Abietane Diterpenoids from the Hairy Roots of Salvia corrugata
Abstract
1. Introduction
2. Results
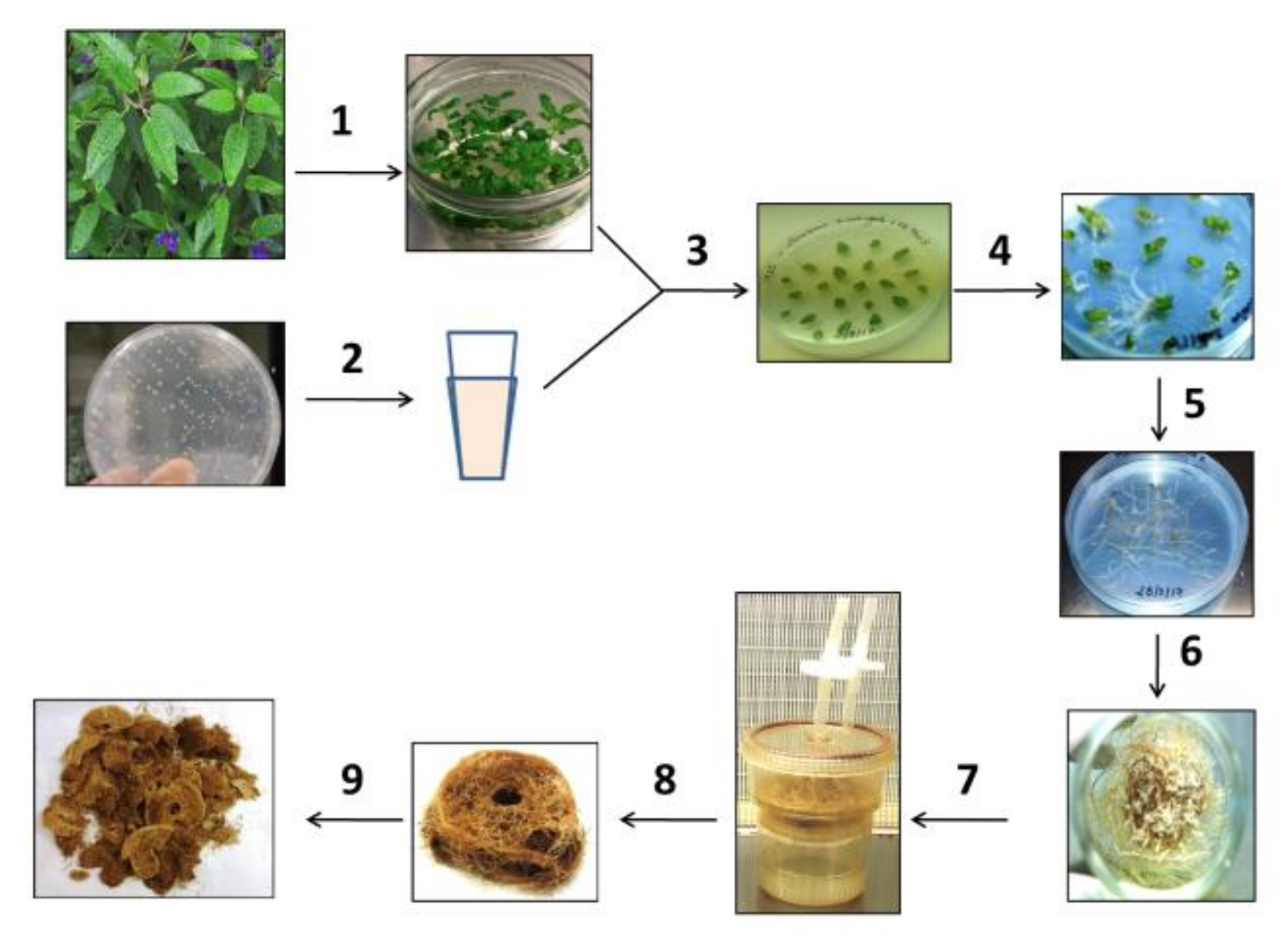
2.1. Establishment of Hairy Root Cultures
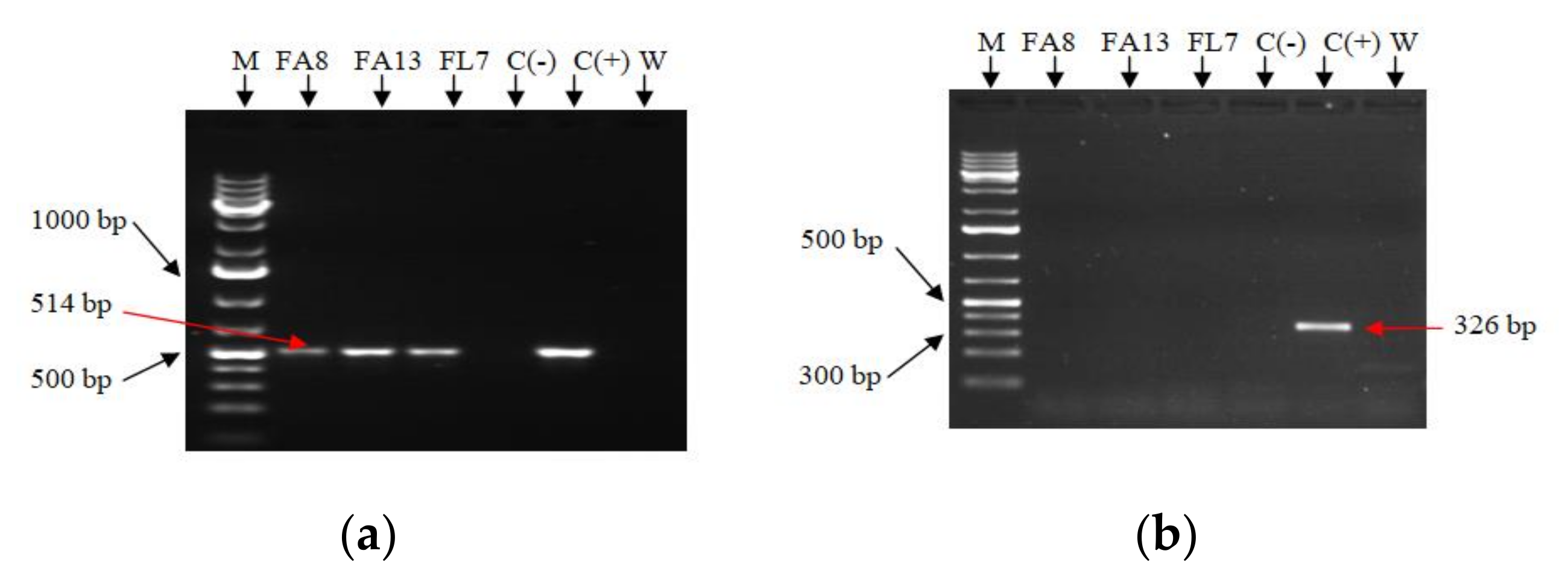
2.2. Confirmation of Transformation
2.3. Growth of Hairy Root Cultures
2.4. Phytochemical Analysis
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Establishment of Hairy Root Cultures
4.4. Confirmation of Transformation
4.5. Growth of Hairy Root Cultures
4.5.1. Effect of Medium Composition
4.5.2. Effect of Initial Sucrose Concentration
4.5.3. Growth Kinetics
4.5.4. Scale up Production
4.6. Phytochemical Analysis
4.6.1. Extraction of the Plant Material
4.6.2. Determination of the Content of Demethylfruticuline A and Fruticuline A in the In Vitro Biomass
4.6.3. Analysis of the Extracts
4.6.4. Determination of the Content of Agastol and Ferruginol
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Halder, M.; Roychowdhury, D.; Jha, S. A critical review on biotechnological interventions for production and yield enhancement of secondary metabolites in hairy root cultures. In Hairy Roots: An Effective Tool of Plant Biotechnology; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Singapore, 2018; pp. 21–44. [Google Scholar]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone diterpenes from Salvia species: Chemistry, botany, and biological activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- Topçu, G.; Yücer, R.; Şenol, H. Bioactive constituents of Anatolian Salvia species. In Salvia Biotechnology; Springer: Cham, Switzerland, 2017; pp. 31–132. [Google Scholar]
- Esquivel, B. Rearranged clerodane and abietane derived diterpenoids from American Salvia species. Nat. Prod. Commun. 2008, 3, 989–1002. [Google Scholar] [CrossRef]
- Esquivel, B.; Sánchez, A.A.; Vergara, F.; Matus, W.; Hernandez-Ortega, S.; Ramírez-Apan, M.T. Abietane Diterpenoids from the Roots of some Mexican Salvia Species (Labiatae): Chemical Diversity, Phytogeographical Significance, and Cytotoxic Activity. Chem. Biodivers. 2005, 2, 738–747. [Google Scholar] [CrossRef]
- Vaccaro, M.C.; Mariaevelina, A.; Malafronte, N.; De Tommasi, N.; Leone, A. Increasing the synthesis of bioactive abietane diterpenes in Salvia sclarea hairy roots by elicited transcriptional reprogramming. Plant Cell Rep. 2017, 36, 375–386. [Google Scholar] [CrossRef]
- Kuźma, Ł. Biosynthesis of biological active abietane diterpenoids in transformed root cultures of Salvia species. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- Kümmritz, S.; Haas, C.; Winkler, K.; Georgiev, V.; Pavlov, A. Hairy roots of Salvia species for bioactive substances production. In Salvia Biotechnology; Georgiev, V., Pavlov, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 271–289. [Google Scholar]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Kabouche, A.; Kabouche, Z. Bioactive diterpenoids of Salvia species. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 35, pp. 753–833. [Google Scholar]
- Topçu, G.; Gören, A.C. Biological activity of diterpenoids isolated from Anatolian Lamiaceae plants. Rec. Nat. Prod. 2007, 1, 1–16. [Google Scholar]
- Becerra, J.; Flores, C.; Mena, J.; Aqueveque, P.; Alarcón, J.; Bittner, M.; Hernández, V.; Hoeneisen, M.; Ruiz, E.; Silva, M. Antifungal and antibacterial activity of diterpenes isolated from wood extractables of Chilean podocarpaceae. Bol. Soc. Chil. Quim. 2002, 47, 151–157. [Google Scholar] [CrossRef]
- Zare, S.; Hatam, G.; Firuzi, O.; Bagheri, A.; Chandran, J.N.; Schneider, B.; Paetz, C.; Pirhadi, S.; Jassbi, A.R. Antileishmanial and pharmacophore modeling of abietane-type diterpenoids extracted from the roots of Salvia hydrangea. J. Mol. Struct. 2021, 1228, 129447. [Google Scholar] [CrossRef]
- González, M.A.; Clark, J.; Connelly, M.; Rivas, F. Antimalarial activity of abietane ferruginol analogues possessing a phthalimide group. Biorg. Med. Chem. Lett. 2014, 24, 5234–5237. [Google Scholar] [CrossRef]
- González-Cardenete, M.A.; Rivas, F.; Basset, R.; Stadler, M.; Hering, S.; Padrón, J.M.; Zaragozá, R.J.; Dea-Ayuela, M.A. Biological profiling of semisynthetic C19-functionalized ferruginol and sugiol analogues. Antibiotics (Basel) 2021, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Roa-Linares, V.C.; Brand, Y.M.; Agudelo-Gomez, L.S.; Tangarife-Castaño, V.; Betancur-Galvis, L.A.; Gallego-Gomez, J.C.; González, M.A. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem. 2016, 108, 79–88. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Perez-Castillo, Y.; Elshabrawy, H.A.; Filho, C.d.S.M.B.; de Sousa, D.P. Bioactive terpenes and their derivatives as potential SARS-CoV-2 proteases inhibitors from molecular modeling studies. Biomolecules 2021, 11, 74. [Google Scholar] [CrossRef]
- Fuzimoto, A.D.; Isidoro, C. The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds—Additional weapons in the fight against the COVID-19 pandemic? J. Tradit. Complement. Med. 2020, 10, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Naguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Luo, D.; Wang, T.; Ji, X.; Xu, G. Vasorelaxant 4,5-seco-abietane diterpenoids with diverse 6/6/6, 6/6/7, and 6/6/8 architectures from Salvia prattii Hemsl. Fitoterapia 2020, 142, 104521. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-J.; Zhou, P.-P.; Lin, C.-J.; Wang, W.-F.; Li, Y.; Gao, K. Diterpenoids from Salvia miltiorrhiza and their immune-modulating activity. J. Agric. Food Chem. 2017, 65, 5985–5993. [Google Scholar] [CrossRef]
- Tabefam, M.; Moridi Farimani, M.; Danton, O.; Ramseyer, J.; Nejad Ebrahimi, S.; Neuburger, M.; Kaiser, M.; Salehi, P.; Potterat, O.; Hamburger, M. Antiprotozoal Isoprenoids from Salvia hydrangea. J. Nat. Prod. 2018, 81, 2682–2691. [Google Scholar] [CrossRef]
- Kadir, A.; Zheng, G.; Zheng, X.; Jin, P.; Maiwulanjiang, M.; Gao, B.; Aisa, H.A.; Yao, G. Structurally diverse diterpenoids from the roots of Salvia deserta based on nine different skeletal types. J. Nat. Prod. 2021, 84, 1442–1452. [Google Scholar] [CrossRef]
- Simmons, E.M.; Sarpong, R. Structure, biosynthetic relationships and chemical synthesis of the icetexane diterpenoids. Nat. Prod. Rep. 2009, 26, 1195–1217. [Google Scholar] [CrossRef]
- Lee, H.-K.; Byon, S.-J.; Oh, S.-R.; Kim, J.-I.; Kim, Y.-H.; Lee, C.-O. Diterpenoids from the roots of Agastache rugosa and their cytotoxic activities. Kor. J. Pharmacogn. 1994, 25, 319–327. [Google Scholar]
- Becker, K.; Schwaiger, S.; Waltenberger, B.; Fuchs, D.; Pezzei, C.K.; Schennach, H.; Stuppner, H.; Gostner, J.M. Immunomodulatory effects of diterpene quinone derivatives from the roots of Horminum pyrenaicum in human PBMC. Oxid. Med. Cell. Longev. 2018, 2018, 2980295. [Google Scholar] [CrossRef]
- Min, B.S.; Hattori, M.; Lee, H.K.; Kim, Y.H. Inhibitory constituents against HIV-1 protease from Agastache rugosa. Arch. Pharm. Res. 1999, 22, 75–77. [Google Scholar] [CrossRef]
- Khan, T.; Khan, M.A.; Karam, K.; Ullah, N.; Mashwani, Z.U.; Nadhman, A. Plant in vitro culture technologies; a promise into factories of secondary metabolites against COVID-19. Front. Plant Sci. 2021, 12, 610194. [Google Scholar] [CrossRef]
- Epling, C. A Revision of Salvia, Subgenus Calosphace; Verlag des Repertoriums: Dahlem dei Berlin, Germany, 1939; Volume 110, p. 383. [Google Scholar]
- Jenks, A.A.; Walker, J.B.; Kim, S.C. Phylogeny of New World Salvia subgenus Calosphace (Lamiaceae) based on cpDNA (psbA-trnH) and nrDNA (ITS) sequence data. J. Plant Res. 2013, 126, 483–496. [Google Scholar] [CrossRef]
- Bisio, A.; Romussi, G.; Russo, E.; Cafaggi, S.; Schito, A.M.; Repetto, B.; De Tommasi, N. Antimicrobial activity of the ornamental species Salvia corrugata, a potential new crop for extractive purposes. J. Agric. Food Chem. 2008, 56, 10468–10472. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bertrand, S.; Nievergelt, A.; Zwick, V.; Simoes-Pires, C.; Marcourt, L.; Rivara-Minten, E.; Cuendet, M.; Bisio, A.; Wolfender, J.L. Cancer chemopreventive diterpenes from Salvia corrugata. Phytochemistry 2013, 96, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Fraternale, D.; Schito, A.M.; Parricchi, A.; Dal Piaz, F.; Ricci, D.; Giacomini, M.; Ruffoni, B.; De Tommasi, N. Establishment and analysis of in vitro biomass from Salvia corrugata Vahl. and evaluation of antimicrobial activity. Phytochemistry 2016, 122, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hedge, I. Labiatae of South-West Asia: Diversity, distribution and endemism. Proc. R. Soc. Edinb. Sect. B Biol. Sci. 1986, 89, 23–35. [Google Scholar] [CrossRef]
- Monticone, M.; Bisio, A.; Daga, A.; Giannoni, P.; Giaretti, W.; Maffei, M.; Pfeffer, U.; Romeo, F.; Quarto, R.; Romussi, G.; et al. Demethyl Fruticulin A (SCO-1) causes apoptosis by inducing reactive oxygen species in mitochondria. J. Cell. Biochem. 2010, 111, 1149–1159. [Google Scholar] [CrossRef]
- Fronza, M.; Murillo, R.; Ślusarczyk, S.; Adams, M.; Hamburger, M.; Heinzmann, B.; Laufer, S.; Merfort, I. In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011, 19, 4876–4881. [Google Scholar] [CrossRef]
- Tezuka, Y.; Kasimu, R.; Li, J.X.; Basnet, P.; Tanaka, K.; Namba, T.; Kadota, S. Constituents of roots of Salvia deserta Schang. (Xinjiang-Danshen). Chem. Pharm. Bull. 1998, 46, 107–112. [Google Scholar] [CrossRef]
- Jonathan, L.T.; Che, C.-T.; Pezzuto, J.M.; Fong, H.H.S.; Farnsworth, N.R. 7-O-Methylhorminone and other cytotoxic diterpene quinones from Lepechinia bullata. J. Nat. Prod. 1989, 52, 571–575. [Google Scholar] [CrossRef]
- Rüedi, P. 8α,9α-Epoxy-7-oxoroyleanon, ein diterpen-epoxychinon aus einer abessinischen Plectranthus-Art (Labiatae). Helv. Chim. Acta 1984, 67, 1116–1120. [Google Scholar] [CrossRef]
- Rodríguez, B. 1H and 13C NMR spectral assignments of some natural abietane diterpenoids. Magn. Reson. Chem. 2003, 41, 741–746. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Zou, Z.-M.; Cong, P.-Z.; Yu, D.-Q. Diterpenoids from the Roots of Agastache rugosa. JCPS 1997, 6, 115–118. [Google Scholar]
- Davies, K.M.; Deroles, S.C. Prospects for the use of plant cell cultures in food biotechnology. Curr. Opin. Biotechnol. 2014, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Giovannini, A.; Ruffoni, B.; Bertoli, A.; Pistelli, L. Hairy root cultures for secondary metabolites production. In Bio-farms for Nutraceuticals. Advances in Experimental Medicine and Biology; Giardi, M.T., Rea, G., Berra, B., Eds.; Springer: Boston, MA, USA, 2010; Volume 698. [Google Scholar]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia officinalis L. hairy root cultures for the production of rosmarinic acid. Z. Naturforsch. C 2006, 61, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Zhi, B.H.; Alfermann, A.W. Diterpenoid production in hairy root cultures of Salvia miltiorrhiza. Phytochemistry 1993, 32, 699–703. [Google Scholar] [CrossRef]
- Ruffoni, B.; Bertoli, A.; Pistelli, L.; Pistelli, L. Micropropagation of Salvia wagneriana Polak and hairy root cultures with rosmarinic acid production. Nat. Prod. Res. 2016, 30, 2538–2544. [Google Scholar] [CrossRef]
- D’Angiolillo, F.; Dos Santos, F.; Barberini, S.; Pistelli, L.; Pistelli, L.; Ruffoni, B. Utilizzo di due ceppi di Agrobacterium rhizogenes per la trasformazione di Ocimum basilicum L: Risultati preliminari. In Proceedings of the 2 Convegno Nazionale di Micropropagazione; Acta Italus Hortus, SOI: Sesto Fiorentino, FI, Italy, 2012; pp. 199–203. [Google Scholar]
- Hu, Z.-B.; Du, M. Hairy root and its application in plant genetic engineering. J. Integr. Plant. Biol. 2006, 48, 121–127. [Google Scholar] [CrossRef]
- Mascheretti, I.; Alfieri, M.; Lauria, M.; Locatelli, F.; Consonni, R.; Cusano, E.; Dougué Kentsop, R.A.; Laura, M.; Ottolina, G.; Faoro, F.; et al. New insight into Justicidin B pathway and production in Linum austriacum. Int. J. Mol. Sci. 2021, 22, 2507. [Google Scholar] [CrossRef]
- Kuźma, L.; Bruchajzer, E.; Wysokińska, H. Diterpenoid production in hairy root culture of Salvia sclarea L. Z. Naturforsch. C 2008, 63, 621–624. [Google Scholar] [CrossRef]
- Sivakumar, G.; Yu, K.W.; Paek, K.Y. Production of biomass and ginsenosides from adventitious roots of Panax ginseng in bioreactor cultures. Eng. Life Sci. 2005, 5, 333–342. [Google Scholar] [CrossRef]
- Bozena, B.; Szczerba, J. Influence of different carbon sources on invertase activity and growth of sour cherry (Prunus cerasus L.) shoot cultures. J. Exp. Bot. 1991, 42, 911–915. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, M.L. Transgenic hairy roots. recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.P.; Chen, F.; Zhang, Y.L.; Song, J.Y. Tanshinone production in Ti-transformed Salvia miltiorrhiza cell suspension cultures. J. Biotechnol. 1997, 58, 147–156. [Google Scholar] [CrossRef]
- Petrova, M.; Zayova, E.; Dincheva, I.; Badjakov, I.; Vlahova, M. Influence of carbon sources on growth and GC-MS based metabolite profiling of Arnica montana L. hairy roots. Turk. J. Biol. 2015, 39, 469–478. [Google Scholar] [CrossRef]
- Ślesak, H.; Skoczowski, A.; Przywara, L. Exogenous carbohydrate utilisation by explants of Brassica napus cultured in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2004, 79, 45–51. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Maria John, K.M.; Yang, Y.-S.; Kim, S.-H.; Chung, I.-M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Taya, M.; Yoyama, A.; Kondo, O.; Kobayashi, T.; Matsui, C. Growth characteristics of plant hairy roots and their cultures in bioreactors. J. Chem. Eng. Jpn. 1989, 22, 84–89. [Google Scholar] [CrossRef]
- Urbańska, N.; Giebułtowicz, J.; Olszowska, O.; Szypuła, W. The growth and saponin production of Platycodon grandiflorum (Jacq.) A. DC. (Chinese bellflower) hairy roots cultures maintained in shake flasks and mist bioreactor. Acta Soc. Bot. Pol. 2014, 83, 229–237. [Google Scholar] [CrossRef][Green Version]
- Georgiev, M.I.; Pavlov, A.I.; Bley, T. Hairy root type plant in vitro systems as sources of bioactive substances. Appl. Microbiol. Biotechnol. 2007, 74, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.N.; Ranjan, R. Growth of hairy-root cultures in various bioreactors for the production of secondary metabolites. Biotechnol. Appl. Biochem. 2008, 49, 1–10. [Google Scholar] [CrossRef]
- Wei, T.; Gao, Y.; Deng, K.; Zhang, L.; Yang, M.; Liu, X.; Qi, C.; Wang, C.; Song, W.; Zhang, Y.; et al. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root cultures by metabolic engineering. Plant Methods 2019, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Leroy, B.; Mariage, P.-A.; Wattiez, R. Proteomic analysis reveals novel insights into tanshinones biosynthesis in Salvia miltiorrhiza hairy roots. Sci. Rep. 2019, 9, 5768. [Google Scholar] [CrossRef]
- Pei, T.; Ma, P.; Ding, K.; Liu, S.; Jia, Y.; Ru, M.; Dong, J.; Liang, Z. SmJAZ8 acts as a core repressor regulating JA-induced biosynthesis of salvianolic acids and tanshinones in Salvia miltiorrhiza hairy roots. J. Exp. Bot. 2017, 69, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced diterpene tanshinone accumulation and bioactivity of transgenic Salvia miltiorrhiza hairy roots by pathway engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Li, G.; Liu, Y.; Gao, W.; Huang, L. Effects of combined elicitors on tanshinone metabolic profiling and SmCPS expression in Salvia miltiorrhiza hairy root cultures. Molecules 2013, 18, 7473–7485. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kwok, K.W.; Wu, J.Y. Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy-root culture by hyperosmotic stress and yeast elicitor. Biotechnol. Appl. Biochem. 2007, 46, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Zheng, L.P.; Tian, H.; Wang, J.W. Synergistic effects of ultraviolet-B and methyl jasmonate on tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. J. Photochem. Photobiol. B 2016, 159, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Zheng, L.P.; Yuan, H.Y.; Wang, J. Propagation of Salvia miltiorrhiza from hairy root explants via somatic embryogenesis and tanshinone content in obtained plants. Ind. Crops Prod. 2013, 50, 648–653. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Shi, M. Ultrahigh diterpenoid tanshinone production through repeated osmotic stress and elicitor stimulation in fed-batch culture of Salvia miltiorrhiza hairy roots. Appl. Microbiol. Biotechnol. 2008, 78, 441–448. [Google Scholar] [CrossRef]
- Xing, B.; Yang, D.; Guo, W.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Ag+ as a more effective elicitor for production of tanshinones than phenolic acids in Salvia miltiorrhiza hairy roots. Molecules 2014, 20, 309–324. [Google Scholar] [CrossRef]
- Zhou, Z.; Tan, H.; Li, Q.; Chen, J.; Gao, S.; Wang, Y.; Chen, W.; Zhang, L. CRISPR/Cas9-mediated efficient targeted mutagenesis of RAS in Salvia miltiorrhiza. Phytochemistry 2018, 148, 63–70. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kaiser, M.; Wysokińska, H. The production and antiprotozoal activity of abietane diterpenes in Salvia austriaca hairy roots grown in shake flasks and bioreactor. Prep. Biochem. Biotechnol. 2017, 47, 58–66. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Wysokińska, H.; Różalski, M.; Krajewska, U.; Kisiel, W. An unusual taxodione derivative from hairy roots of Salvia austriaca. Fitoterapia 2012, 83, 770–773. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kisiel, W.; Królicka, A.; Wysokińska, H. Genetic transformation of Salvia austriaca by Agrobacterium rhizogenes and diterpenoid isolation. Pharmazie 2011, 66, 904–907. [Google Scholar]
- Fraga, B.M.; Díaz, C.E.; Guadaño, A.; González-Coloma, A. Diterpenes from Salvia broussonetii transformed roots and their insecticidal activity. J. Agric. Food Chem. 2005, 53, 5200–5206. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag⁺, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, R.; Babalar, M.; Mirmasoumi, M. Investigation of hairy root induction in some Salvia L. species. Nova Biologica Reperta 2017, 4, 173–180. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Skrzypek, Z.; Wysokińska, H. Diterpenoids and triterpenoids in hairy roots of Salvia sclarea. Plant Cell Tissue Organ. Cult. 2006, 84, 171–179. [Google Scholar] [CrossRef]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and aethiopinone from Salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Med. 2007, 73, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.; Georgiev, V.; Ivanov, I.; Badjakov, I.; Pavlov, A. Two-phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.). Biotechnol. Lett. 2011, 33, 1873–1878. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Skała, E.; Kiss, A.K. Hairy root cultures of Salvia viridis L. for production of polyphenolic compounds. Ind. Crops Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1986, 3, 307–322. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1987, 4, 399–413. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1990, 7, 149–164. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1992, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1994, 11, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1996, 13, 59–71. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2002, 19, 125–132. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2003, 20, 70–78. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2004, 21, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2005, 22, 594–602. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2006, 23, 875–885. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2009, 26, 1156–1171. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2011, 28, 1755–1772. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2015, 32, 1654–1663. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2015, 32, 76–87. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2016, 33, 1227–1238. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2017, 34, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-M.; Kachroo, A.; Kachroo, P. Chemical inducers of systemic immunity in plants. J. Exp. Bot. 2014, 65, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Bathe, U. The Role of Cytochrome P450 Enzymes in the Biosynthesis of Abietane Diterpenes from Rosemary and Sage. Kumulative Dissertation, Martin-Luther-Universität Halle-Wittenberg, Halle (Saale), Germany, 2019. [Google Scholar]
- Liang, Z.-S.; Yang, D.-F.; Liang, X.; Zhang, Y.-J.; Liu, Y.; Liu, F.-H. Roles of reactive oxygen species in methyl jasmonate and nitric oxide-induced tanshinone production in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2012, 31, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Will, M.; Claßen-Bockhoff, R. Time to split Salvia sl (Lamiaceae)—New insights from Old World Salvia phylogeny. Mol. Phylogenet. Evol. 2017, 109, 33–58. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant. Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Harnessing the potential of hairy roots: Dawn of a new era. Trends Biotechnol. 2006, 24, 403–409. [Google Scholar] [CrossRef]
- Pedreño, M.A.; Almagro, L. Carrot hairy roots: Factories for secondary metabolite production. J. Exp. Bot. 2020, 71, 6861–6864. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mascarello, C.; Mantovani, E.; Ruffoni, B. In vitro culture of several ornamental and medicinal Salvia species. In Proceedings of the I International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation; Acta Horticulturae, ISHS: Sanremo, Italy, 2006; pp. 375–380. [Google Scholar]
- Figlan, S.; Makunga, N.P. Genetic transformation of the medicinal plant Salvia runcinata L. f. using Agrobacterium rhizogenes. S. Afr. J. Bot. 2017, 112, 193–202. [Google Scholar] [CrossRef]
- Hooykaas, P.; Klapwijk, P.; Nuti, M.; Schilperoort, R.; Rörsch, A. Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent agrobacteria and to Rhizobium ex planta. Microbiology 1977, 98, 477–484. [Google Scholar] [CrossRef]
- Bisio, A.; Corallo, A.; Gastaldo, P.; Romussi, G.; Ciarallo, G.; Fontana, N.; De Tommasi, N.; Profumo, P. Glandular hairs and secreted material in Salvia blepharophylla Brandegee ex Epling grown in Italy. Ann. Bot. 1999, 83, 441–452. [Google Scholar] [CrossRef]
- David, R.; Carde, J.P. Coloration différentielle dês inclusions lipidique et terpeniques dês pseudophylles du pin maritime au moyen du reactif Nadi. Comptes Rendus Acad. Sci. Paris 1964, 258, 1338–1340. [Google Scholar]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. J. Vis. Exp. 2010, 45, e2565. [Google Scholar] [CrossRef]
- Klimyuk, V.I.; Carroll, B.J.; Thomas, C.M.; Jones, J.D. Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J. 1993, 3, 493–494. [Google Scholar] [CrossRef]
- Vaira, A.; Semeria, L.; Crespi, S.; Lisa, V.; Allavena, A.; Accotto, G. Resistance to tospoviruses in Nicotiana benthamiana transformed with the N gene of tomato spotted wilt virus: Correlation between transgene expression and protection in primary transformants. Mol. Plant-Microbe Interact. 1995, 8, 66–73. [Google Scholar] [CrossRef]
- Scorza, R.; Zimmerman, T.; Cordts, J.; Footen, K.; Ravelonandro, M. Horticultural characteristics of transgenic tobacco expressing the rolC gene from Agrobacterium rhizogenes. J. Am. Soc. Hort. Sci. 1994, 119, 1091–1098. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. In Proceedings of the International Plant Propagators’ Society Congress; IPPS: Louth, Lincolnshire, UK, 1980; pp. 421–427. [Google Scholar]
- Okamura, N.; Kobayashi, K.; Yagi, A.; Kitazawa, T.; Shimomura, K. High-performance liquid chromatography of abietane-type compounds. J. Chromatogr. A 1991, 542, 317–326. [Google Scholar] [CrossRef]
- D’Amelia, V.; Ruggiero, A.; Tranchida-Lombardo, V.; Leone, A.; Tucci, M.; Docimo, T. Biosynthesis of salvia specialized metabolites and biotechnological approaches to increase their production. In Salvia Biotechnology; Georgiev, V., Pavlov, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 241–270. [Google Scholar]
- Shi, M.; Liao, P.; Nile, S.H.; Georgiev, M.I.; Kai, G. Biotechnological exploration of transformed root culture for value-added products. Trends Biotechnol. 2021, 39, 137–149. [Google Scholar] [CrossRef] [PubMed]

| A. rhizogenes Strain | N° of Treated Explants | N° of Explants Producing Roots and Relative Percentage |
|---|---|---|
| ATCC 15834 | 16 | 12 (75%) |
| LBA 9402 | 21 | 8 (38.1%) |
| Control | 21 | 3 (14.3%) |
| Clones | Number of Bioreactors (Total Liquid Volume) | FW (g)/Bioreactor (FW/L of Total MS Used) | DW (g)/Bioreactor) (DW/L of Total MS Used) | Total FW Produced (g) | Total DW Produced (g) |
|---|---|---|---|---|---|
| SCO-HR-FA8 | 13 (15.6 L) | 37.5 ± 2.3 a (31.23 ± 1.9) | 3.5 ± 0.1 b (2.9 ± 0.1) | 487.8 | 46.0 |
| SCO-HR-FA13 | 4 (4.8 L) | 35.4 ± 2.4 a (29.5 ± 2) | 4.16 ± 0.38 c (3.5 ± 0.3) | 143.4 | 16.5 |
| SCO-HR-FL7 | 1 (1.2 L) | 23.3 | 2.4 | 23.3 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kentsop, R.A.D.; Iobbi, V.; Donadio, G.; Ruffoni, B.; De Tommasi, N.; Bisio, A. Abietane Diterpenoids from the Hairy Roots of Salvia corrugata. Molecules 2021, 26, 5144. https://doi.org/10.3390/molecules26175144
Kentsop RAD, Iobbi V, Donadio G, Ruffoni B, De Tommasi N, Bisio A. Abietane Diterpenoids from the Hairy Roots of Salvia corrugata. Molecules. 2021; 26(17):5144. https://doi.org/10.3390/molecules26175144
Chicago/Turabian StyleKentsop, Roméo Arago Dougué, Valeria Iobbi, Giuliana Donadio, Barbara Ruffoni, Nunziatina De Tommasi, and Angela Bisio. 2021. "Abietane Diterpenoids from the Hairy Roots of Salvia corrugata" Molecules 26, no. 17: 5144. https://doi.org/10.3390/molecules26175144
APA StyleKentsop, R. A. D., Iobbi, V., Donadio, G., Ruffoni, B., De Tommasi, N., & Bisio, A. (2021). Abietane Diterpenoids from the Hairy Roots of Salvia corrugata. Molecules, 26(17), 5144. https://doi.org/10.3390/molecules26175144











