Semi-Synthesis and In Vitro Anti-Cancer Evaluation of Magnolol Derivatives
Abstract
:1. Introduction
2. Results and Discussion
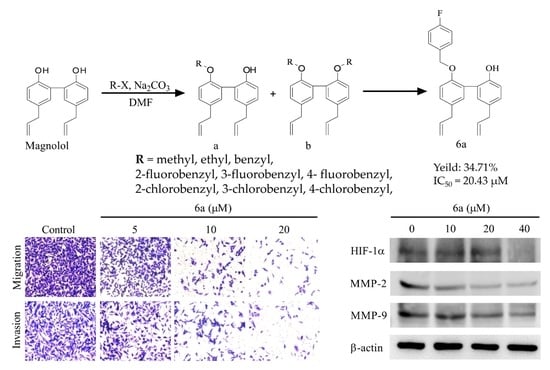
2.1. Synthesis and Characterization of the Derivatives
2.2. In Vitro Cytotoxic Activity
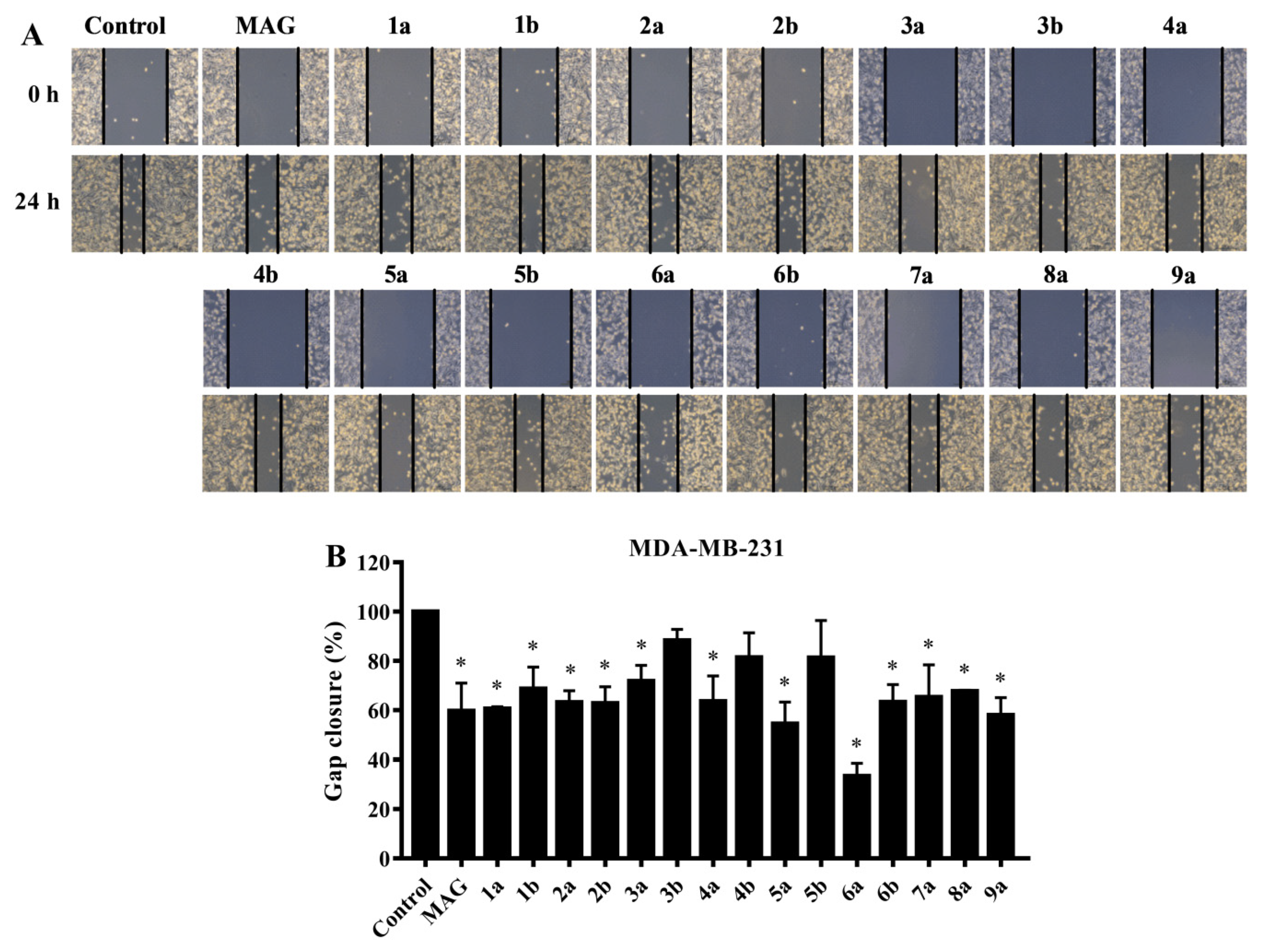
2.3. Preliminary Screening of the Inhibitory Effect on MDA-MB-231 Cell Migration
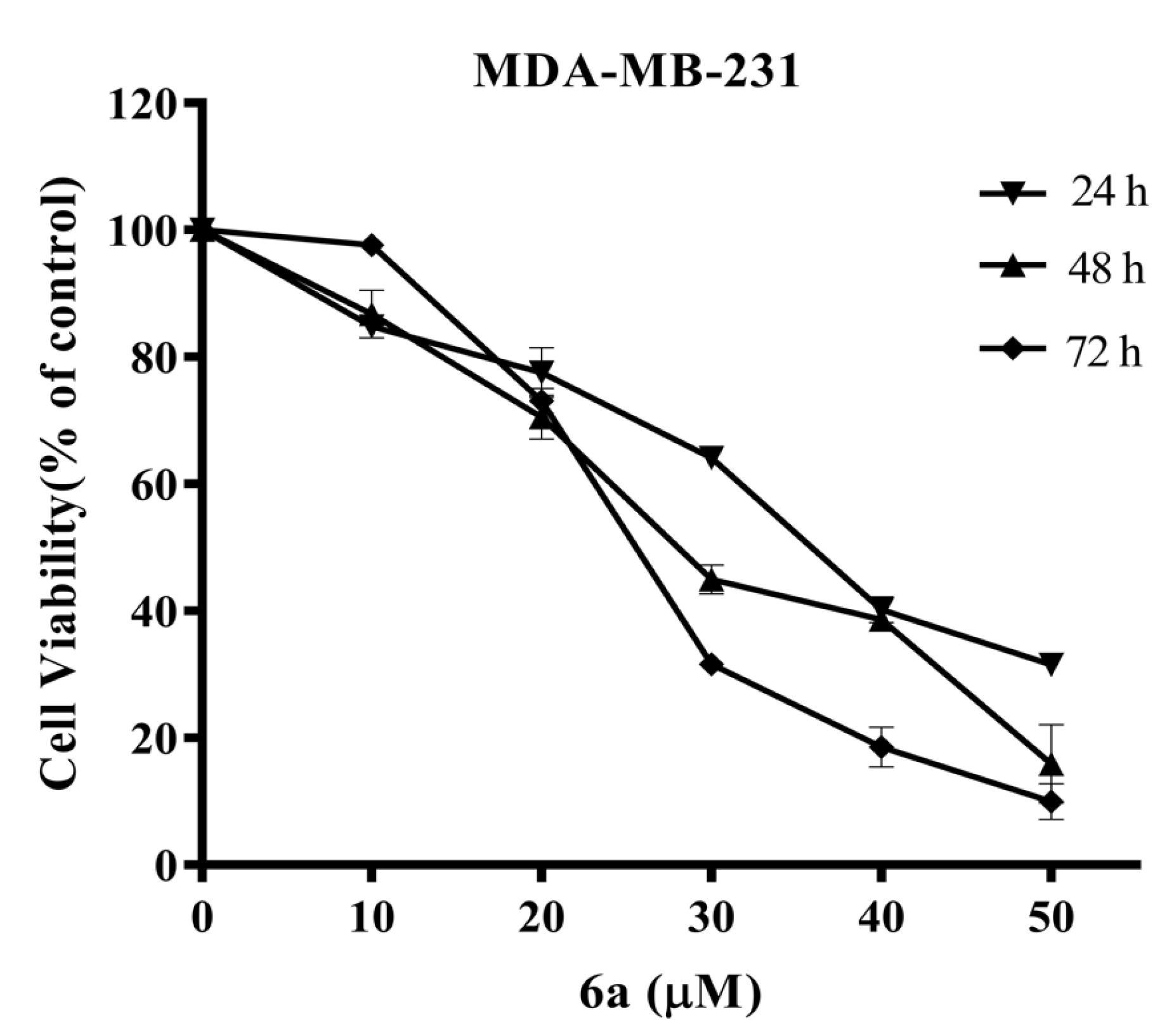
2.4. Anti-Proliferative Activity of Compound 6a in MDA-MB-231 Cells
2.5. Suppressed Migration and Invasion of MDA-MB-231 Cells by Compound 6a
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Preparation of 1a–9a and 1b–6b
3.3. MTT Assay
3.4. Wound-Healing Assay
3.5. Cell Migration Assay
3.6. Cell Invasion Assay
3.7. Western Blotting
3.8. Statiscal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Loi, S.; Pommey, S.; Haibe-Kains, B.; Beavis, P.A.; Darcy, P.K.; Smyth, M.J.; Stagg, J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 11091–11096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyslop, T.; Michael, Y.; Avery, T.; Rui, H. Population and target considerations for triple-negative breast cancer clinical trials. Biomark. Med. 2013, 7, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. Scaffold Diversity Inspired by the Natural Product Evodiamine: Discovery of Highly Potent and Multitargeting Antitumor Agents. J. Med. Chem. 2015, 58, 6678–6696. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Xu, X.; Lu, Q.; Pan, Q.; Hu, X. Schisandrin B: A dual inhibitor of P-glycoprotein and multidrug resistance-associated protein 1. Cancer Lett. 2007, 246, 300–307. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Stone, R. Biochemistry. Lifting the veil on traditional Chinese medicine. Science 2008, 319, 709–710. [Google Scholar] [CrossRef]
- He, L.; Fan, T.; Hu, J.; Zhang, L. Polyethylene glycol-based ultrasound-assisted extraction of magnolol and honokiol from Cortex Magnoliae Officinalis. Nat. Prod. Res. 2015, 29, 31–36. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; He, Y.; Gu, C.; Zhu, K.; Li, S.; Li, Y.; Lu, X.; Liu, J.; Zhang, N.; et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018, 196, 69–76. [Google Scholar] [CrossRef]
- Huang, S.Y.; Tai, S.H.; Chang, C.C.; Tu, Y.F.; Chang, C.H.; Lee, E.J. Magnolol protects against ischemic-reperfusion brain damage following oxygen-glucose deprivation and transient focal cerebral ischemia. Int. J. Mol. Med. 2018, 41, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhou, Y.; Niu, X.; Wang, T.; Li, J.; Liu, Z.; Wang, J.; Tang, S.; Wang, Y.; Deng, X. Magnolol restores the activity of meropenem against NDM-1-producing Escherichia coli by inhibiting the activity of metallo-beta-lactamase. Cell Death Discov. 2018, 4, 28. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.J.; Park, H.J.; Chung, H.J.; Min, H.Y.; Park, E.J.; Lee, M.A.; Shin, Y.; Lee, S.K. Wnt/β-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol. Pharmacol. 2012, 82, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Park, S.S.; Lee, U.S.; Kim, W.J.; Moon, S.K. Signaling pathway for TNF-alpha-induced MMP-9 expression: Mediation through p38 MAP kinase, and inhibition by anti-cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int. Immunopharmacol. 2008, 8, 1821–1826. [Google Scholar] [CrossRef]
- Kim, G.D.; Oh, J.; Park, H.J.; Bae, K.; Lee, S.K. Magnolol inhibits angiogenesis by regulating ROS-mediated apoptosis and the PI3K/AKT/mTOR signaling pathway in mES/EB-derived endothelial-like cells. Int. J. Oncol. 2013, 43, 600–610. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Chemical composition, antioxidant activity and cytotoxicity on tumour cells of the essential oil from flowers of Magnolia grandiflora cultivated in Iran. Nat. Prod. Res. 2017, 31, 2857–2864. [Google Scholar] [CrossRef]
- Zhao, M.; Zheng, Y.H.; Zhao, Q.Y.; Zheng, W.; Yang, J.H.; Pei, H.Y.; Liu, L.; Liu, K.J.; Xue, L.L.; Deng, D.X.; et al. Synthesis and evaluation of new compounds bearing 3-(4-aminopiperidin-1-yl)methyl magnolol scaffold as anticancer agents for the treatment of non-small cell lung cancer via targeting autophagy. Eur. J. Med. Chem. 2021, 209, 112922. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, Z.; Guo, Y.; Bai, L.P. Semisynthesis of novel magnolol-based Mannich base derivatives that suppress cancer cells via inducing autophagy. Eur. J. Med. Chem. 2020, 205, 112663. [Google Scholar] [CrossRef]
- Ren, C.; Wang, J.; Tan, Y.; Guo, M.; Guo, J.; Liu, Y.; Wu, X.; Feng, Y. Synthesis, Characterization and Biological Evaluation of Magnolol and Honokiol Derivatives with 1,3,5-Triazine of Metformin Cyclization. Molecules 2020, 25, 5779. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Y.; Li, D.; Fu, S.; Tang, M.; Wan, L.; Chen, K.; Liu, Z.; Xue, L.; Peng, A.; et al. Discovery and synthesis of novel magnolol derivatives with potent anticancer activity in non-small cell lung cancer. Eur. J. Med. Chem. 2018, 156, 190–205. [Google Scholar] [CrossRef]
- Amblard, F.; Govindarajan, B.; Lefkove, B.; Rapp, K.L.; Detorio, M.; Arbiser, J.L.; Schinazi, R.F. Synthesis, cytotoxicity, and antiviral activities of new neolignans related to honokiol and magnolol. Bioorg. Med. Chem. Lett. 2007, 17, 4428–4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Li, B.; Ma, H.; Huang, X.; Wang, H.; Dai, Y.; Li, Y.; Li, H.M.; Wu, C.Z. Synthesis and in vitro antitumor evaluation of honokiol derivatives. Bioorg. Med. Chem. Lett. 2020, 30, 126849. [Google Scholar] [CrossRef]
- Singh, T.; Katiyar, S.K. Honokiol inhibits non-small cell lung cancer cell migration by targeting PGE₂-mediated activation of β-catenin signaling. PLoS ONE 2013, 8, e60749. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Seo, N.J.; Jeong, S.J.; Park, Y.; Jung, D.B.; Koh, W.; Lee, H.J.; Lee, E.O.; Ahn, K.S.; Ahn, K.S.; et al. Oral administration of penta-O-galloyl-β-d-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis 2011, 32, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Lee, J.K.; Jang, E.H.; Jeong, S.Y.; Kim, J.H. Shikonin blocks migration and invasion of human breast cancer cells through inhibition of matrix metalloproteinase-9 activation. Oncol. Rep. 2014, 31, 2827–2833. [Google Scholar] [CrossRef] [Green Version]
- Shahi Thakuri, P.; Gupta, M.; Singh, S.; Joshi, R.; Glasgow, E.; Lekan, A.; Agarwal, S.; Luker, G.D.; Tavana, H. Phytochemicals inhibit migration of triple negative breast cancer cells by targeting kinase signaling. BMC Cancer 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. HIF-1 and tumor progression: Pathophysiology and therapeutics. Trends Mol. Med. 2002, 8, S62–S67. [Google Scholar] [CrossRef]
- Xu, Q.H.; Xiao, Y.; Li, X.Q.; Fan, L.; Zhou, C.C.; Cheng, L.; Jiang, Z.D.; Wang, G.H. Resveratrol Counteracts Hypoxia-Induced Gastric Cancer Invasion and EMT through Hedgehog Pathway Suppression. Anticancer Agents Med. Chem. 2020, 20, 1105–1114. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Bramhachari, P.V.; Raghu, G.; El-Rayes, B.F. Hypoxia inducible factor-1α: Its role in colorectal carcinogenesis and metastasis. Cancer Lett. 2015, 366, 11–18. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Sherapura, A.; Vigneshwaran, V.; Basappa, G.; Vivek, H.K.; Prabhakar, B.T.; Khanum, S.A. Targeting HIF-1α by newly synthesized Indolephenoxyacetamide (IPA) analogs to induce anti-angiogenesis-mediated solid tumor suppression. Pharmacol. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, Y.S.; Min, S.Y.; Song, J.Y. Overexpression of MMP-9 and HIF-1α in Breast Cancer Cells under Hypoxic Conditions. J. Breast Cancer 2011, 14, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojima, I. Use of fluorine in the medicinal chemistry and chemical biology of bioactive compounds—A case study on fluorinated taxane anticancer agents. ChemBioChem 2004, 5, 628–635. [Google Scholar] [CrossRef]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]



| Compound | IC50 Values (μM) | |||
|---|---|---|---|---|
| MDA-MB-231 a | MCF-7 b | CNE-2Z c | SMMC-7721 d | |
| 1a | >50 e | 34.7 ± 0.87 f | 45.87 ± 0.51 | >50 |
| 1b | >50 | >50 | >50 | >50 |
| 2a | >50 | 29.76 ± 1.06 | 47 ± 1.29 | 44.49 ± 4.41 |
| 2b | >50 | >50 | >50 | >50 |
| 3a | 22.85 ± 2.15 | 26.32 ± 2.39 | 25.85 ± 4.47 | 30.21 ± 3.06 |
| 3b | >50 | >50 | >50 | >50 |
| 4a | 26.95 ± 1.04 | 26.77 ± 2.12 | 27.2 ± 2.31 | 28.08 ± 3.64 |
| 4b | >50 | >50 | >50 | >50 |
| 5a | 24.51 ± 0.62 | 23.61 ± 1.03 | 27.81 ± 2.74 | 27.94 ± 1.08 |
| 5b | >50 | >50 | >50 | >50 |
| 6a | 20.43 ± 2.17 | 28.27 ± 2.89 | 22.35 ± 2.55 | 23.07 ± 1.95 |
| 6b | >50 | >50 | 36.47 ± 4.20 | >50 |
| 7a | >50 | 32.83 ± 0.28 | 45.06 ± 2.69 | 34.35 ± 4.00 |
| 8a | 48.93 ± 4.49 | 30.90 ± 0.47 | 46.64 ± 0.69 | 32.97 ± 5.39 |
| 9a | >50 | 30.65 ± 0.98 | 36.95 ± 0.56 | 32.30 ± 1.59 |
| MAG | 54.70 ± 0.95 | 46.37 ± 1.75 | 57.52 ± 3.22 | 58.24 ± 4.78 |
| Taxol | 0.69 ± 0.06 | 0.43 ± 0.04 | 1.71 ± 0.19 | 0.22 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.-L.; Zhu, M.-L.; Dai, Y.-Q.; Li, H.-M.; Li, B.-H.; Ma, H.; Zhang, C.-H.; Wu, C.-Z. Semi-Synthesis and In Vitro Anti-Cancer Evaluation of Magnolol Derivatives. Molecules 2021, 26, 4302. https://doi.org/10.3390/molecules26144302
Sun X-L, Zhu M-L, Dai Y-Q, Li H-M, Li B-H, Ma H, Zhang C-H, Wu C-Z. Semi-Synthesis and In Vitro Anti-Cancer Evaluation of Magnolol Derivatives. Molecules. 2021; 26(14):4302. https://doi.org/10.3390/molecules26144302
Chicago/Turabian StyleSun, Xiao-Long, Mei-Lin Zhu, Yi-Qun Dai, Hong-Mei Li, Bo-Han Li, Hui Ma, Chang-Hao Zhang, and Cheng-Zhu Wu. 2021. "Semi-Synthesis and In Vitro Anti-Cancer Evaluation of Magnolol Derivatives" Molecules 26, no. 14: 4302. https://doi.org/10.3390/molecules26144302
APA StyleSun, X.-L., Zhu, M.-L., Dai, Y.-Q., Li, H.-M., Li, B.-H., Ma, H., Zhang, C.-H., & Wu, C.-Z. (2021). Semi-Synthesis and In Vitro Anti-Cancer Evaluation of Magnolol Derivatives. Molecules, 26(14), 4302. https://doi.org/10.3390/molecules26144302