hao, X.; Dou, H.; Xia, Y.; Zhang, X. Pseudocapacitive materials for electrochemical capacitors: From rational synthesis to capacitance optimization. Nat. Sci. Rev. 2017, 4, 71–90. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, W.; Wang, D.; Li, F.; Liu, B.; Cheng, H. High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano 2010, 4, 5835–5842. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, H.; Cheng, Q.; Wang, G.; Petr, S.; Li, C. Amorphous vanadium oxides with metallic character for asymmetric supercapacitors. Chem. Eng. J. 2021, 403, 126380. [Google Scholar]
- Zhang, H.; Lin, L.; Wu, B.; Hu, N. Vertical carbon skeleton introduced three-dimensional MnO2 nanostructured composite electrodes for high-performance asymmetric supercapacitors. J. Power Sources 2020, 476, 228527. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Li, B.; Xue, H.; Pang, H. Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors. J. Mater. Chem. A 2017, 5, 8155–8186. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Yu, F.; Zhao, X.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Wang, C.; Li, H.; Deng, X.; Xu, X.; Zhai, T. Ultrathin and porous Ni3S2/CoNi2S4 3D-network structure for superhigh energy density asymmetric supercapacitors. Adv. Energy Mater. 2017, 7, 1700983. [Google Scholar] [CrossRef]
- Li, S.; Yu, C.; Yang, J.; Zhao, C.; Zhang, M.; Huang, H.; Liu, Z.; Guo, W.; Qiu, J. A superhydrophilic “nanoglue” for stabilizing metal hydroxides onto carbon materials for high-energy and ultralong-life asymmetric supercapacitors. Energy. Environ. Sci. 2017, 10, 1958–1965. [Google Scholar] [CrossRef]
- Li, X.; Elshahawy, A.; Guan, C.; Wang, J. Metal phosphides and phosphates-based electrodes for electrochemical supercapacitors. Small 2017, 13, 1701530. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xia, C.; Jiang, Q.; Gandi, A.; Schwingenschlögl, U.; Alshareef, H. Low temperature synthesis of ternary metal phosphides using plasma for asymmetric supercapacitors. Nano Energy 2017, 35, 331–340. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, A.; Wang, X.; Tian, C.; An, R.; Fu, H. Porous NiCoP nanosheets as efficient and stable positive electrodes for advanced asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 17905–17914. [Google Scholar] [CrossRef]
- Gu, Y.; Fan, L.; Huang, J.; Geng, C.; Lin, J.; Huang, M.; Huang, Y.; Wu, J. N-doped reduced graphene oxide decorated NiSe2 nanoparticles for high-performance asymmetric supercapacitors. J. Power. Sources 2019, 425, 60–68. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Ma, Z.; Zhu, T.; Liu, L.; Zheng, J.; Gong, X. All-solid-state asymmetric supercapacitors with metal selenides electrodes and ionic conductive composites electrolytes. Adv. Funct. Mater. 2019, 29, 1904182. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Li, S.; Urbankowski, P.; Li, J.; Xu, Y. Gogotsi. An ultrafast conducting polymer@MXene positive electrode with high volumetric capacitance for advanced asymmetric supercapacitors. Small 2020, 16, 1906851. [Google Scholar] [CrossRef]
- Snook, G.; Kao, P.; Best, A. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Li, K.; Wang, X.; Wang, X.; Liang, M.; Nicolosi, V.; Xu, Y.; Gogotsi, Y. All-pseudocapacitive asymmetric MXene-carbon-conducting polymer supercapacitors. Nano Energy 2020, 75, 104971. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, Y.; Wang, C.; Guo, L. NiCo-MOF nanosheets wrapping polypyrrole nanotubes for high-performance supercapacitors. Appl. Surf. Sci. 2020, 507, 145089. [Google Scholar] [CrossRef]
- Dai, F.; Wang, X.; Zheng, S.; Sun, J.; Huang, Z.; Xu, B.; Fan, L.; Wang, R.; Sun, D.; Wu, Z. Toward high-performance and flexible all-solid-state micro-supercapacitors: MOF bulk vs. MOF nanosheets. Chem. Eng. J. 2021, 413, 127520. [Google Scholar] [CrossRef]
- Feng, C.; Lv, C.; Zhao, H.; Li, Z.; Xie, W.; Sun, L.; Wang, Y. Structural elucidation and supercapacitive performance on a Mn (II)-based MOF. Cryst. Growth Des. 2020, 20, 5682–5687. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Lu, W.; Lei, D.; Tian, Y.; Guo, M.; Mi, P.; Qu, N.; Zhao, Y. MXenes induced formation of Ni-MOF microbelts for high-performance supercapacitors. J. Colloid. Interf. Sci. 2021, 592, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Q.; Long, Y.; Wang, F.; Wu, L.; Pan, J.; Han, J.; Lei, Y.; Shi, W.; Song, S. In situ formation of Co9S8 quantum dots in MOF-derived ternary metal layered double hydroxide nanoarrays for high-performance hybrid supercapacitors. Adv. Energy Mater. 2020, 10, 1903193. [Google Scholar] [CrossRef]
- Gu, J.; Sun, L.; Zhang, Y.; Zhang, Q.; Li, X.; Si, H.; Shi, Y.; Sun, C.; Gong, Y.; Zhang, Y. MOF-derived Ni-doped CoP@C grown on CNTs for high-performance supercapacitors. Chem. Eng. J. 2020, 385, 123454. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Huang, J.; Li, D.; Liu, J.; Xu, Y.; Ke, H.; Wei, Q. “One for two” strategy to prepare MOF-derived NiCo2S4 nanorods grown on carbon cloth for high-performance asymmetric supercapacitors and efficient oxygen evolution reaction. Electrochim. Acta 2020, 334, 135636. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.; Elias, J.; Sun, C.; Shao-Horn, Y.; Dincă, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Azadfalah, M.; Sedghi, A.; Hosseini, H.; Kashani, H. Cobalt based metal organic framework/graphene nanocomposite as high performance battery-type electrode materials for asymmetric supercapacitors. J. Energy Storage 2021, 33, 101925. [Google Scholar] [CrossRef]
- Xu, S.; Liu, R.; Shi, X.; Ma, Y.; Hong, M.; Chen, X.; Wang, T.; Li, F.; Hu, N.; Yang, Z. A dual CoNi MOF nanosheet/nanotube assembled on carbon cloth for high performance hybrid supercapacitors. Electrochim. Acta 2020, 342, 136124. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, H.; Ma, M.; Wang, W.; Yang, L.; Wang, L.; Shi, X.; Cheng, X.; Sun, X.; Yao, Y. A self-supported hierarchical Co-MOF as a supercapacitor electrode with ultrahigh areal capacitance and excellent rate performance. Chem. Commun. 2018, 54, 10499–10502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, C.; Liu, Y.; Li, D.; Chen, W.; Ma, Y.; Wang, C.; Zhang, J. Electrochemical performance and transformation of Co-MOF/reduced graphene oxide composite. Mater. Lett. 2017, 193, 26–219. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, N.; Yang, S.; Lei, D.; Liu, A.; Zhou, Q. Cobalt induced growth of hollow MOF spheres for high performance supercapacitors. Mater. Chem. Front. 2021, 5, 482–491. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, H.; Liu, W.; Li, Y.; Zhang, J.; Hou, H.; Yang, J. Ultrathin NiCo-MOF nanosheets for high-performance supercapacitor electrodes. ACS Appl. Energy Mater. 2019, 2, 2063–2071. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. Wire spherical-shaped Co-MOF electrode materials for high-performance all-solid-state flexible asymmetric supercapacitor device. J. Alloy. Comp. 2021, 879, 160423. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Yu, L.; Liu, X.; Yang, J.; Wei, D. A new promising Ni-MOF superstructure for high-performance supercapacitors. Chem. Commun. 2020, 56, 1803–1806. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. A new straightforward uncalcined approach for morphology modulating to enhance the electrical capacity performance of Co-MOF. Electrochim. Acta 2021, 389, 138684. [Google Scholar] [CrossRef]
- Jia, R.; Zhao, C.; Huang, Z.; Liu, X.; Wang, D.; Hui, Z.; Xu, X. An in situ growth strategy of NiCo-MOF nanosheets with more activity sites for asymmetric supercapacitors. Ionics 2020, 26, 6309–6318. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Xiong, Y.; Cheng, D.; Zeng, Y.; Bu, Y. Fabrication of 3D Co-doped Ni-based MOF hierarchical micro-flowers as a high-performance electrode material for supercapacitors. Appl. Surf. Sci. 2019, 483, 1158–1165. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Z.; Gao, W.; Wei, M. Layered structural Co-based MOF with conductive network frames as a new supercapacitor electrode. Chem. Eur. J. 2017, 23, 631–636. [Google Scholar] [CrossRef]
- Chu, X.; Meng, F.; Deng, T.; Lu, Y.; Bondarchuk, O.; Sui, M.; Feng, M.; Li, H.; Zhang, W. Mechanistic insight into bimetallic CoNi-MOF arrays with enhanced performance for supercapacitors. Nanoscale 2020, 12, 5669–5677. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Xin, J.; Li, B.; Pang, H.; Ma, H.; Li, K.; Xiao, B.; Wang, X.; Tan, L. Mo-Based crystal POMOFs with a high electrochemical capacitor performance. Dalton Trans. 2019, 48, 13026–13033. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal-organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.; Kim, J.; Taberna, P.; Tolbert, S.; Abruña, H.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Brezesinski, T.; Wang, J.; Tolbert, S.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C. 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, H.; Chao, D.; Wang, R.; He, B.; Gong, Y.; Fan, H. Al2O3-assisted confinement synthesis of oxide/carbon hollow composite nanofibers and application in metal-ion capacitors. Small 2020, 16, 2001950. [Google Scholar] [CrossRef]
- Xu, D.; Chao, D.; Wang, H.; Gong, Y.; Wang, R.; He, B.; Hu, X.; Fan, H. Flexible quasi-solid-state sodium-ion capacitors developed using 2D metal-organic-framework array as reactor. Adv. Energy Mater. 2018, 8, 1702769. [Google Scholar] [CrossRef]
- Banerjee, P.; Lobo, D.; Middag, R.; Ng, W.; Shaibani, M.; Majumder, M. Electrochemical capacitance of Ni-doped metal organic framework and reduced graphene oxide composites: More than the sum of its parts. ACS Appl. Mater. Interfaces 2015, 7, 3655–3664. [Google Scholar] [CrossRef]
- Choi, K.; Jeong, H.; Park, J.; Zhang, Y.; Kang, J.; Yaghi, O. Supercapacitors of nanocrystalline metal-organic frameworks. ACS Nano 2014, 8, 7451–7457. [Google Scholar] [CrossRef] [PubMed]



| Electrode | Synthetic Method | Morphology | Electrolyte | Capacitance/Capacity | Reference |
|---|---|---|---|---|---|
| NiCo-MOF | Chemical method | Nanosheets | 2 M KOH | 1109 F g−1 at 0.5 A g−1 | [26] |
| Mn-MOF | Hydrothermal | Crystalline | 6 M KOH | 433 F g−1 at 1 A g−1 | [28] |
| Co-MOF@Graphene | Ultrasonicate and centrifugation | Nanoparticles | 6 M KOH | 549.96 F g−1 at 10 mV s−1 | [36] |
| Co-MOF | Hydrothermal | Nanospheres | 1 M NaOH | 657 F g−1 at 0.5 A g−1 | [40] |
| Co-MOF | Hydrothermal | Microspheres | 1 M LiOH | 553 F g−1 at 1 A g−1 | [42] |
| Co-doped Ni-based MOF | Hydrothermal | Nanoflowers | 6 M KOH | 100 F g−1 at 1 A g−1 | [44] |
| Mo&polyoxometalate-MOF | Hydrothermal | 2D layered microstructure | 1 M H2SO4 | 249.0 F g−1 at 3 A g−1 | [47] |
| MOF-5 | Incipient wetness technique | Nanoparticles | 1 M H2SO4 | 100 F g−1 at 5 mV s−1 | [48] |
| Co-MOF | Hydrothermal | nanoarrays | 2 M KOH | 946 F g−1 at 1.63 A g−1 582.0 mC cm−2 at 2 mA cm−2 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Mi, H.; Jin, Y.; Ren, D.; Zhou, K.; Zhang, Q.; Liu, J.; Wang, H. Fabrication of Vertical-Standing Co-MOF Nanoarrays with 2D Parallelogram-like Morphology for Aqueous Asymmetric Electrochemical Capacitors. Molecules 2021, 26, 5394. https://doi.org/10.3390/molecules26175394
Li L, Mi H, Jin Y, Ren D, Zhou K, Zhang Q, Liu J, Wang H. Fabrication of Vertical-Standing Co-MOF Nanoarrays with 2D Parallelogram-like Morphology for Aqueous Asymmetric Electrochemical Capacitors. Molecules. 2021; 26(17):5394. https://doi.org/10.3390/molecules26175394
Chicago/Turabian StyleLi, Leyuan, Hongtian Mi, Yuhong Jin, Dayong Ren, Kailing Zhou, Qianqian Zhang, Jingbing Liu, and Hao Wang. 2021. "Fabrication of Vertical-Standing Co-MOF Nanoarrays with 2D Parallelogram-like Morphology for Aqueous Asymmetric Electrochemical Capacitors" Molecules 26, no. 17: 5394. https://doi.org/10.3390/molecules26175394
APA StyleLi, L., Mi, H., Jin, Y., Ren, D., Zhou, K., Zhang, Q., Liu, J., & Wang, H. (2021). Fabrication of Vertical-Standing Co-MOF Nanoarrays with 2D Parallelogram-like Morphology for Aqueous Asymmetric Electrochemical Capacitors. Molecules, 26(17), 5394. https://doi.org/10.3390/molecules26175394