Electrochemical Detection of Dopamine at Fe3O4/SPEEK Modified Electrode
Abstract
1. Introduction
2. Results
2.1. Characterization of Fe3O4 and Fe3O4/SPEEK
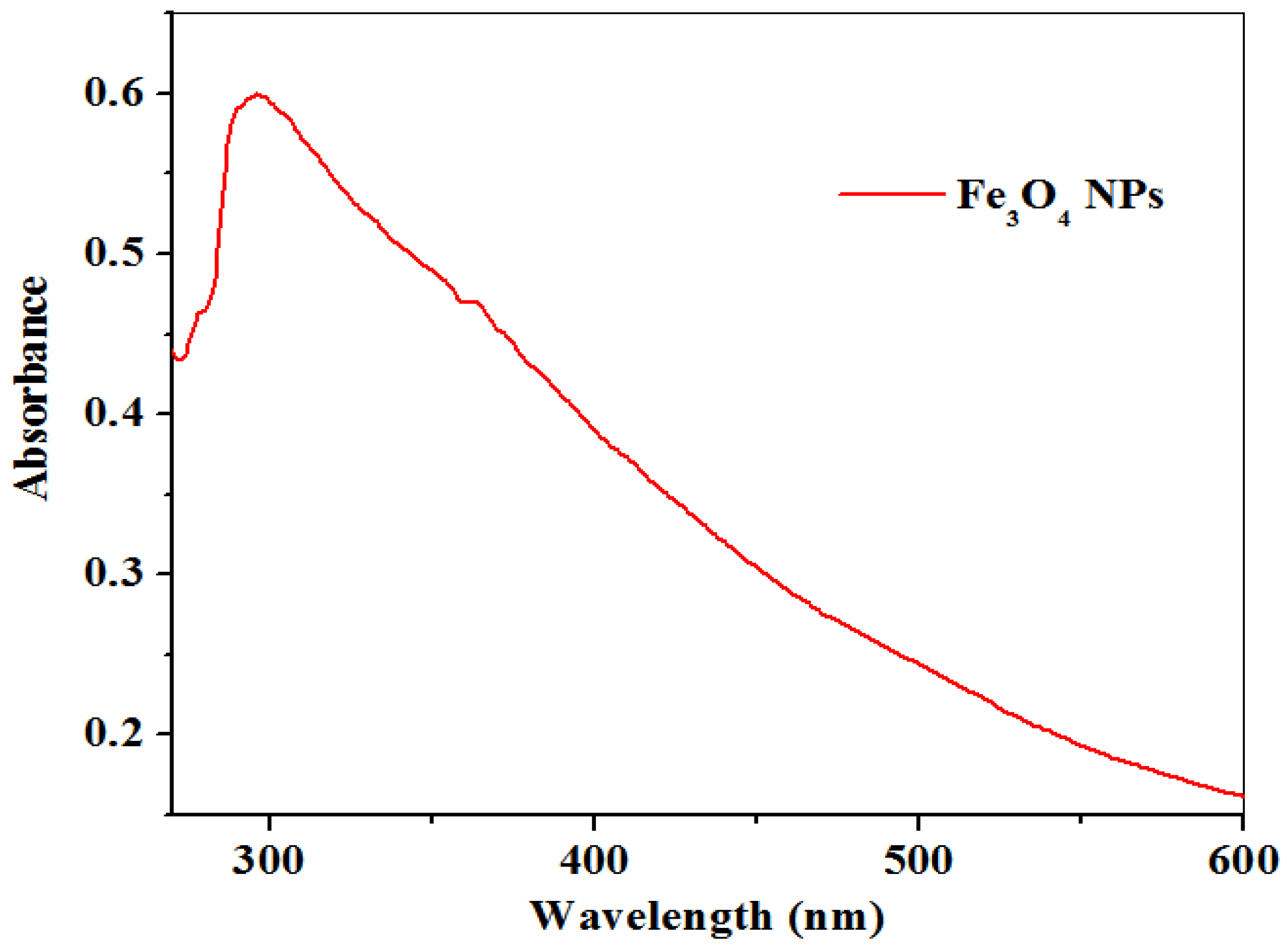
2.1.1. UV–Visible Study
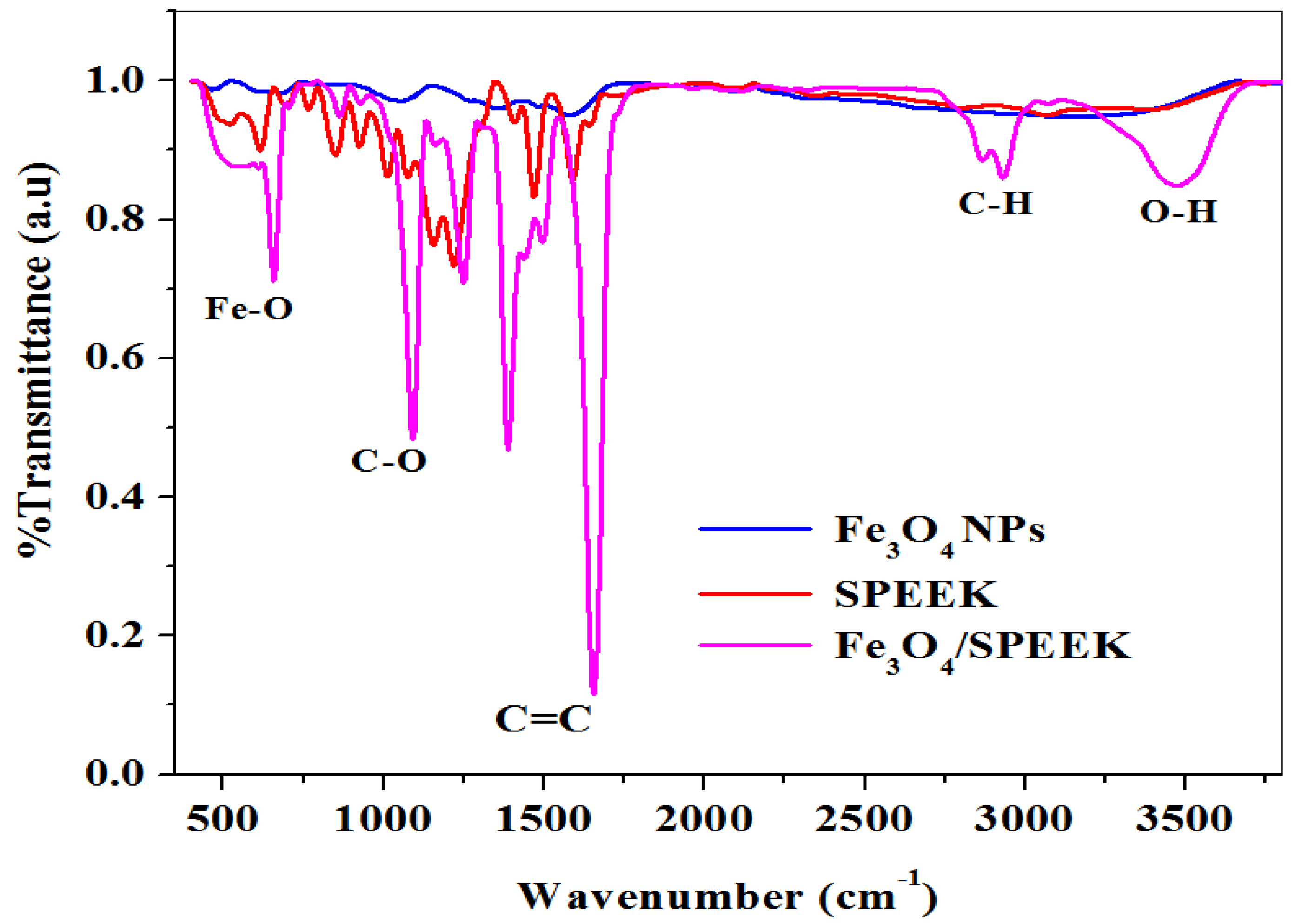
2.1.2. FTIR Study
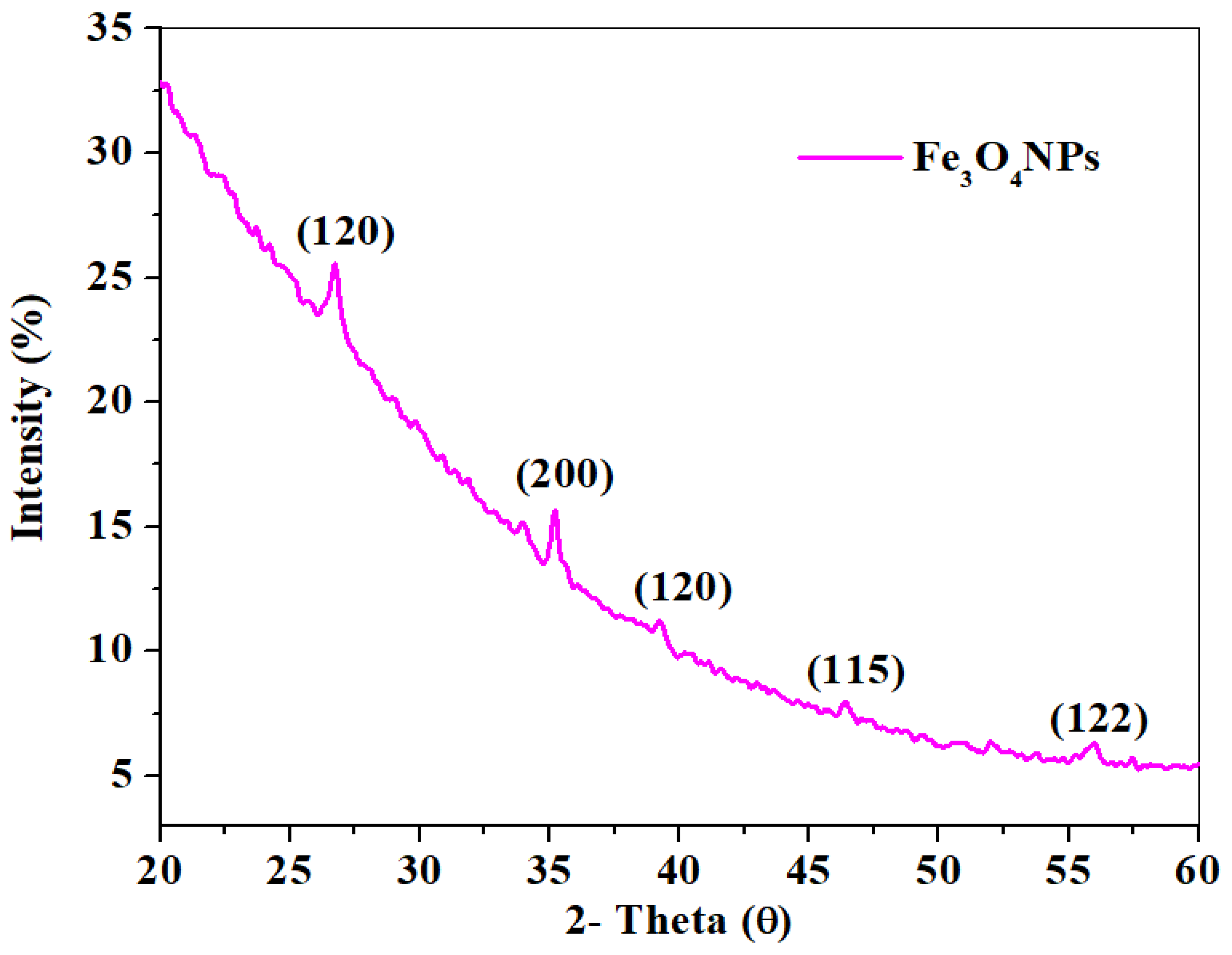
2.1.3. XRD Study
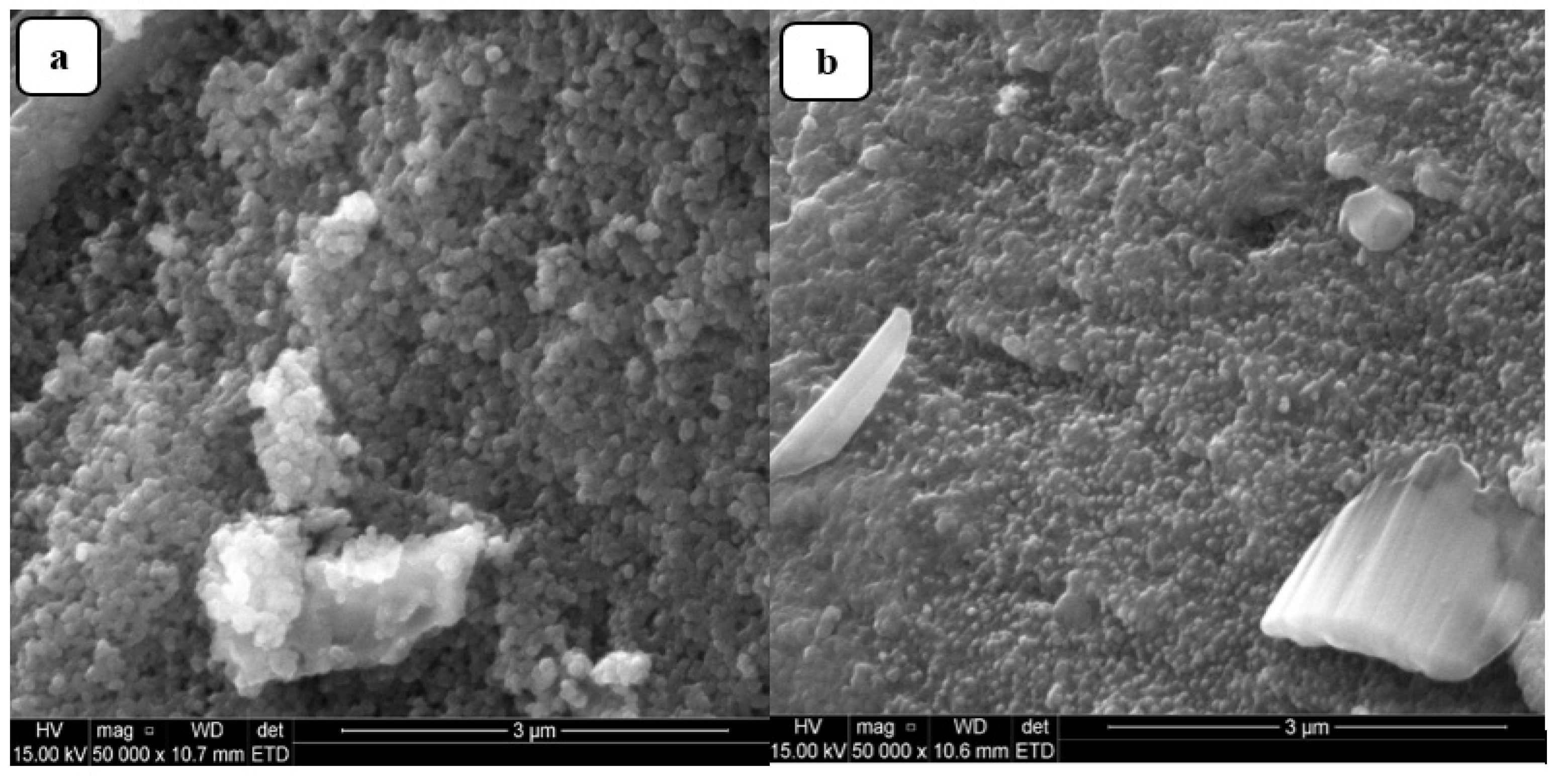
2.1.4. SEM Study
2.2. Electrochemical Characterization
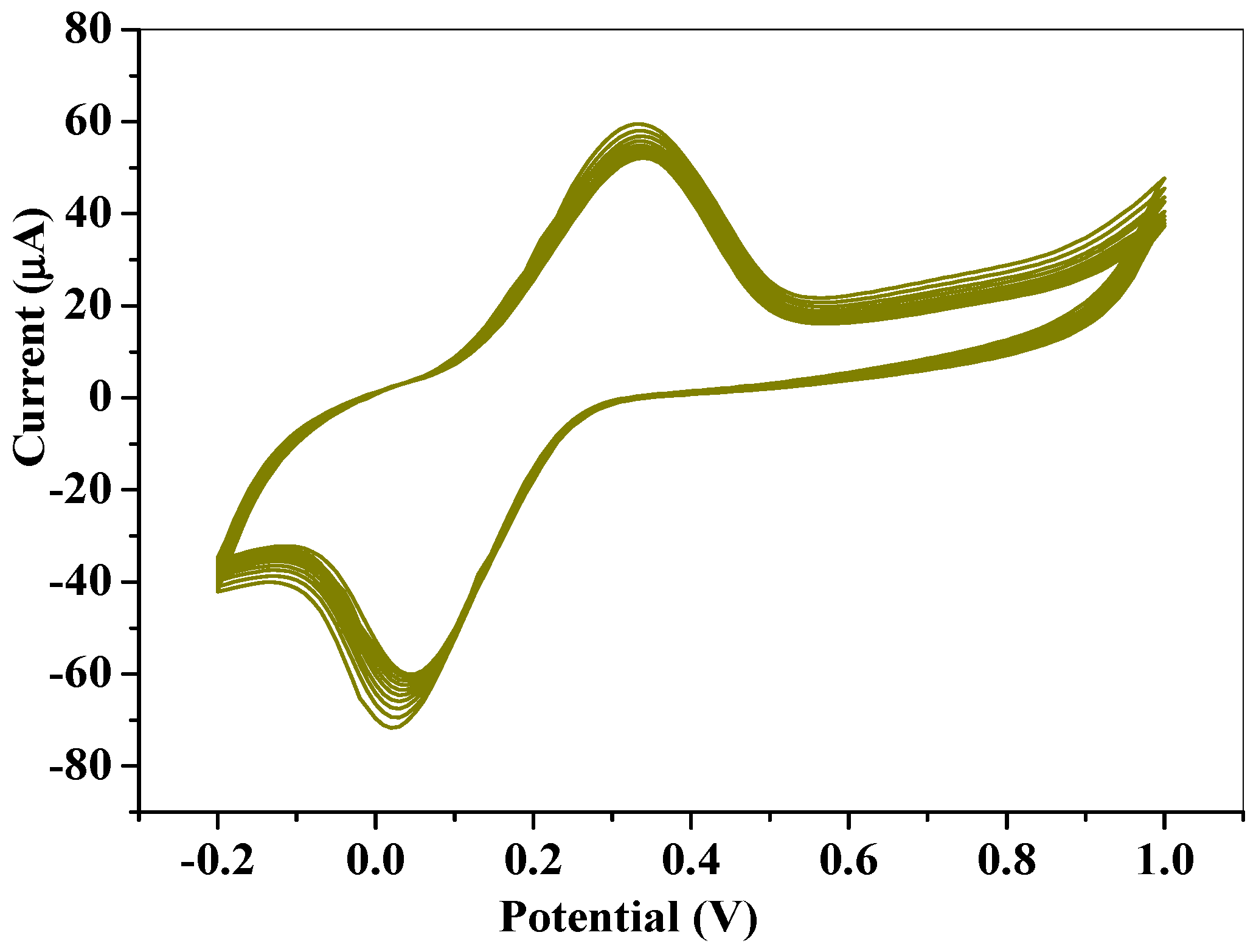
2.2.1. Electrochemical Characterization of Electrodes
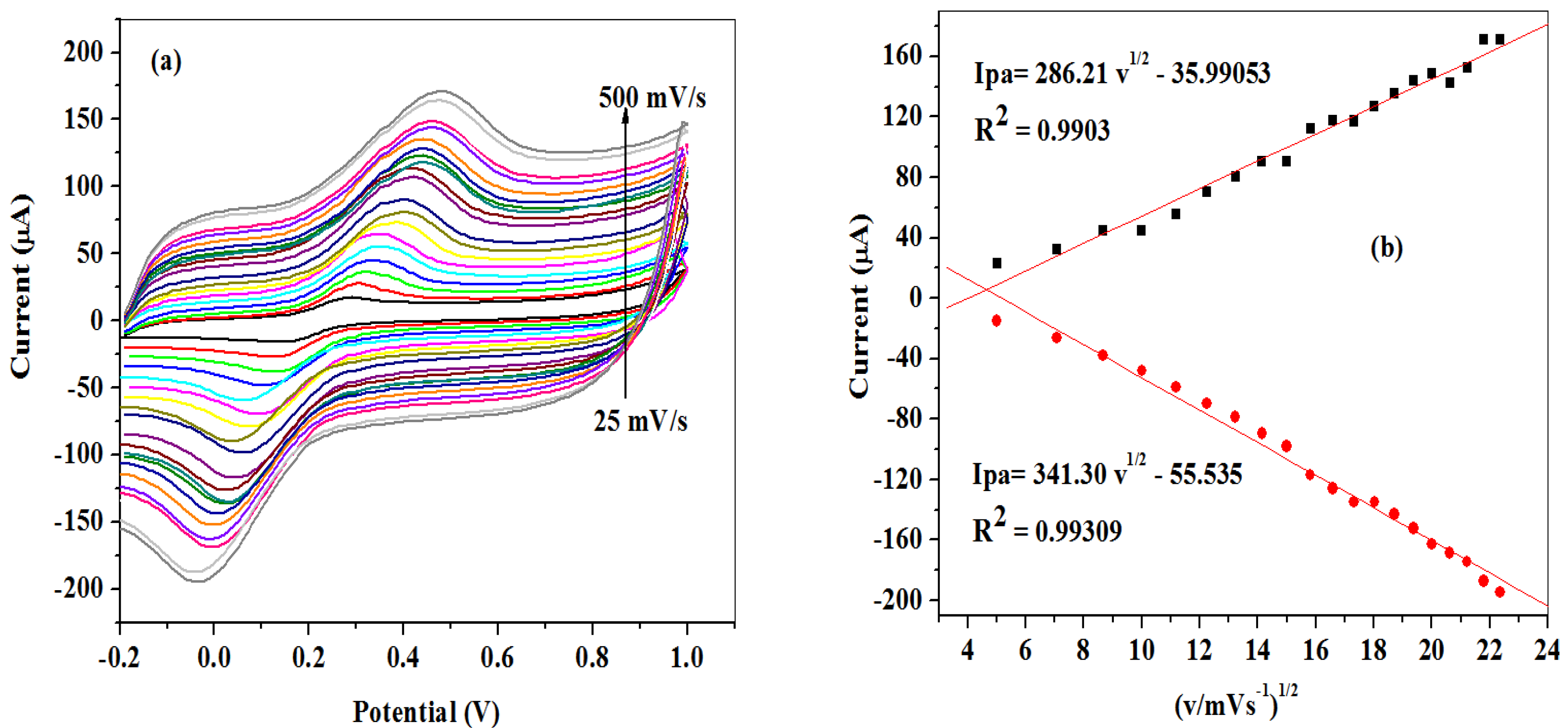
2.2.2. Scan Rate Study at SPCE-Fe3O4/SPEEK Electrode
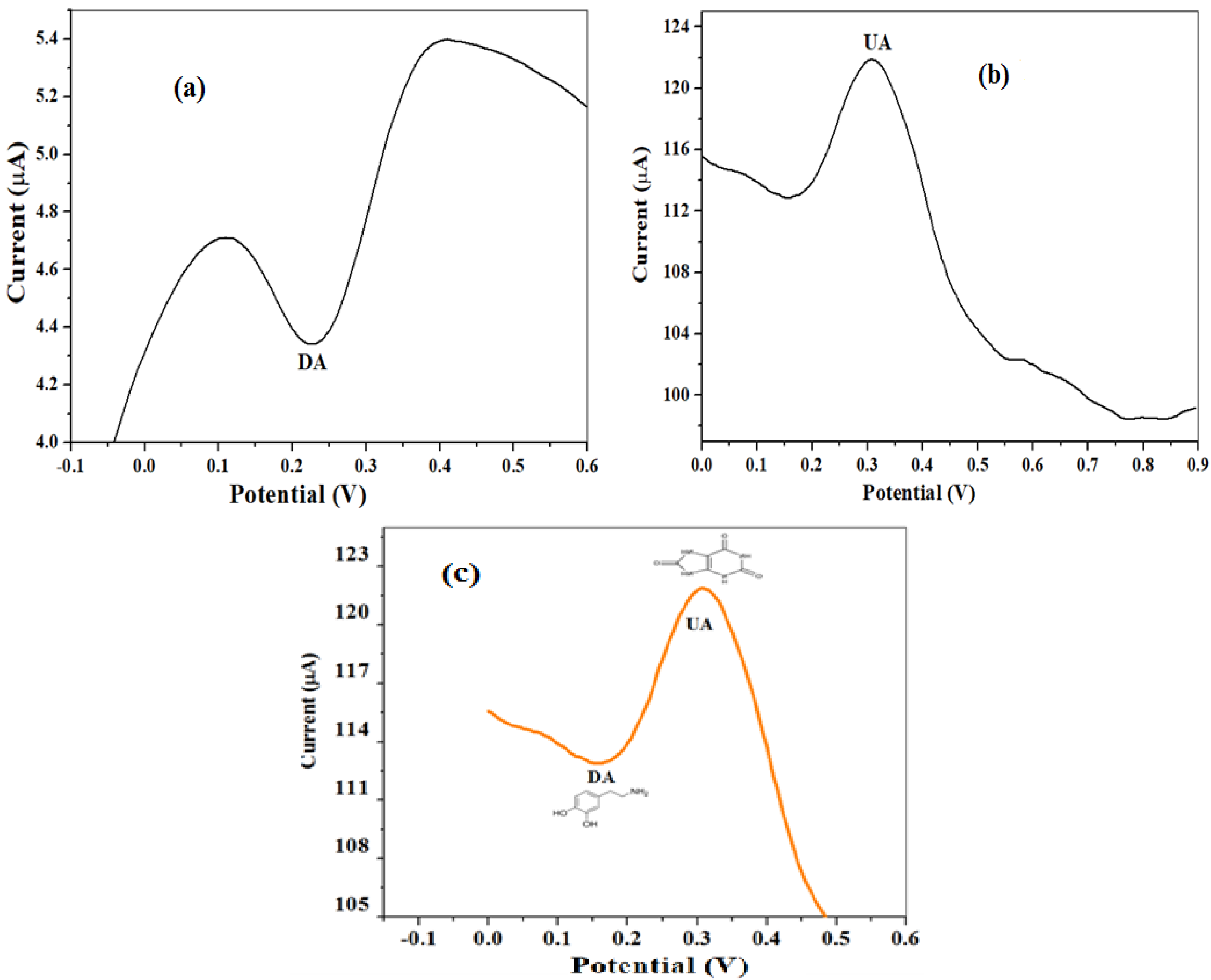
2.2.3. Electrocatalysis of Dopamine
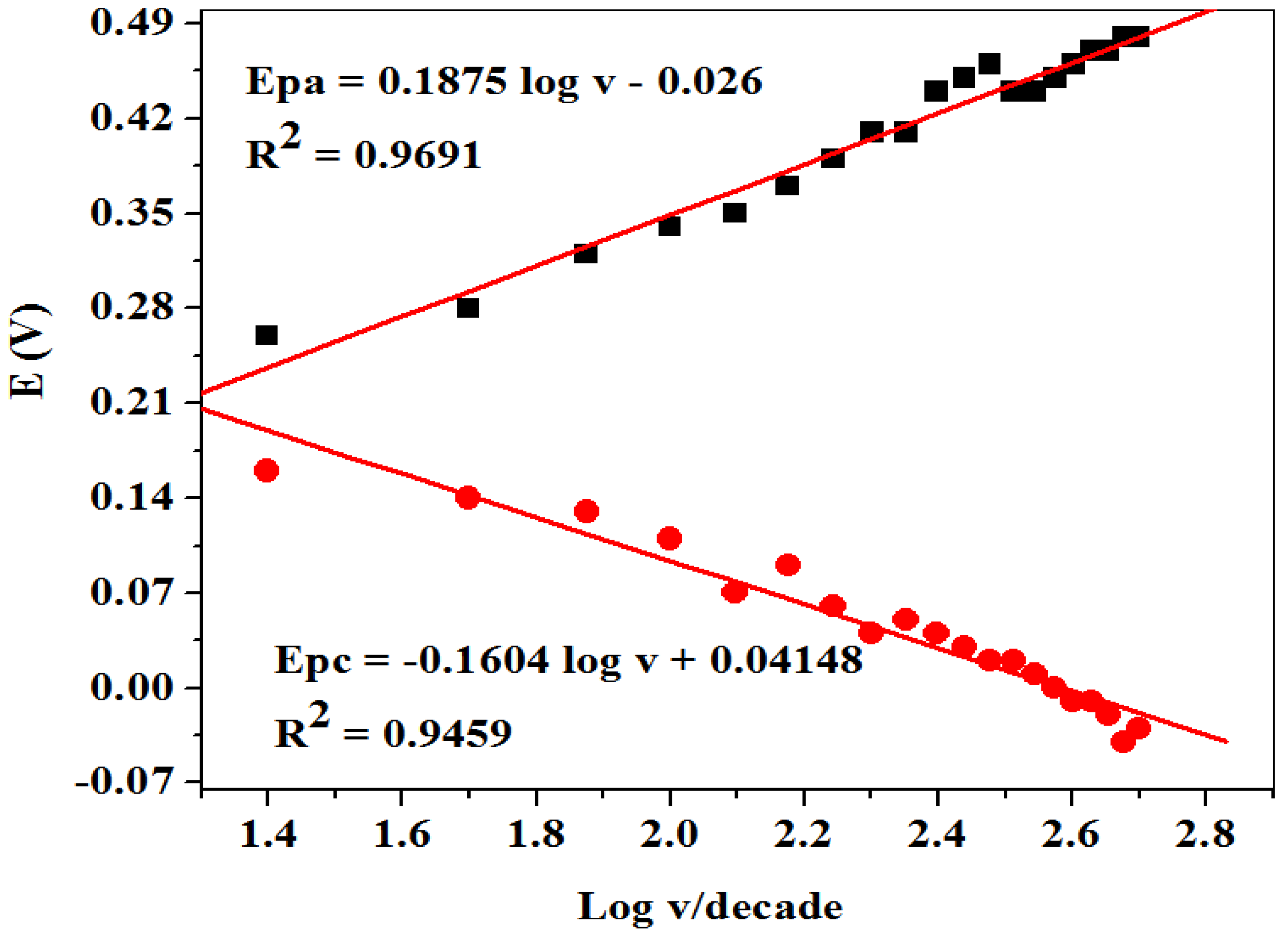
2.2.4. Scan Rate Study on Dopamine
2.3. Electro-Analysis of DA
Concentration Study of DA
2.4. Analytical Application of the Proposed Sensor for Determination of DA in Pharmaceutical Sample
2.5. Repeatability and Stability Study for Fe3O4/SPEEK
3. Materials and Methods
3.1. Preparation of Plant Leaf Extract
3.2. Synthesis of Iron Oxide Nanoparticles
3.3. Preparation of SPEEK Polymer
3.4. Synthesis of Iron Oxide/SPEEK Nanocomposites
3.5. Characterization of Nanomaterials Synthesized
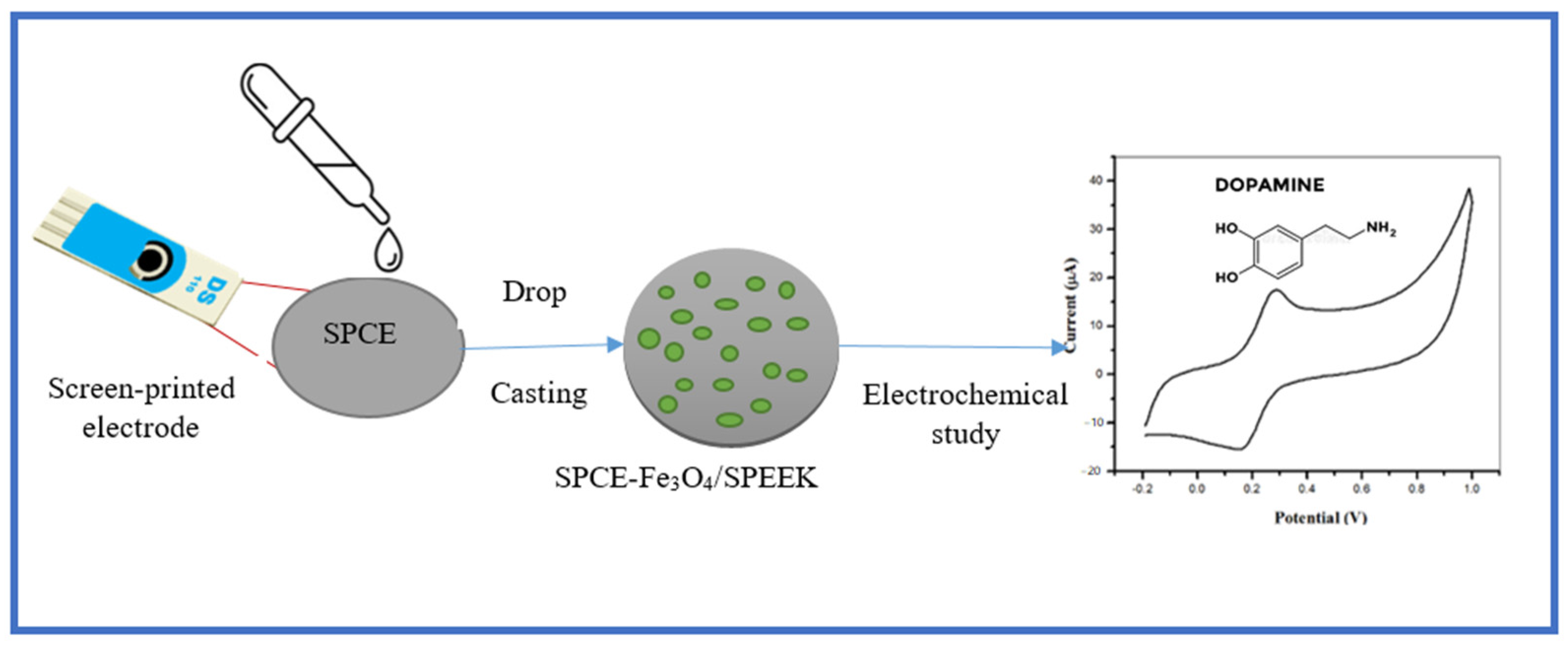
3.6. Electrode Modification and Electrochemical Studies
3.7. Preparation of Real Sample for Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kim, B.; Song, H.S.; Jin, H.J.; Park, E.J.; Lee, S.H.; Lee, B.Y.; Park, T.H.; Hong, S. Highly selective and sensitive detection of neurotransmitters using receptor-modified single-walled carbon nanotube sensors. Nanotechnology 2013, 24, 285501. [Google Scholar] [CrossRef]
- Palanisamy, S. Simultaneous electrochemical detection of dopamine and uric acid over ceria supported three dimensional gold nanoclusters. Mater. Res. Express 2014, 1, 045020. [Google Scholar] [CrossRef]
- Howell, L.L.; Cunningham, K.A. Serotonin 5-HT2 receptor interactions with dopamine function: Implications for therapeutics in cocaine use disorder. Pharmacol. Rev. 2015, 67, 176–197. [Google Scholar] [CrossRef]
- Kaya, C.; Block, E.R.; Sorkin, A.; Faeder, J.R.; Bahar, I. Multi-scale spatial simulations reveal the effect of dopamine transporter localization on dopamine neurotransmission. Biophys. J. 2016, 110, 632a. [Google Scholar] [CrossRef][Green Version]
- Meyyappan, M. Nano biosensors for neurochemical monitoring. Nano Converg. 2015, 2, 18. [Google Scholar] [CrossRef]
- Sangamithirai, D.; Munusamy, S.; Narayanan, V.; Stephen, A. Fabrication of neurotransmitter dopamine electrochemical sensor based on poly(o-anisidine)/CNTs nanocomposite. Surf. Interfaces 2016, 4, 27–34. [Google Scholar] [CrossRef]
- Dhanasekaran, T.; Padmanaban, A.; Gnanamoorthy, G.; Manigandan, R.; Sivakumar, P.K.; Stephen, A.; Narayanan, V. Recent advances in polymer supporting layered double hydroxides nanocomposite for electrochemical biosensors. Mater. Res. Express 2018, 5, 014011. [Google Scholar] [CrossRef]
- Buddhala, C.; Loftin, S.K.; Kuley, B.M.; Cairns, N.J.; Campbell, M.; Perlmutter, J.S.; Kotzbauer, P.T. Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann. Clin. Transl. Neurol. 2015, 2, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; McCutcheon, R.; Owen, M.J.; Murray, R. The role of genes, stress, and dopamine in the development of schizophrenia. Biol. Psychiatry 2017, 81, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Schaeffer, W.J. Is cell death primary or secondary in the pathophysiology of idiopathic Parkinson’s disease? Biomolecules 2015, 5, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Petzinger, G.; Holschneider, D.; Fisher, B.; McEwen, S.; Kintz, N.; Halliday, M.; Toy, W.; Walsh, J.; Beeler, J.; Jakowec, M. The effects of exercise on dopamine neurotransmission in Parkinson’s disease: Targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast. 2015, 1, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sitte, H.H.; Pifl, C.; Rajput, A.H.; Hörtnagl, H.; Tong, J.; Lloyd, G.K.; Kish, S.J.; Hornykiewicz, O. Dopamine and noradrenaline, but not serotonin, in the human claustrum are greatly reduced in patients with Parkinson’s disease: Possible functional implications. Eur. J. Neurosci. 2017, 45, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-S.; Kim, H.-W.; Tronche, F.; Palmiter, R.D.; Storm, D.R.; Xia, Z. Conditional deletion of Ndufs4 in dopaminergic neurons promotes Parkinson’s disease-like non-motor symptoms without loss of dopamine neurons. Sci. Rep. 2017, 7, 44989. [Google Scholar] [CrossRef]
- Yi, X.; Wu, Y.; Tan, G.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Wang, Z.; Pang, J.; Ning, C. Palladium nanoparticles entrapped in a self-supporting nanoporous gold wire as sensitive dopamine biosensor. Sci. Rep. 2017, 7, 7941. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, D.; Carlsen, S.; Khemani, L.; Miotto, J.; Alkalay, D. Determination of the dopamine agonist CGS 15855A in human plasma by gas chromatography/mass spectrometry. Anal. Lett. 1989, 22, 2307–2321. [Google Scholar] [CrossRef]
- Li, N.; Guo, J.; Liu, B.; Yu, Y.; Cui, H.; Mao, L.; Lin, Y. Determination of monoamine neurotransmitters and their metabolites in a mouse brain microdialysate by coupling high-performance liquid chromatography with gold nanoparticle-initiated chemiluminescence. Anal. Chim. Acta 2009, 645, 48–55. [Google Scholar] [CrossRef]
- Gao, W.; Qi, L.; Liu, Z.; Majeed, S.; Kitte, S.A.; Xu, G. Efficient lucigenin/thiourea dioxide chemiluminescence system and its application for selective and sensitive dopamine detection. Sens. Actuators B Chem. 2017, 238, 468–472. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Li, X.; Guo, X.; Zhang, B.; Jia, X.; Dai, B. A simple, fast and low-cost turn-on fluorescence method for dopamine detection using in situ reaction. Anal. Chim. Acta 2016, 944, 51–56. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.; Swamy, B.; Ebenso, E.E. Electrochemical sensor for the detection of dopamine in real samples using polyaniline/NiO, ZnO, and Fe3O4 nanocomposites on glassy carbon electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Baskar, R.; Adekunle, A.S.; Sherif, E.M.; Ebenso, E.E. SPEEK/ZnO Nanocomposite modified gold electrode for electrochemical detection of dopamine. Electroanalysis 2020, 32, 2713–2722. [Google Scholar] [CrossRef]
- Manbohi, A.; Ahmadi, S.H. Sensitive and selective detection of dopamine using electrochemical microfluidic paper-based analytical nanosensor. Sens. Bio-Sens. Res. 2019, 23, 100270. [Google Scholar] [CrossRef]
- Zablocka, I.; Wysocka-Zolopa, M.; Winkler, K. Electrochemical detection of dopamine at a gold electrode modified with a polypyrrole–mesoporous silica molecular sieves (MCM-48) film. Int. J. Mol. Sci. 2019, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.-H. Green biosynthesis of magnetic iron oxide (Fe3O4) nanoparticles using the aqueous extracts of food processing wastes under photo-catalyzed condition and investigation of their antimicrobial and antioxidant activity. J. Photochem. Photobiol. B Biol. 2017, 173, 291–300. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Devi, D.R.; Botsa, S.M.; Basavaiah, K. Facile green synthesis of Fe3O4 nanoparticles using aqueous leaf extract of Zanthoxylum armatum DC. For efficient adsorption of methylene blue. J. Asian Ceram. Soc. 2018, 6, 145–155. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, J.; Liu, X.; Chan, H.S.O. Synthesis of Fe3O4 nanoparticles from emulsions. J. Mater. Chem. 2001, 11, 1704–1709. [Google Scholar] [CrossRef]
- Wang, W.-W.; Zhu, Y.-J.; Ruan, M.-L. Microwave-assisted synthesis and magnetic property of magnetite and hematite nanoparticles. J. Nanopart. Res. 2007, 9, 419–426. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, F.; Hong, R. Solvothermal synthesis of magnetic Fe3O4 microparticles via self-assembly of Fe3O4 nanoparticles. Particuology 2011, 9, 179–186. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Javanbakht, M.; Pourmahdian, S. Enhancing the performance of SPEEK polymer electrolyte membranes using functionalized TiO2 nanoparticles with proton hopping sites. RSC Adv. 2017, 7, 8303–8313. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Fayemi, O.E. Electrochemical detection of serotonin in banana at green mediated PPy/Fe3O4NPs nanocomposites modified electrodes. Sens. Bio-Sens. Res. 2020, 28, 100338. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, D.C.; Shrivastava, S. Polyaniline-bismuth oxide nanocomposite sensor for quantification of anti-parkinson drug pramipexole in solubilized system. Mater. Sci. Eng. B 2014, 185, 53–59. [Google Scholar] [CrossRef]
- Ghanbari, K.; Babaei, Z. Fabrication and characterization of non-enzymatic glucose sensor based on ternary NiO/CuO/polyaniline nanocomposite. Anal. Biochem. 2016, 498, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-G.; Peng, Y.-T. Fabrication of a room-temperature H2S gas sensor based on PPy/WO3 nanocomposite films by in-situ photopolymerization. Sens. Actuators B Chem. 2014, 193, 637–643. [Google Scholar] [CrossRef]
- Ngaboyamahina, E.; Cachet, H.; Pailleret, A.; Sutter, E. Photo-assisted electrodeposition of an electrochemically active polypyrrole layer on anatase type titanium dioxide nanotube arrays. Electrochim. Acta 2014, 129, 211–221. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.; Fatibello-Filho, O. Nanostructured carbon black for simultaneous sensing in biological fluids. Sens. Actuators B Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Thenmozhi, B.; Suryakiran, S.; Sudha, R.; Revath, B. Green synthesis and comparative study of silver and iron nanoparticle from leaf extract. Int. J. Inst. Pharm. Life Sci. 2014, 4, 5–12. [Google Scholar]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Shameli, K.; Miyake, M.; Hara, H.; Mohamad, S.E.B.; Kalantari, K.; Taib, S.H.M.; Rasouli, E. Cytotoxicity assay of plant-mediated synthesized iron oxide nanoparticles using Juglans regia green husk extract. Arab. J. Chem. 2020, 13, 2011–2023. [Google Scholar] [CrossRef]
- Qin, H.; Wang, C.; Dong, Q.; Zhang, L.; Zhang, X.; Ma, Z.; Han, Q. Preparation and characterization of magnetic Fe3O4–chitosan nanoparticles loaded with isoniazid. J. Magn. Magn. Mater. 2015, 381, 120–126. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, Y.; Xie, A.; Li, S.; Wang, X. Green synthesis of soya bean sprouts-mediated superparamagnetic Fe3O4 nanoparticles. J. Magn. Magn. Mater. 2010, 322, 2938–2943. [Google Scholar] [CrossRef]
- Quadri, T.W.; Olasunkanmi, L.O.; Fayemi, O.E.; Solomon, M.M.; Ebenso, E.E. Zinc oxide nanocomposites of selected polymers: Synthesis, characterization, and corrosion inhibition studies on mild steel in HCL solution. ACS Omega 2017, 2, 8421–8437. [Google Scholar] [CrossRef]
- Kim, D.-S.; Kang, E.-S.; Baek, S.; Choo, S.-S.; Chu, S.S.; Lee, D.; Min, J.; Kim, T.-H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Gadi, S.; Cherukuri, C.S.; Barla, S.; Pammi, S. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 2019, 5, e02765. [Google Scholar] [CrossRef]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective coating of superparamagnetic iron oxide nanoparticles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Hou, S.; Kasner, M.L.; Su, S.; Patel, K.; Cuellari, R. Highly sensitive and selective dopamine biosensor fabricated with silanized graphene. J. Phys. Chem. C 2010, 114, 14915–14921. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Rabia, M.K.; Newair, E. Electrochemical behaviour of antioxidants: Part 2. Electrochemical oxidation mechanism of quercetin at glassy carbon electrode modified with multi-wall carbon nanotubes. Arab. J. Chem. 2016, 9, 350–356. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Xiang, H.; Li, Z.; Yang, W.; Wang, H. Enhanced cycling performance of Fe3O4–graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 56, 834–840. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Rashid-Nadimi, S. Voltammetric determination of ascorbic acid and dopamine in the same sample at the surface of a carbon paste electrode modified with polypyrrole/ferrocyanide films. Electrochim. Acta 2005, 50, 4694–4698. [Google Scholar] [CrossRef]
- Feng, X.; Huang, H.; Ye, Q.; Zhu, A.J.-J.; Hou, W. Ag/Polypyrrole core−shell nanostructures: Interface polymerization, characterization, and modification by gold nanoparticles. J. Phys. Chem. C 2007, 111, 8463–8468. [Google Scholar] [CrossRef]
- Doyle, R.; Breslin, C.B.; Rooney, A.D. A simple but highly selective electrochemical sensor for dopamine. Chem. Biochem. Eng. Q. 2009, 23, 93–98. [Google Scholar]
- Liu, X.; Zhu, H.; Yang, X. An electrochemical sensor for dopamine based on poly(o-phenylenediamine) functionalized with electrochemically reduced graphene oxide. RSC Adv. 2014, 4, 3743–3749. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Lee, K.X. Green synthesis of magnetite (Fe3O4) nanoparticles using seaweed (Kappaphycus alvarezii) extract. Nanoscale Res. Lett. 2016, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.U.; Kareem, A.; Nami, S.; Khan, M.S.; Rehman, S.; Bhat, S.A.; Mohammad, A.; Nishat, N. Biogenic synthesis of iron oxide nanoparticles using Agrewia optiva and Prunus persica phyto species: Characterization, antibacterial and antioxidant activity. J. Photochem. Photobiol. B Biol. 2018, 185, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, H.; Matheswaran, M. Amaranthus spinosus leaf extract mediated FeO nanoparticles: Physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain. Chem. Eng. 2015, 3, 3149–3156. [Google Scholar] [CrossRef]
- Ramaganthan, B.; Sivakumar, P.M.; Dharmalingam, S. Synthesis, characterization of novel silicotungstic acid incorporated SPEEK/PVA-co-ethylene-based composite membranes for fuel cell. J. Mater. Sci. 2011, 46, 1741–1748. [Google Scholar] [CrossRef]
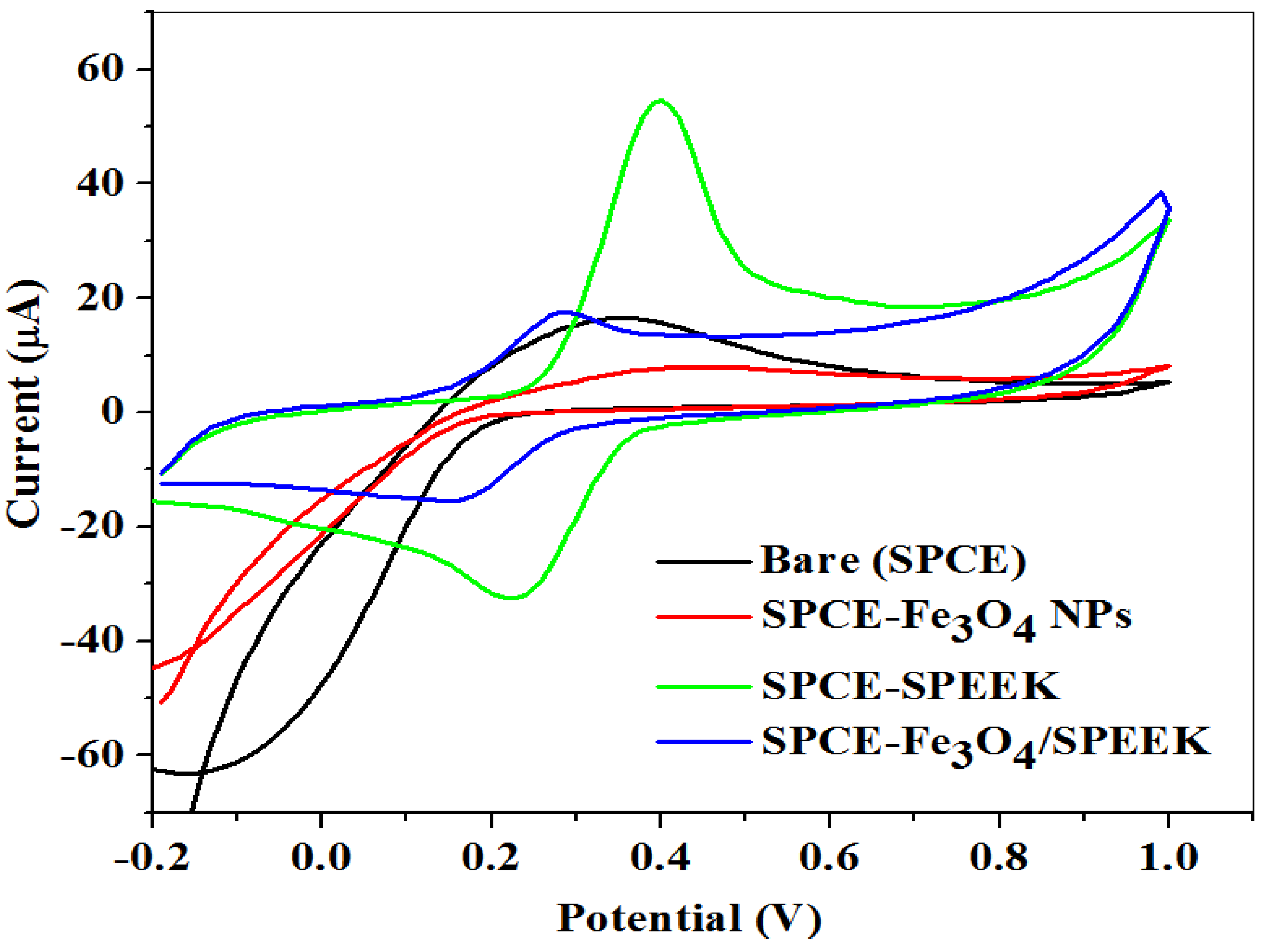
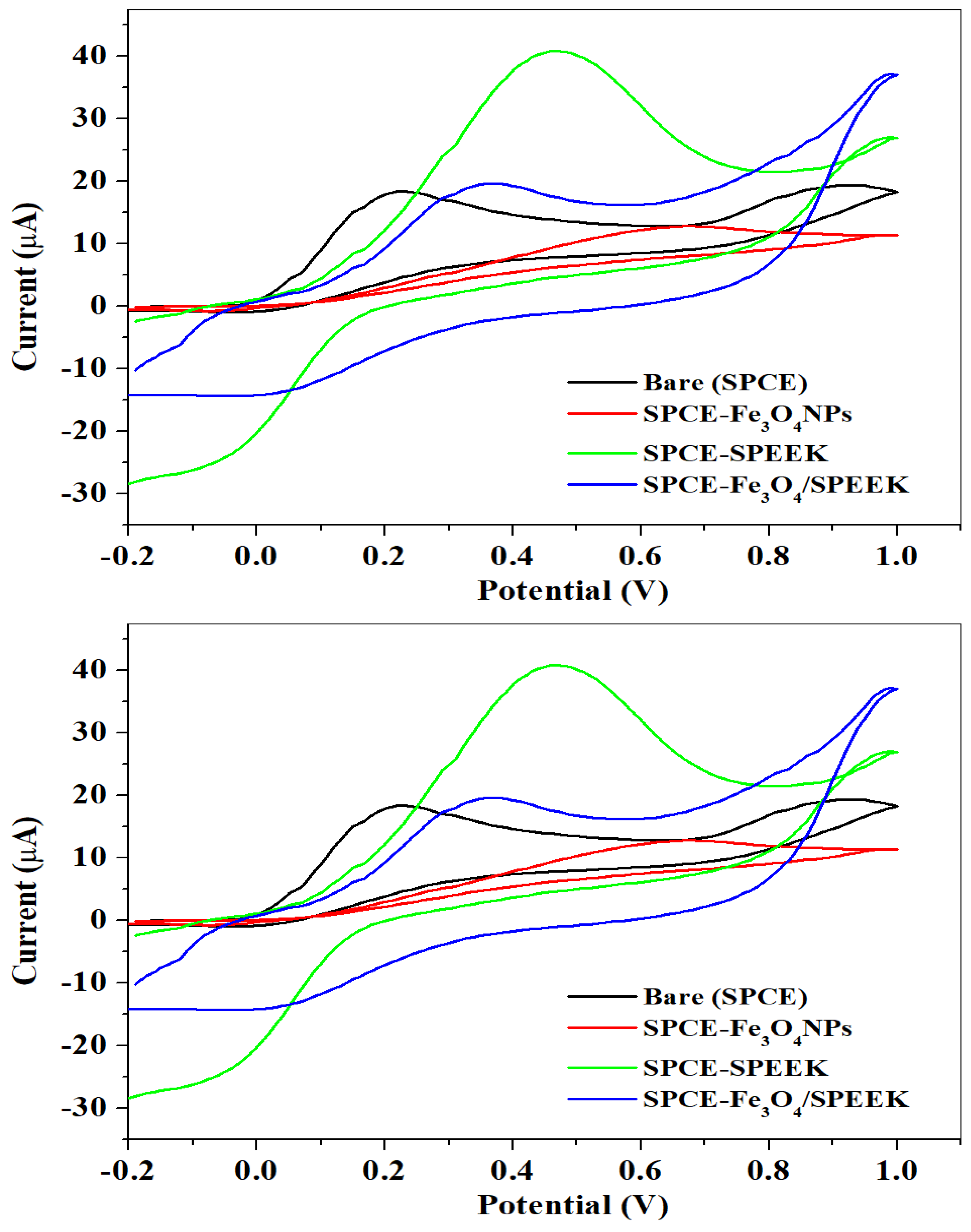
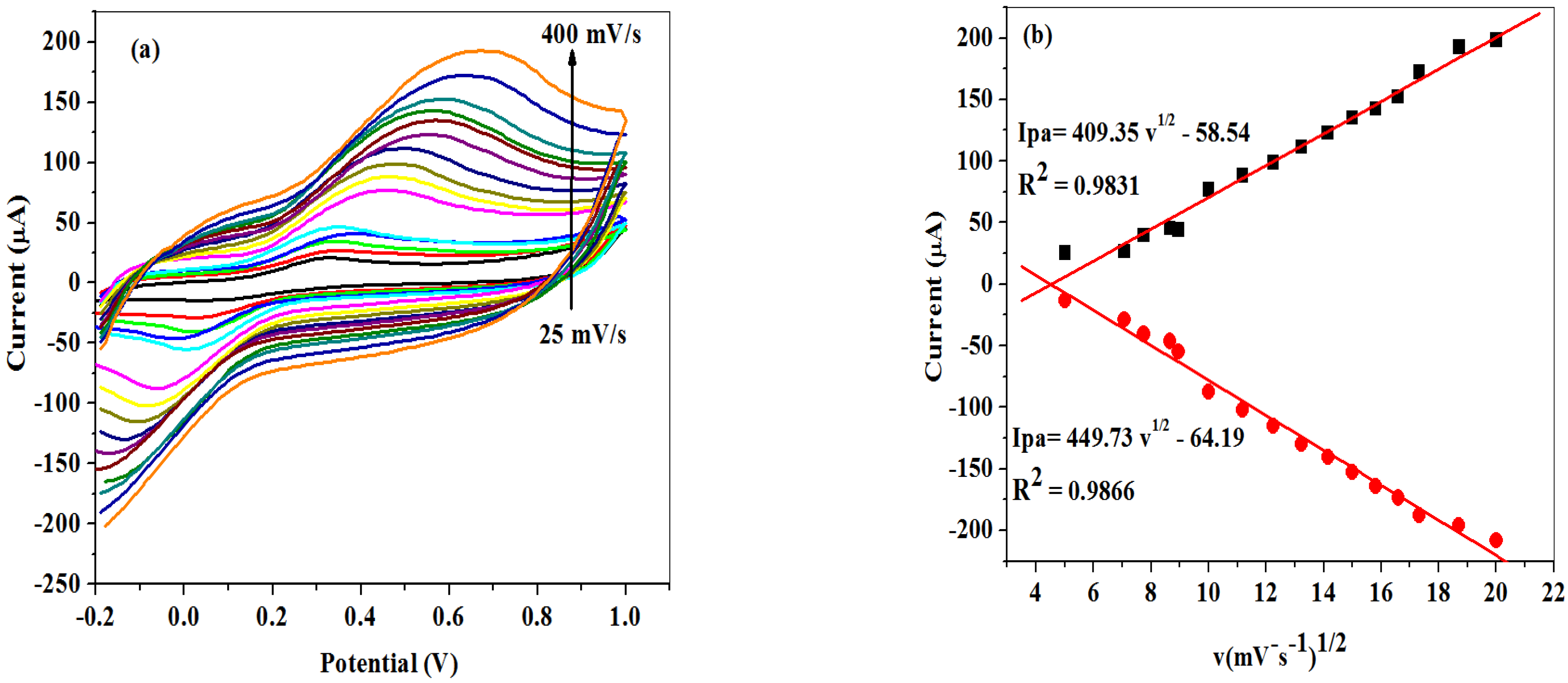
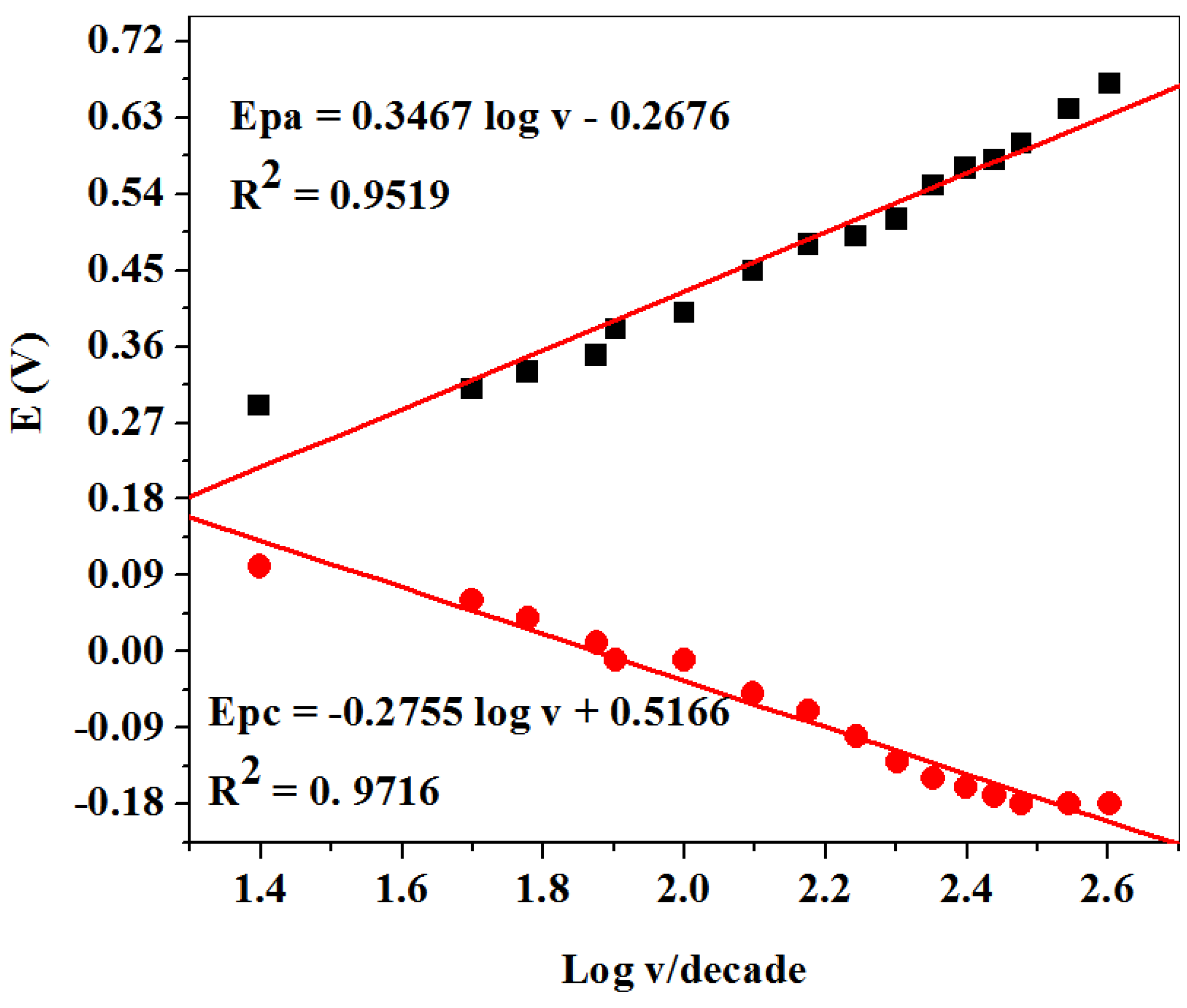
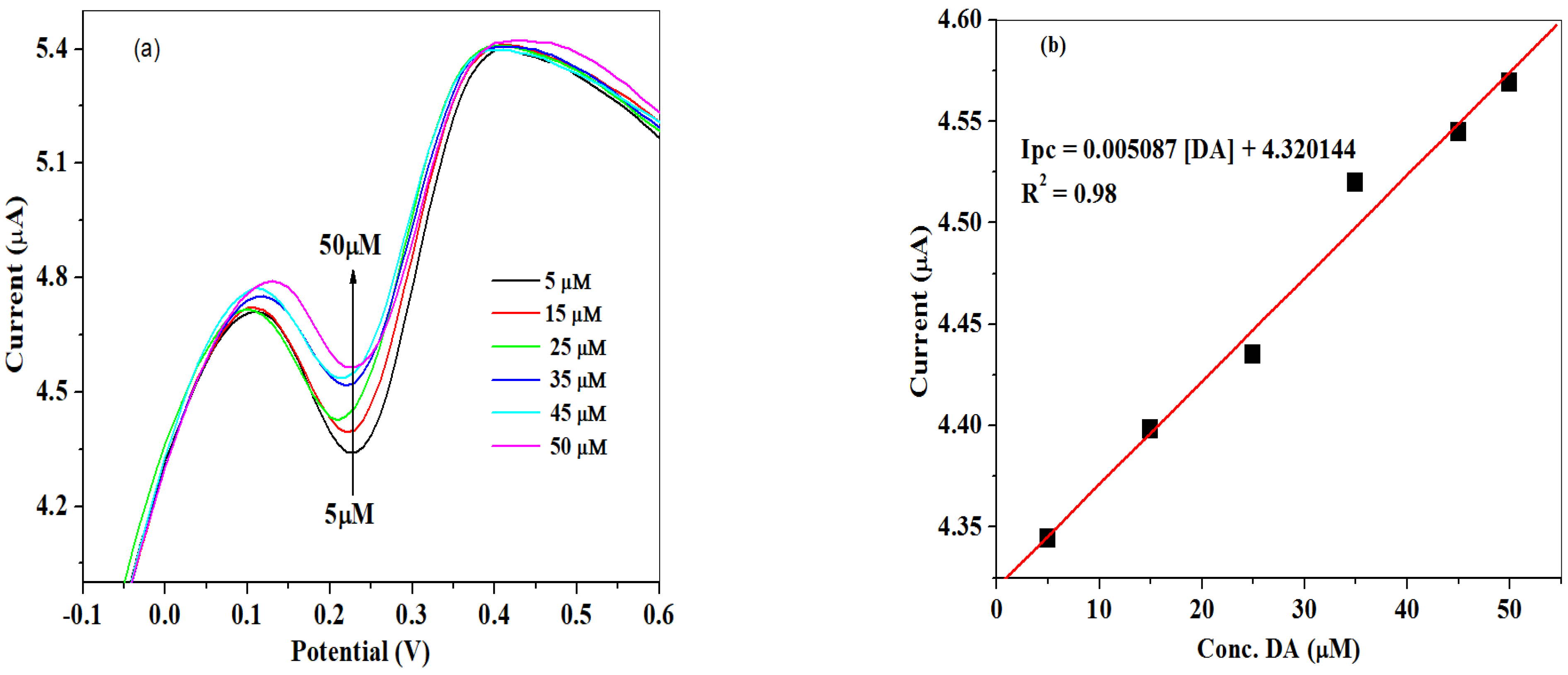
| Electrode | Ipa (μA) | Ipc (μA) | Ipa/Ipc | Epa (V) | Epc (V) | E1/2 (V) | ΔEp (V) |
|---|---|---|---|---|---|---|---|
| Bare-SPCE | 16.40 | −16.97 | −0.26 | 0.36 | −0.16 | 0.10 | 0.52 |
| SPCE-Fe3O4NPs | 7.99 | −40.56 | −0.11 | 0.42 | −0.14 | 0.14 | 0.56 |
| SPCE-SPEEK | 54.48 | −32.69 | −1.67 | 0.31 | 0.22 | 0.26 | 0.09 |
| SPCE-Fe3O4/SPEEK | 17.93 | −15.18 | −1.18 | 0.29 | 0.18 | 0.22 | 0.11 |
| Dopamine | Ipa (μA) | Ipc (μA) | Ipa/Ipc | Epa (V) | Epc (V) | E1/2 (V) | ΔEp (V) |
|---|---|---|---|---|---|---|---|
| Bare-SPCE | 19.78 | −1.12 | −17.66 | 0.19 | 0.02 | 0.11 | 0.17 |
| SPCE-Fe3O4NPs | 12.56 | 0.51 | 24.63 | 0.66 | 0.09 | 0.37 | 0.57 |
| SPCE-SPEEK | 42.00 | −25.33 | −1.66 | 0.48 | −0.04 | 0.22 | 0.52 |
| SPCE-Fe3O4/SPEEK | 20.94 | −14.13 | −1.48 | 0.34 | 0.06 | 0.20 | 0.28 |
| Modified Electrode | Methods | Linear Range (μM) | Analyte | LoD (μM) | R2 | Ref. |
|---|---|---|---|---|---|---|
| Ppy/Ferro-cyanide/carbon paste electrode | LSV | 100–1200 | DA | 38.6 | 0.9984 | [47] |
| DPV | 200–950 | DA | 15 | 0.9998 | ||
| Au/Ppy/Ag/GCE | Amperometry | 100–5000 | DA | 50 | [48] | |
| p-Sulphonatocalix [6]arene/polypyrrole | 75–1000 | DA | 20 | [49] | ||
| PoPD/E-RGO/GCE | 10–400 | DA | 7.5 | [50] | ||
| SPCE-Fe3O4/SPEEK | SWV | 5–50 | DA | 7.1 | 0.9831 | This work |
| Sample | Added (μM) | Detected (μM) | Recovery (%) | RSD |
|---|---|---|---|---|
| Dopamine HCl-Fresenius 200 mg/5 mL injection | 40 | 39.97 | 99.9 | 3.90 |
| 80 | 80.01 | 100 | 0.05 | |
| 120 | 119.4 | 99.9 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranku, M.N.; Uwaya, G.E.; Fayemi, O.E. Electrochemical Detection of Dopamine at Fe3O4/SPEEK Modified Electrode. Molecules 2021, 26, 5357. https://doi.org/10.3390/molecules26175357
Ranku MN, Uwaya GE, Fayemi OE. Electrochemical Detection of Dopamine at Fe3O4/SPEEK Modified Electrode. Molecules. 2021; 26(17):5357. https://doi.org/10.3390/molecules26175357
Chicago/Turabian StyleRanku, Mogomotsi N., Gloria E. Uwaya, and Omolola E. Fayemi. 2021. "Electrochemical Detection of Dopamine at Fe3O4/SPEEK Modified Electrode" Molecules 26, no. 17: 5357. https://doi.org/10.3390/molecules26175357
APA StyleRanku, M. N., Uwaya, G. E., & Fayemi, O. E. (2021). Electrochemical Detection of Dopamine at Fe3O4/SPEEK Modified Electrode. Molecules, 26(17), 5357. https://doi.org/10.3390/molecules26175357







