Anthocyanin Color Stabilization by Host-Guest Complexation with p-Sulfonatocalix[n]arenes
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. J. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.A.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937. [Google Scholar] [CrossRef] [Green Version]
- Brouillard, R.; Delaporte, B. Chemistry of anthocyanin pigments. 2. Kinetic and thermodynamic study of proton transfer, hydration, and tautomeric reactions of malvidin 3-glucoside. J. Am. Chem. Soc. 1977, 99, 8461. [Google Scholar] [CrossRef]
- Jurd, L. Anthocyanins and related compounds. I. Structural transformations of flavylium salts in acidic solutions. J. Org. Chem. 1963, 28, 987. [Google Scholar] [CrossRef]
- Mendoza, J.; Basílio, N.; de Freitas, V.; Pina, F. New procedure to calculate all equilibrium constants in flavylium compounds: Application to the copigmentation of anthocyanins. ACS Omega 2019, 4, 12058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina, F.; Parola, A.; Melo, M.; Lima, J.; De Freitas, V. Chapter 2. Chemistry of Anthocyanins. In Anthocyanins from Natural Sources; En Brooks, M., Celli, G., Eds.; Royal Society of Chemistry: London, UK, 2019; pp. 34–76. [Google Scholar]
- Ju, Y.L.; Yang, B.H.; He, S.; Tu, T.Y.; Min, Z.; Fang, Y.L.; Sun, X.Y. Anthocyanin accumulation and biosynthesis are modulated by regulated deficit irrigation in Cabernet Sauvignon (Vitis Vinifera L.) grapes and wines. Plant Physiol. Biochem. 2019, 135, 469. [Google Scholar] [CrossRef]
- Basílio, N.; Pina, F. Flavylium Network of Chemical Reactions in Confined Media: Modulation of 3′,4′,7-Trihydroxyflavilium Reactions by Host–Guest Interactions with Cucurbit[7]uril. Chem. Phys. Chem. 2014, 15, 2295. [Google Scholar] [CrossRef]
- Basílio, N.; Cabrita, L.; Pina, F. Mimicking Positive and Negative Copigmentation Effects in Anthocyanin Analogues by Host–Guest Interaction with Cucurbit [7] uril and β-Cyclodextrins. J. Agric. Food Chem. 2015, 63, 7624. [Google Scholar] [CrossRef]
- Cruz, L.; Basílio, N.; de Freitas, V. Color stabilization of cyanidin-3-glucoside-based dyes by encapsulation with biocompatible PEGylated phospholipid micelles. Dyes Pigm. 2020, 181, 108592. [Google Scholar] [CrossRef]
- Araújo, P.; Basílio, N.; Fernandes, A.; Mateus, N.; de Freitas, V.; Pina, F.; Oliveira, J. Impact of Lignosulfonates on the Thermodynamic and Kinetic Parameters of Malvidin-3-O-glucoside in Aqueous Solutions. J. Agric. Food Chem. 2018, 66, 6382. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Basílio, N.; Mendoza, J.; Mateus, N.; de Freitas, V.; Tawara, M.H.; Correa, J.; Fernandez-Megia, E. Impact of a water-soluble Gallic acid-based dendrimer on the color-stabilizing mechanisms of anthocyanins. Chem. Eur. J. 2019, 25, 11696. [Google Scholar] [CrossRef] [PubMed]
- McIldowie, M.; Mocerino, M.; Ogden, M. A brief review of C n-symmetric calixarenes and resorcinarenes. Supramol. Chem. 2010, 22, 13. [Google Scholar] [CrossRef]
- Atwood, J.L.; Dalgarno, S.J.; Hardie, M.J.; Raston, C.L. Selective single crystal complexation of L-or D-leucine by p-sulfonatocalix [6] arene. Chem. Commun. 2005, 3, 337. [Google Scholar] [CrossRef]
- Nau, W.M.; Ghale, G.; Hennig, A.; Bakirci, H.; Bailey, D.M. Substrate-selective supramolecular tandem assays: Monitoring enzyme inhibition of arginase and diamine oxidase by fluorescent dye displacement from calixarene and cucurbituril macrocycles. J. Am. Chem. Soc. 2009, 131, 11558. [Google Scholar] [CrossRef]
- Mutihac, L.; Lee, J.H.; Kim, J.S.; Vicens, J. Recognition of amino acids by functionalized calixarenes. Chem. Soc. Rev. 2011, 40, 2777. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.D.; Raston, C.L. Multifunctional p-phosphonated calixarenes. Chem. Commun. 2011, 47, 9764. [Google Scholar] [CrossRef]
- Paclet, M.-H.; Rousseau, C.F.; Yannick, C.; Morel, F.; Coleman, A.W. An absence of non-specific immune response towards para-sulphonato-calix [n] arenes. J. Inc. Phenom. Macrocycl. Chem. 2006, 55, 353. [Google Scholar] [CrossRef]
- Da Silva, E.; Shahgaldian, P.; Coleman, A.W. Haemolytic properties of some water-soluble para-sulphonato-calix-[n]-arenes. Int. J. Pharm. 2004, 273, 57. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.; Lazar, A.; Coleman, A. Biopharmaceutical applications of calixarenes. J. Drug Del. Sci. Tech. 2004, 14, 3. [Google Scholar] [CrossRef]
- Shinkai, S.; Araki, K.; Tsubaki, T.; Arimura, T.; Manabe, O. New syntheses of calixarene-p-sulphonates and p-nitrocalixarenes. J. Chem. Soc. (Perkin 1) 1987, 1, 2297. [Google Scholar] [CrossRef]
- Wang, G.-S.; Zhang, H.-Y.; Li, D.; Wang, P.-Y.; Liu, Y. Characterisation and antiproliferative activity of irinotecan and sulphonatocalixarene inclusion complex. Supramol. Chem. 2011, 23, 441. [Google Scholar] [CrossRef]
- Wang, K.; Guo, D.-S.; Zhang, H.-Q.; Li, D.; Zheng, X.-L.; Liu, Y. Highly effective binding of viologens by p-sulfonatocalixarenes for the treatment of viologen poisoning. J. Med. Chem. 2009, 52, 6402. [Google Scholar] [CrossRef]
- McGovern, R.E.; Fernandes, H.; Khan, A.R.; Power, N.P.; Crowley, P.B. Protein camouflage in cytochrome c–calixarene complexes. Nat. Chem. 2012, 4, 527. [Google Scholar] [CrossRef] [PubMed]
- Basilio, N.; Gómez, B.; Garcia-Rio, L.; Francisco, V. Using calixarenes to model polyelectrolyte surfactant nucleation sites. Chem. Eur. J. 2013, 19, 4570. [Google Scholar] [CrossRef]
- Harangozó, J.G.; Wintgens, V.; Miskolczy, Z.; Guigner, J.-M.; Amiel, C.; Biczók, L. Effect of macrocycle size on the self-assembly of methylimidazolium surfactant with sulfonatocalix [n] arenes. Langmuir 2016, 32, 10651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basilio, N.; García-Río, L. Sulfonated Calix [6] arene Host–Guest Complexes Induce Surfactant Self-Assembly. Chem. Eur. J. 2009, 15, 9315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y. Macrocycle crosslinked mesoporous polymers for ultrafast separation of organic dyes. Chem. Commun. 2018, 54, 7362. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv. Colloid Interface Sci. 2019, 269, 187. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, S.; Wang, W.; Yuan, Z.; Ravoo, B.J.; Guo, D.-S. Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation. Nat. Chem. 2019, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Baldini, L.; Casnati, A.; Sansone, F.; Ungaro, R. Calixarene-based multivalent ligands. Chem. Soc. Rev. 2007, 36, 254. [Google Scholar] [CrossRef]
- Guo, D.-S.; Wang, K.; Liu, Y. Selective binding behaviors of p-sulfonatocalixarenes in aqueous solution. J. Incl. Phenom. Macrocyc. Chem. 2008, 62, 1. [Google Scholar] [CrossRef]
- Kalyani, V.S.; Malkhede, D.D. p-Sulfonatocalix [8] arene and vitamin C complexation: Assessment of photophysical, pKa and antioxidant property. J. Incl. Phenom. Macrocyc. Chem. 2017, 87, 179. [Google Scholar] [CrossRef]
- Romero, M.A.; Mateus, P.; Matos, B.; Acuna, A.; Garcia-Rio, L.; Arteaga, J.F.; Pischel, U.; Basílio, N. Binding of flavylium ions to sulfonatocalix [4] arene and implication in the photorelease of biologically relevant guests in water. J. Org. Chem. 2019, 84, 10852. [Google Scholar] [CrossRef]
- McClelland, R.A.; McGall, G.H. Hydration of the flavylium ion. 2. The 4′-hydroxyflavylium ion. J. Org. Chem. 1982, 47, 3730. [Google Scholar] [CrossRef]
- Pissarra, J.; Mateus, N.; Rivas-Gonzalo, J.; Santos Buelga, C.; De Freitas, V. Reaction between malvidin 3-glucoside and (+)-catechin in model solutions containing different aldehydes. J. Food Sci. 2003, 68, 476. [Google Scholar] [CrossRef]
- Basilio, N.; Francisco, V.; Garcia-Rio, L. Aggregation of p-sulfonatocalixarene-based amphiphiles and supra-amphiphiles. Int. J. Mol. Sci. 2013, 14, 3140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chawla, H.; Varadarajan, R. A single step preparation of p-sulphonated calixarenes. Indian J. Chem B. 2003, 42, 2863. [Google Scholar]
- Basilio, N.; García-Río, L.; Martin-Pastor, M. Counterion Binding in Solutions of p-Sulfonatocalix [4] arene. J.Phys. Chem. B 2010, 114, 7201. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Azevedo, J.; Teixeira, N.; Araújo, P.; de Freitas, V.; Basílio, N.; Pina, F. On the Limits of Anthocyanins Co-Pigmentation Models and Respective Equations. J. Agric. Food Chem. 2021, 69, 1359. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Meric, R.; Vigneron, J.-P.; Cesario, M.; Guilhem, J.; Pascard, C.; Asfari, Z.; Vicens, J. Binding of acetylcholine and other quaternary ammonium cations by sulfonated calixarenes. Crystal structure of a [choline-tetrasulfonated calix [4] arene] complex. Supramol. Chem. 1995, 5, 97. [Google Scholar] [CrossRef]
- Wang, K.; Yang, E.-C.; Zhao, X.-J.; Dou, H.-X.; Liu, Y. Molecular binding behaviors of sulfonated calixarenes with phenanthroline-diium in aqueous solution and solid state: Cavity size governing capsule formation. Cryst. Growth Des. 2014, 14, 4631. [Google Scholar] [CrossRef]
- Leydet, Y.; Gavara, R.; Petrov, V.; Diniz, A.M.; Parola, A.J.; Lima, J.C.; Pina, F. The effect of self-aggregation on the determination of the kinetic and thermodynamic constants of the network of chemical reactions in 3-glucoside anthocyanins. Phytochemistry 2012, 83, 125. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R.; Delaporte, B.; Dubois, J.E. Chemistry of anthocyanin pigments. 3. Relaxation amplitudes in pH-jump experiments. J. Am. Chem. Soc. 1978, 100, 6202. [Google Scholar] [CrossRef]

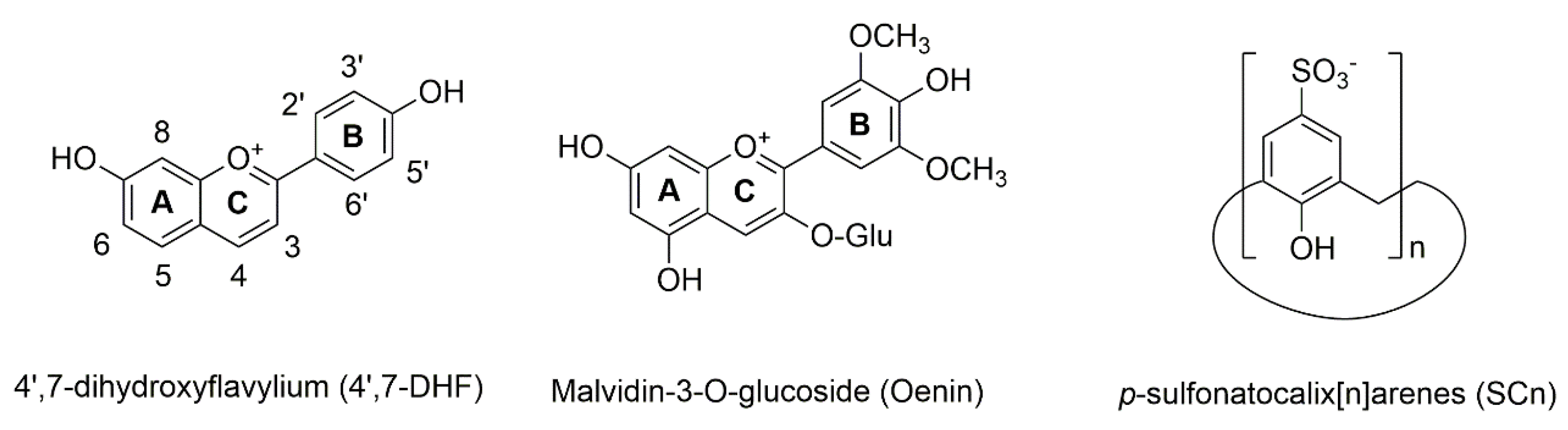
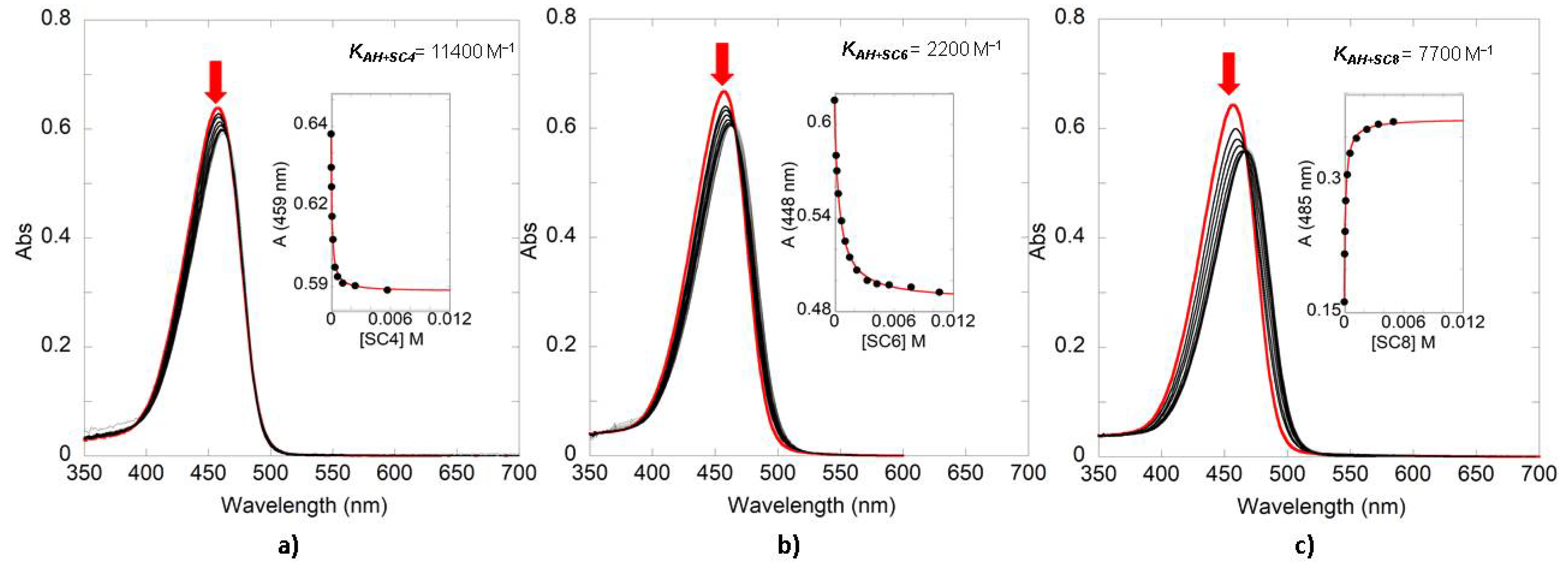
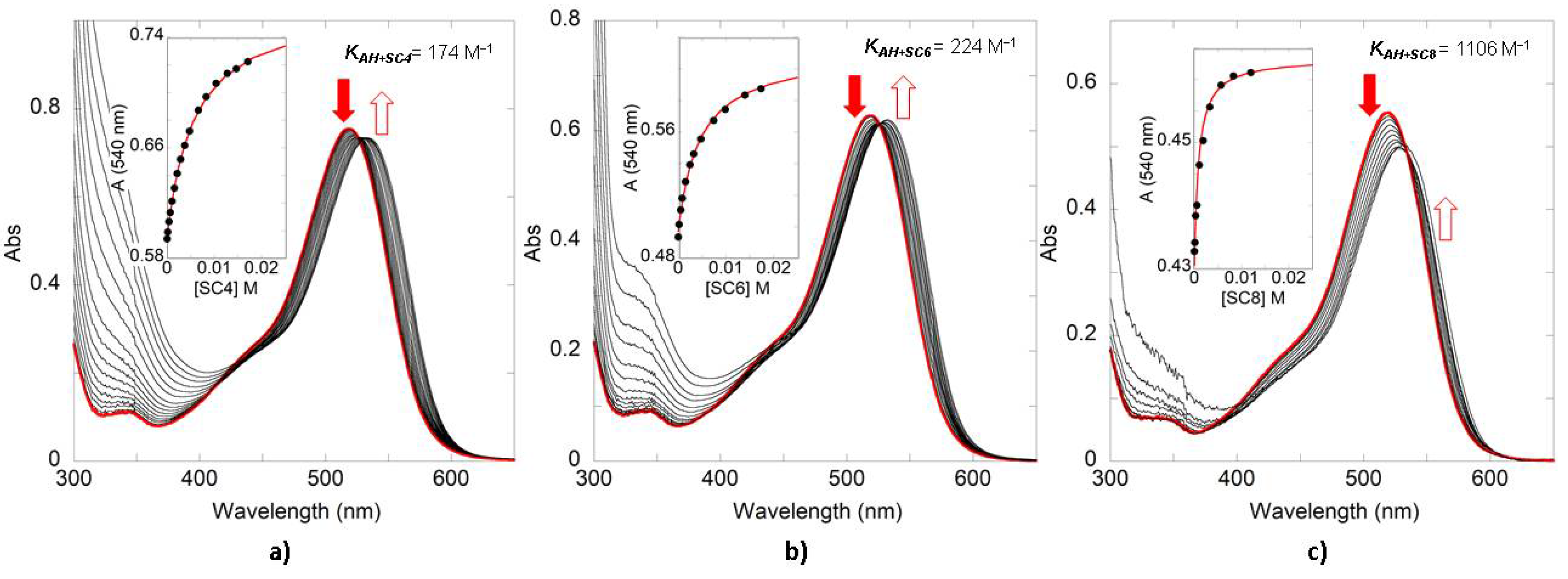
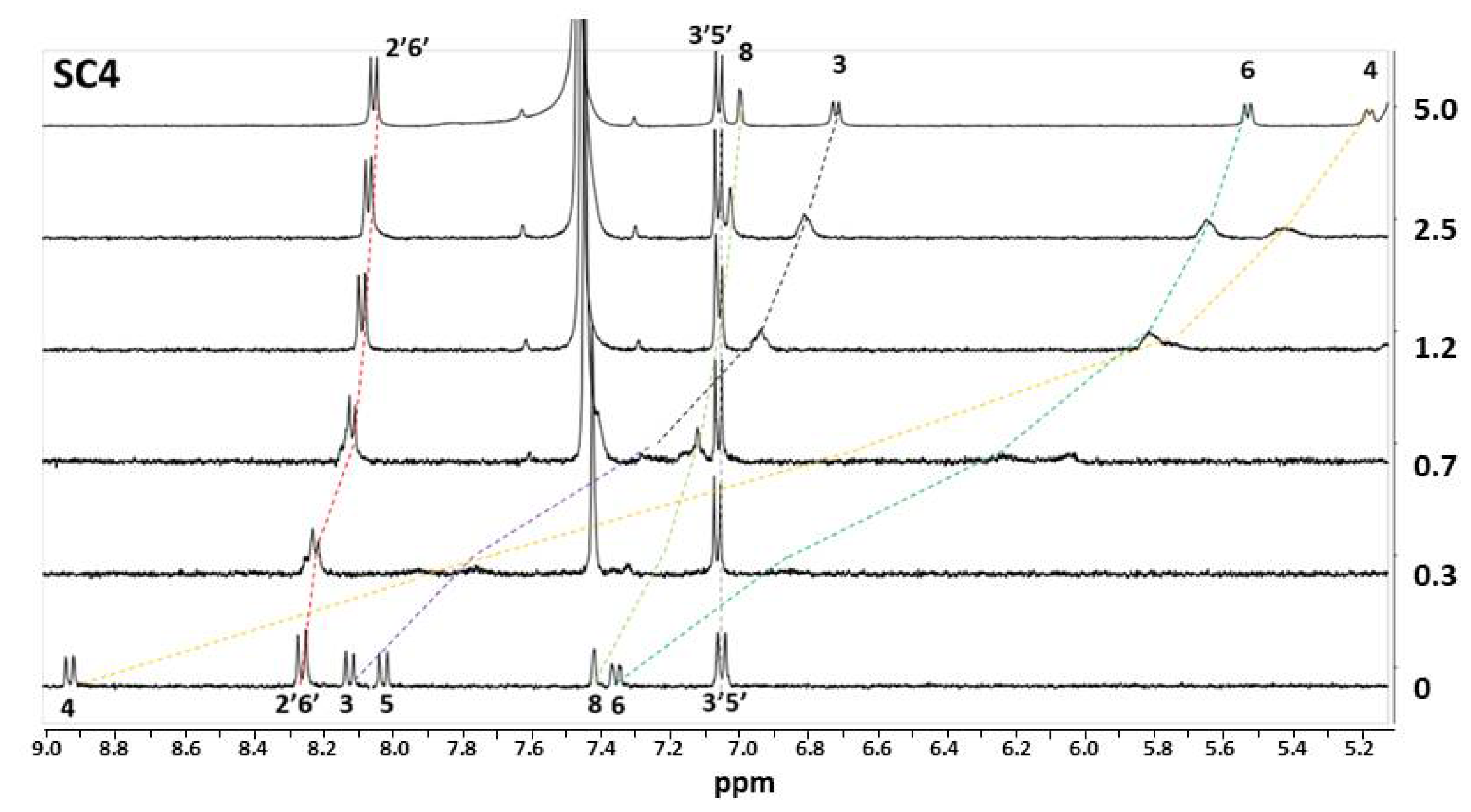
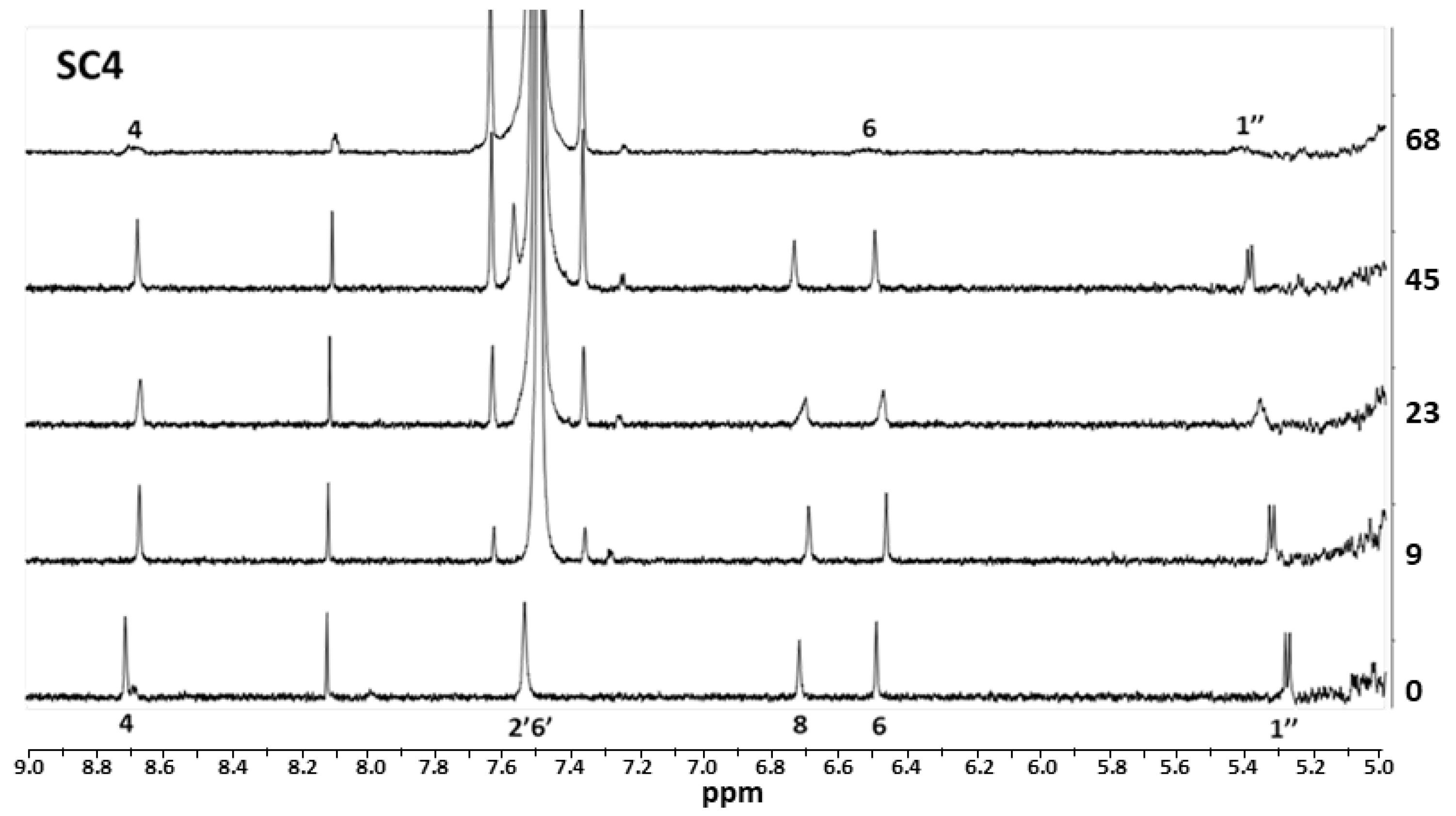
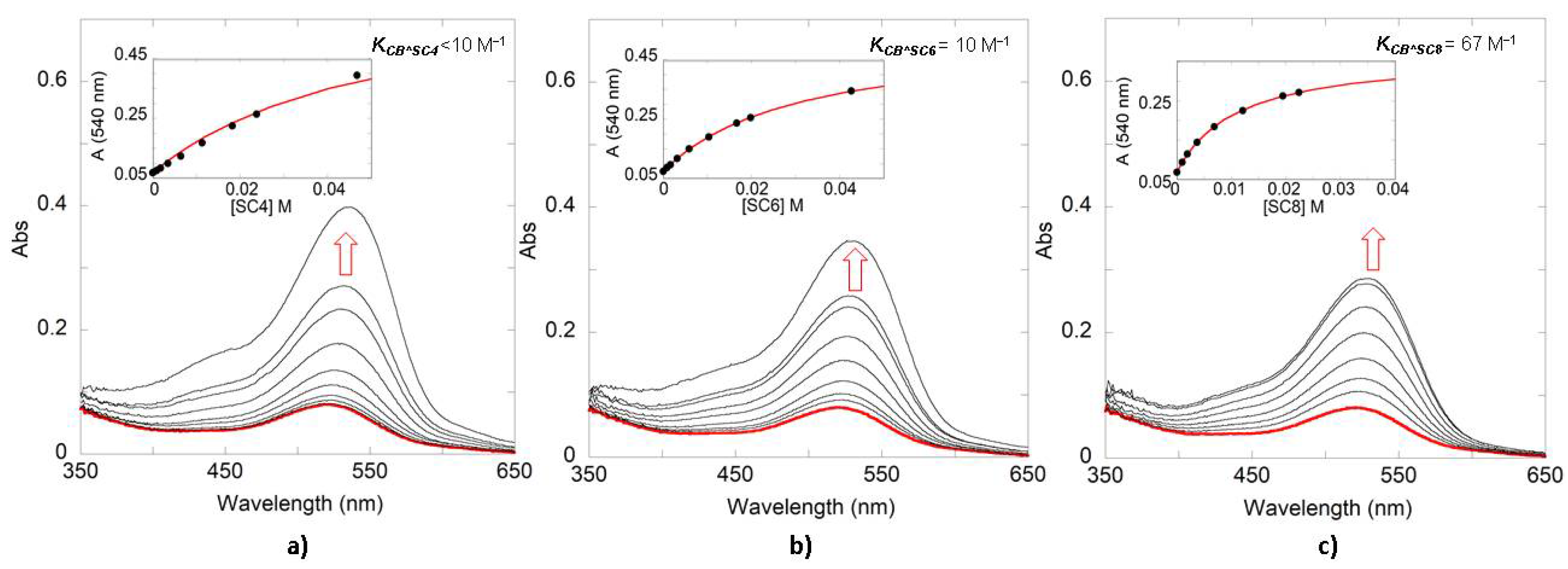
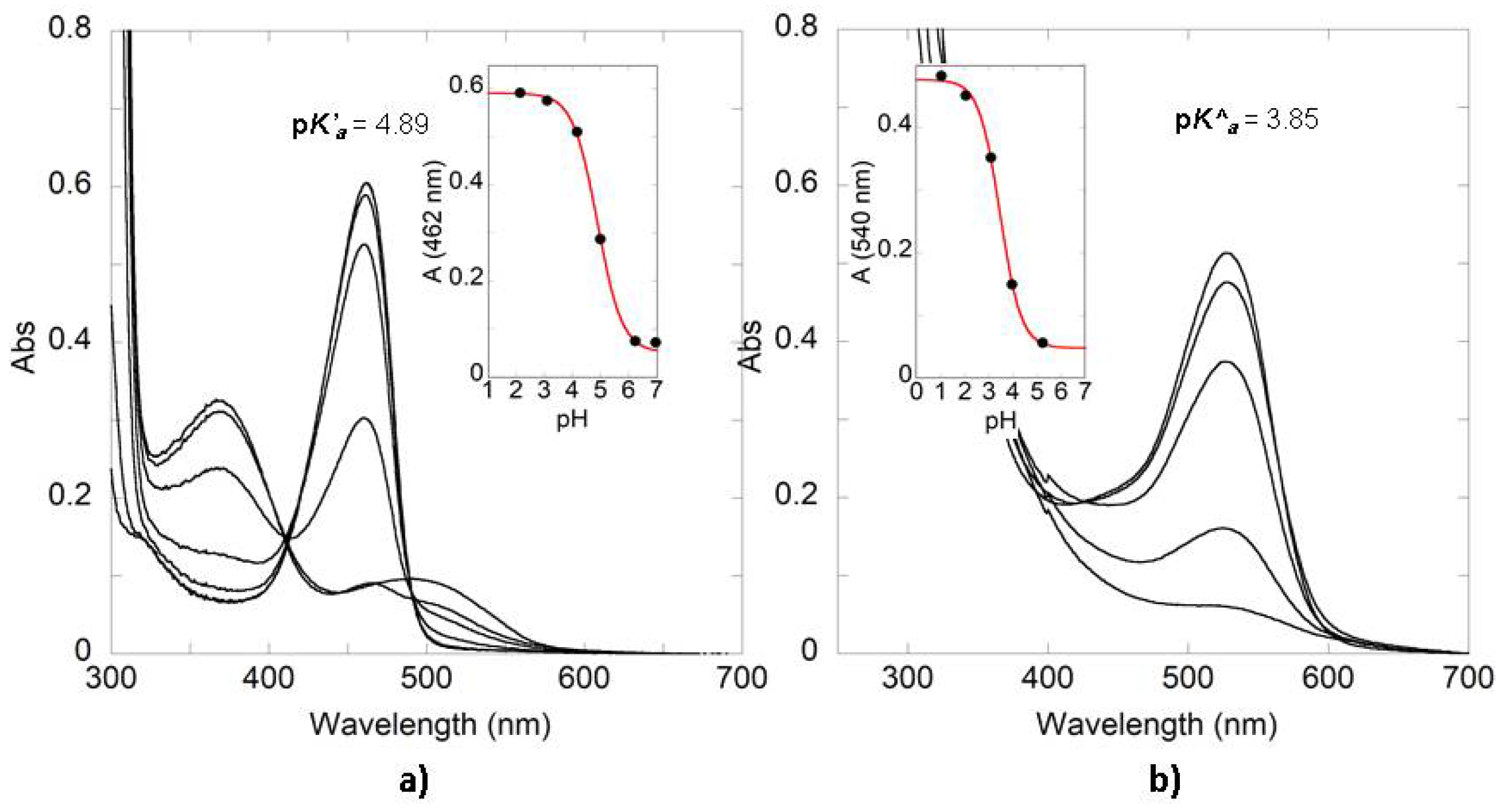
| Compound | SC4 | SC6 | SC8 |
|---|---|---|---|
| 4′7-DHF | 11400 | 2200 | 7700 |
| Oenin | 174 | 224 | 1100 |
| Compound | SC4 | SC6 | SC8 |
|---|---|---|---|
| Oenin | <10 | 10 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, J.; Cruz, L.; Freitas, V.d.; Pina, F.; Basílio, N. Anthocyanin Color Stabilization by Host-Guest Complexation with p-Sulfonatocalix[n]arenes. Molecules 2021, 26, 5389. https://doi.org/10.3390/molecules26175389
Mendoza J, Cruz L, Freitas Vd, Pina F, Basílio N. Anthocyanin Color Stabilization by Host-Guest Complexation with p-Sulfonatocalix[n]arenes. Molecules. 2021; 26(17):5389. https://doi.org/10.3390/molecules26175389
Chicago/Turabian StyleMendoza, Johan, Luis Cruz, Victor de Freitas, Fernando Pina, and Nuno Basílio. 2021. "Anthocyanin Color Stabilization by Host-Guest Complexation with p-Sulfonatocalix[n]arenes" Molecules 26, no. 17: 5389. https://doi.org/10.3390/molecules26175389
APA StyleMendoza, J., Cruz, L., Freitas, V. d., Pina, F., & Basílio, N. (2021). Anthocyanin Color Stabilization by Host-Guest Complexation with p-Sulfonatocalix[n]arenes. Molecules, 26(17), 5389. https://doi.org/10.3390/molecules26175389








