Oleogel Formulations for the Topical Delivery of Betulin and Lupeol in Skin Injuries—Preparation, Physicochemical Characterization, and Pharmaco-Toxicological Evaluation
Abstract
1. Introduction
2. Results
2.1. Physicochemical Properties of Oleogels
2.1.1. Macroscopic Examination
2.1.2. Determination of pH
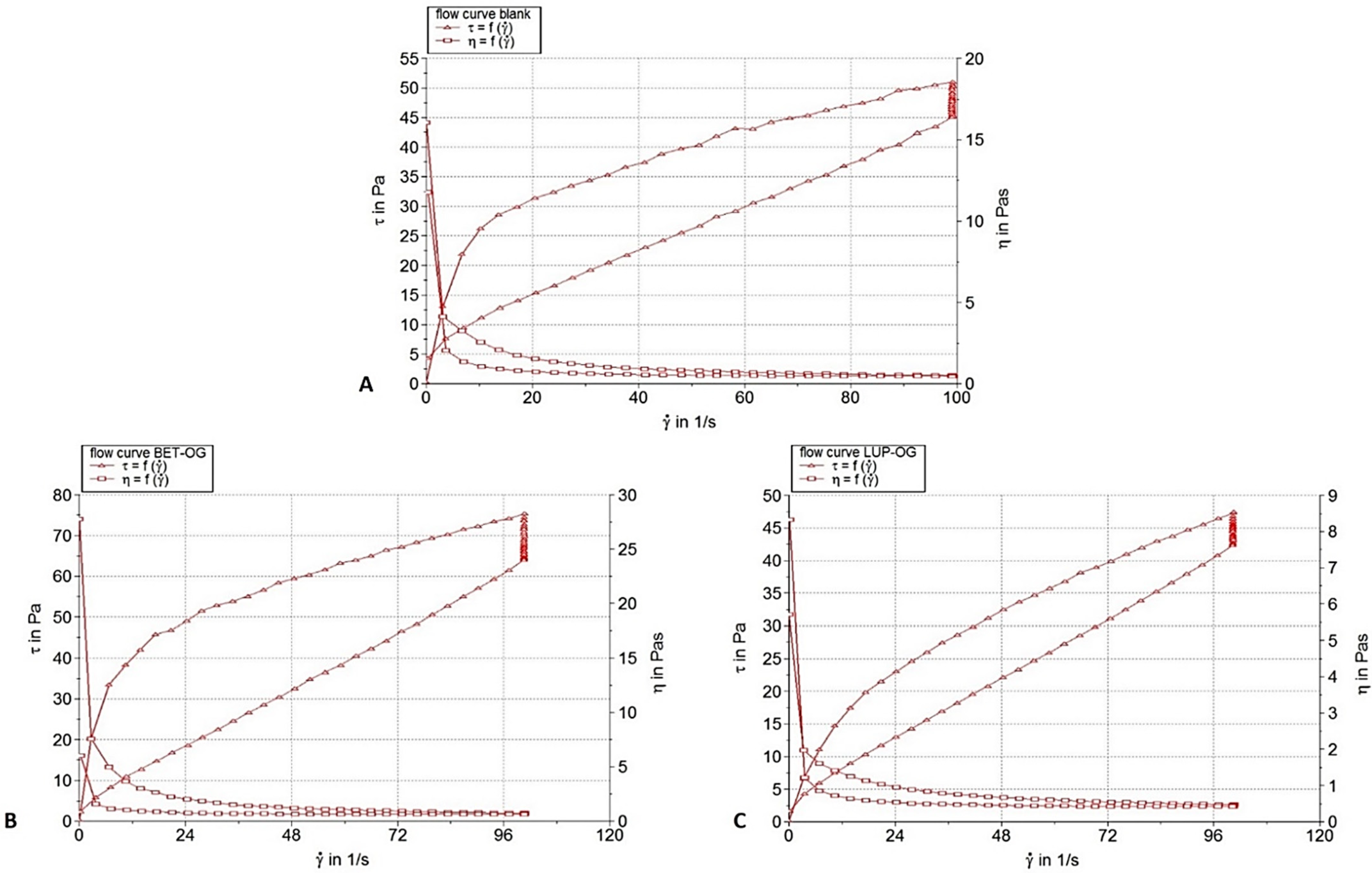
2.1.3. Rheological Characterization
2.1.4. Textural Measurements
2.1.5. FT-IR Investigations of Oleogels
2.2. Skin Permeation Study
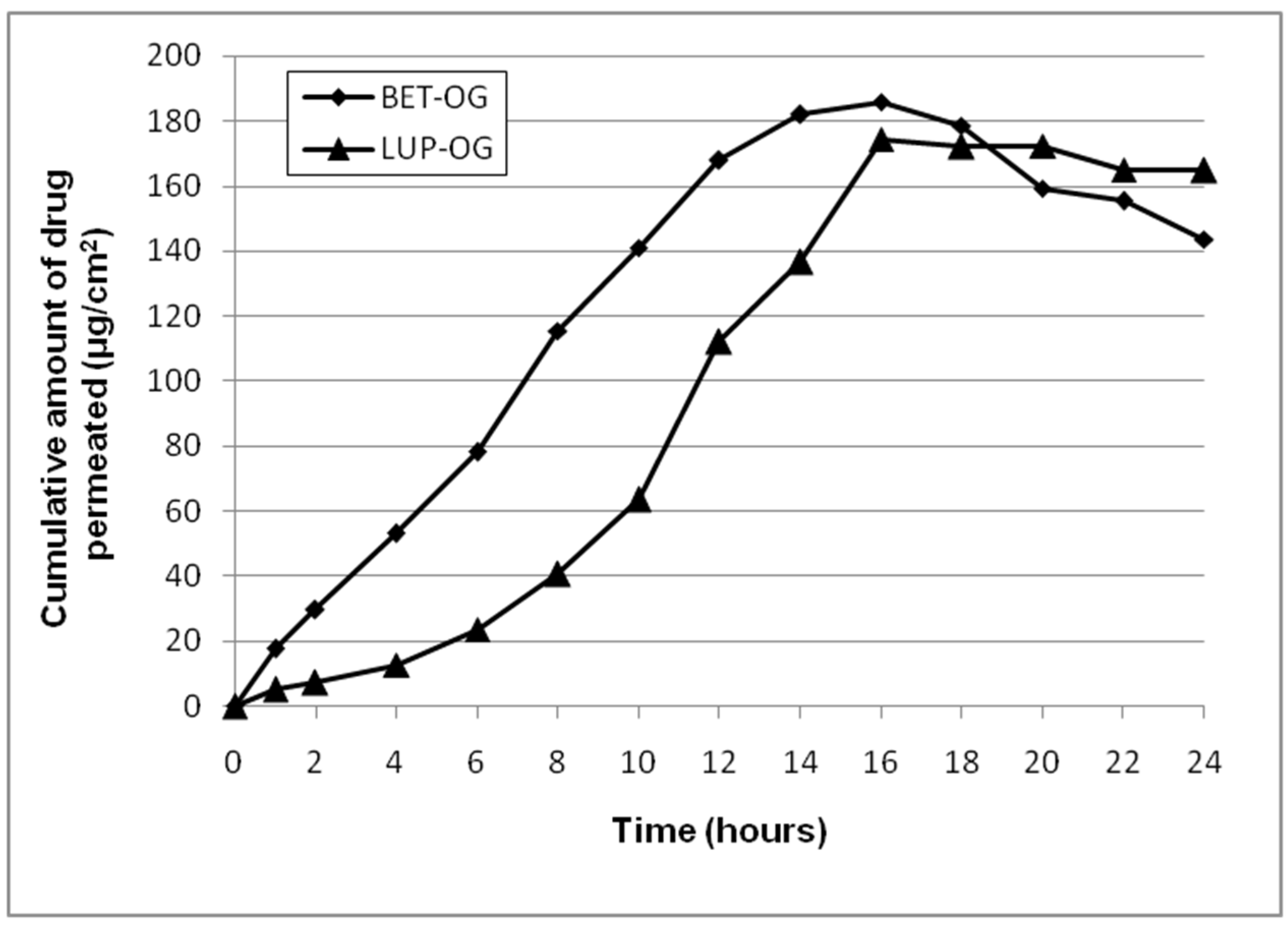
2.2.1. Ex Vivo Permeation Test
2.2.2. Evaluation of Drug Retention on Skin
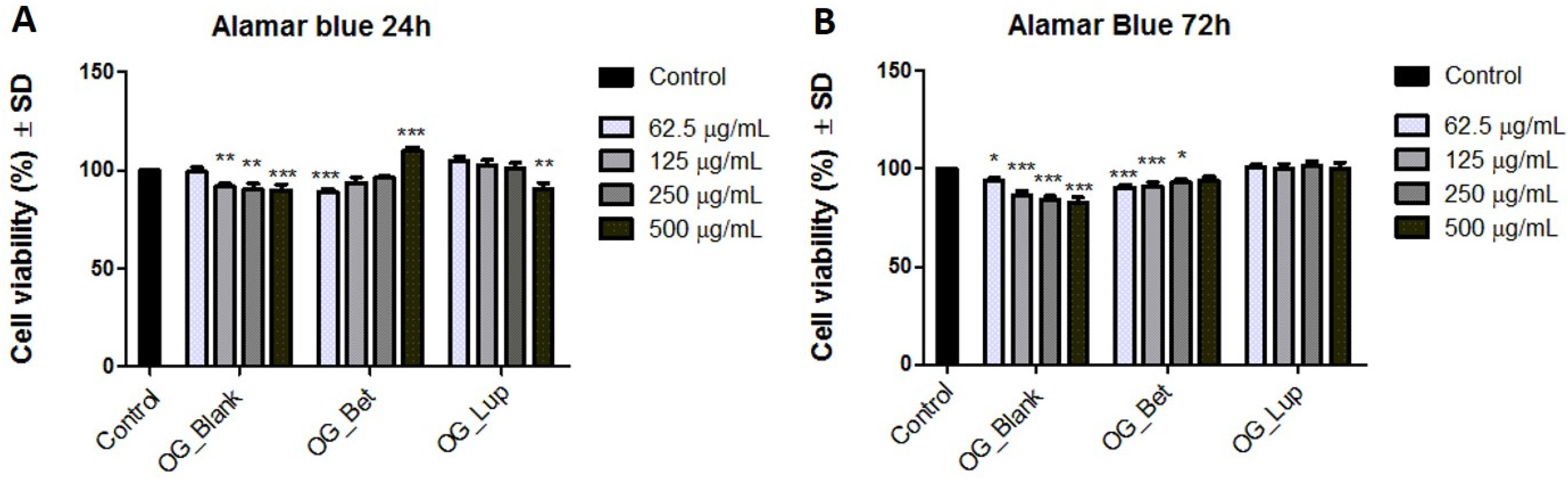
2.3. Cellular Viability Assessment on Human Immortalized Keratinocytes (HaCaT)
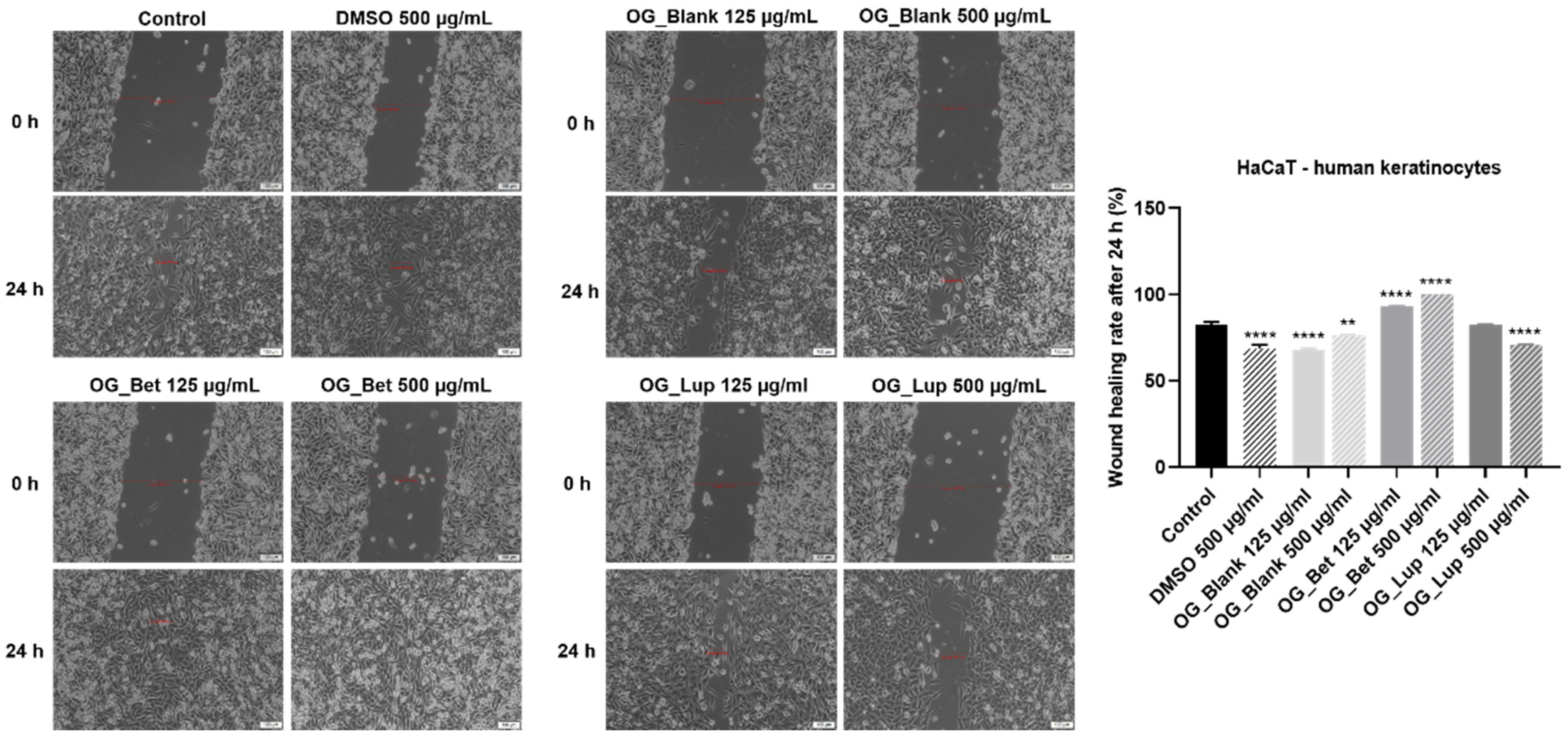
2.4. Wound Healing or Scratch Assay
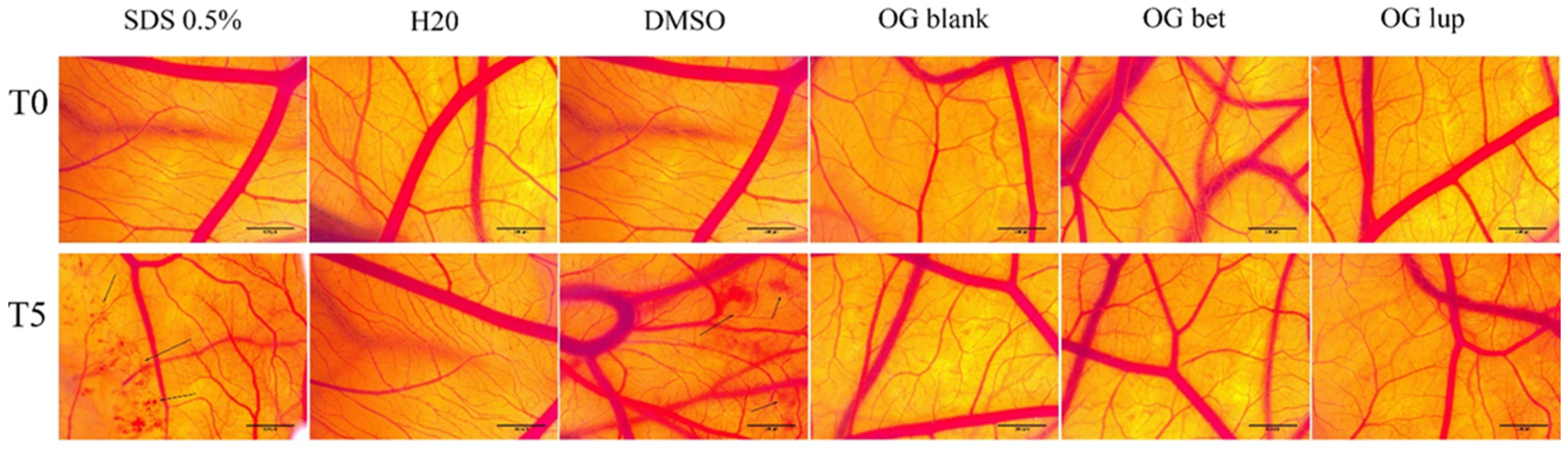
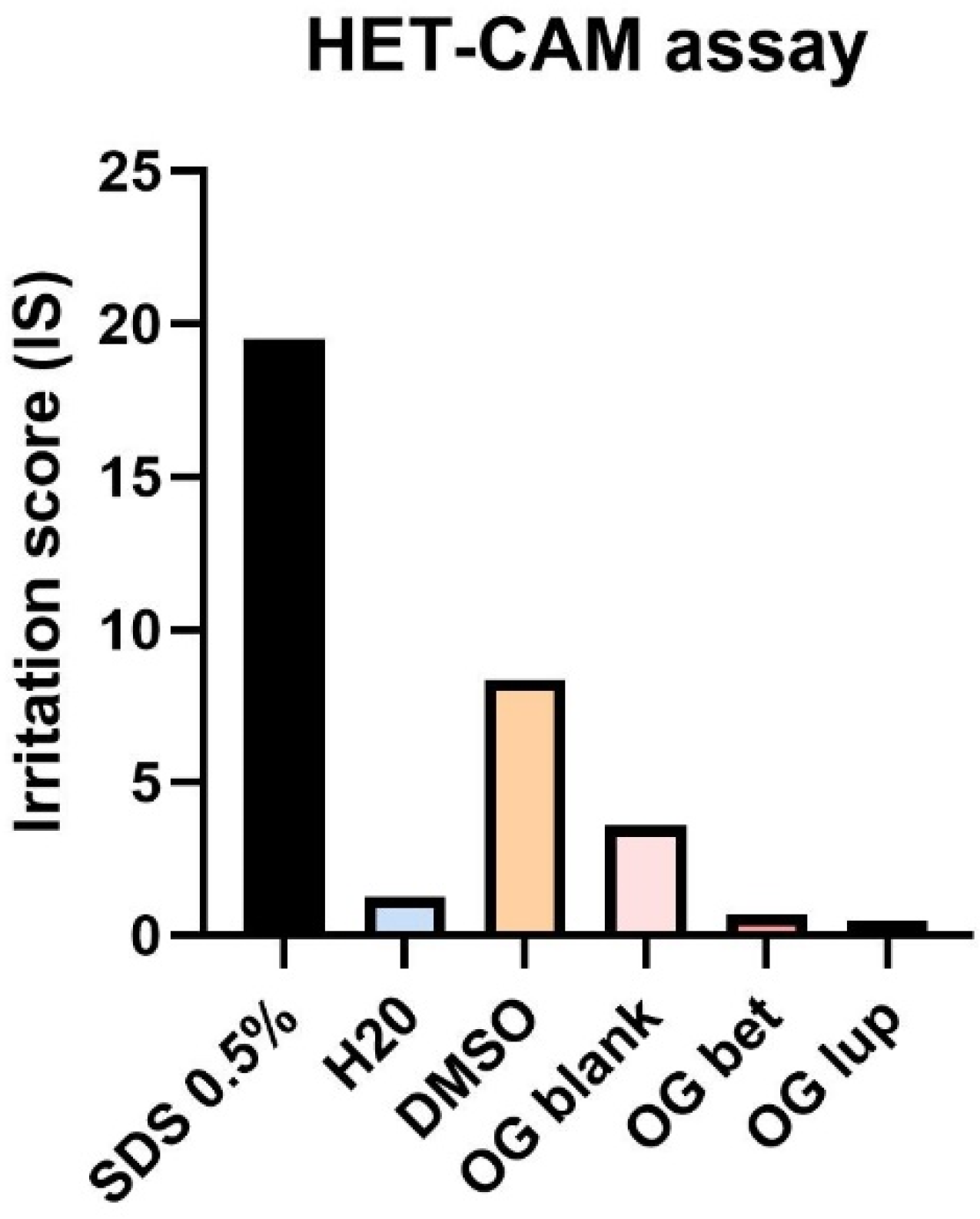
2.5. Hen Embryo Chorioallantoic Membrane Assay (HET-CAM)
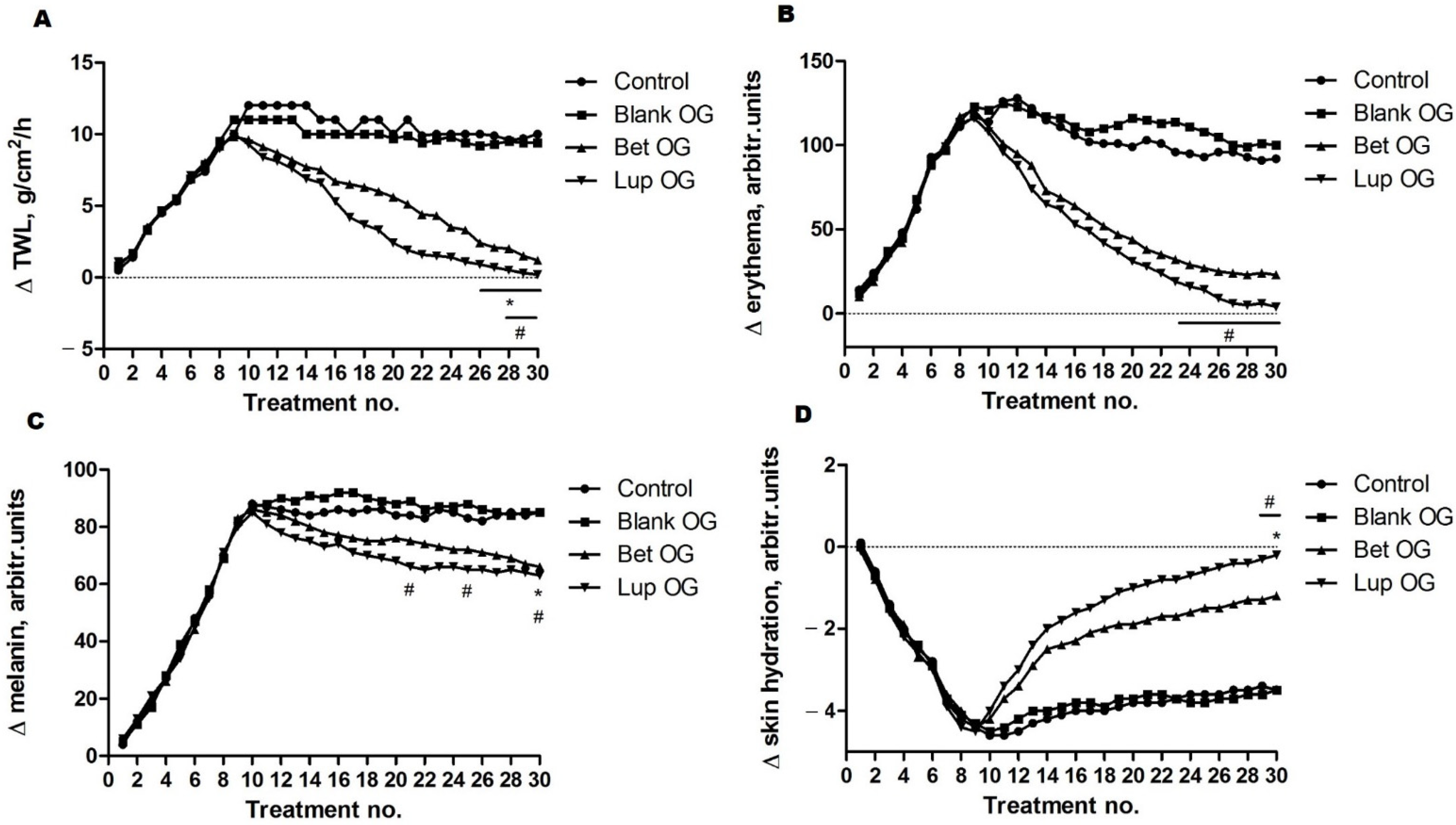
2.6. Skin Biophysical Parameters Assessment
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Chemical and Reagents
4.1.2. In Vitro Evaluations
4.2. Animals
4.3. Methods
4.3.1. Preparation of Betulin/Lupeol Oleogels and Blank Formulations
4.3.2. Physicochemical Characterization of Oleogels
pH Measurement
Rheological Characterization
Textural Measurements
FT-IR Spectroscopy
4.3.3. Ex Vivo Skin Permeation Studies
Preparation of the Skin Samples
Ex Vivo Permeation Tests
Evaluation of Drug Retention in the Skin
Data Analysis of Ex Vivo Drug Permeation Studies
4.3.4. Cell Viability Assessment
4.3.5. Wound Healing or Scratch Assay
4.3.6. Hen Embryo Chorioallantoic Membrane Assay
4.3.7. Skin Biophysical Parameters Assessment
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Nuschke, A.; Yates, C.C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 2016, 49, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nat. Cell Biol. 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Bosanquet, D.C.; Harding, K.G. Wound duration and healing rates: Cause or effect? Wound Repair Regen. 2014, 22, 143–150. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hy-pothesis. Plast Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [PubMed]
- Vahidnezhad, H.; Youssefian, L.; Saeidian, A.H.; Uitto, J. Phenotypic Spectrum of Epidermolysis Bullosa: The Paradigm of Syndromic versus Non-Syndromic Skin Fragility Disorders. J. Investig. Dermatol. 2019, 139, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Bauer, J.; Bodemer, C.; Bolling, M.; Bruckner-Tuderman, L.; Diem, A.; Fine, J.-D.; Heagerty, A.; Hovnanian, A.; Marinkovich, M.; et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br. J. Dermatol. 2020, 183, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.-D.; Bruckner-Tuderman, L.; Eady, R.A.; Bauer, E.A.; Bauer, J.W.; Has, C.; Heagerty, A.; Hintner, H.; Hovnanian, A.; Jonkman, M.F.; et al. Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. J. Am. Acad. Dermatol. 2014, 70, 1103–1126. [Google Scholar] [CrossRef]
- Has, C.; He, Y. Research Techniques Made Simple: Immunofluorescence Antigen Mapping in Epidermolysis Bullosa. J. Investig. Dermatol. 2016, 136, e65–e71. [Google Scholar] [CrossRef]
- Has, C.; Liu, L.; Bolling, M.; Charlesworth, A.; El Hachem, M.; Escámez, M.J.; Fuentes, I.; Büchel, S.; Hiremagalore, R.; Pohla-Gubo, G.; et al. Clinical practice guidelines for laboratory diagnosis of epidermolysis bullosa. Br. J. Dermatol. 2019, 182, 574–592. [Google Scholar] [CrossRef]
- Uitto, J.; Atanasova, V.S.; Jiang, Q.; South, A.P. Precision Medicine for Heritable Skin Diseases—The Paradigm of Epidermolysis Bullosa. J. Investig. Dermatol. Symp. Proc. 2018, 19, S74–S76. [Google Scholar] [CrossRef]
- Bradford, A.; Barlow, A.; Chazot, P.L. Probing the differential effects of infrared light sources IR1072 and IR880 on human lymphocytes: Evidence of selective cytoprotection by IR1072. J. Photochem. Photobiol. B Biol. 2005, 81, 9–14. [Google Scholar] [CrossRef]
- Jobe, N.P.; Živicová, V.; Mifková, A.; Rösel, D.; Dvoˇránková, B.; Kodet, O.; Strnad, H.; Koláˇr, M.; Šedo, A.; Smetana, K.; et al. Fibroblasts potentiate melanoma cells In Vitro invasiveness induced by UV-irradiated keratinocytes. Histochem. Cell Biol. 2018, 149, 503–516. [Google Scholar] [CrossRef]
- Pacholczyk, M.; Czernicki, J.; Ferenc, T. The effect of solar ultraviolet radiation (UVR) on induction of skin cancers. Med. Pr. 2016, 67, 255–266. [Google Scholar] [CrossRef]
- Micka-Michalak, K.; Biedermann, T.; Reichmann, E.; Meuli, M.; Klar, A.S. Induction of angiogenic and inflamma-tion-associated dermal biomarkers following acute UVB exposure on bio-engineered pigmented dermo-epidermal skin substi-tutes In Vivo. Pediatric Surg. Int. 2019, 35, 129–136. [Google Scholar] [CrossRef]
- Cios, A.; Ciepielak, M.; Szyma’nski, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Dif-ferent Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Zhang, J.; Sun, P.; Qian, Y.; Zhao, X. Protective Effects of Kuding Tea (Ilex kudingcha C. J. Tseng) Polyphenols on UVB-Induced Skin Aging in SKH1 Hairless Mice. Molecules 2019, 24, 1016. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Penna, I.; Albanesi, E.; Bertorelli, R.; Bandiera, T.; Russo, D. Cytoprotective, anti-inflammatory, and antioxidant properties of high-molecular-weight hyaluronan enriched with red orange extract in human fibroblasts exposed to ultra violet light b irra-diation: Cytoprotective, anti-inflammatory, and antioxidant properties. Biotechnol. Appl. Biochem. 2019, 66, 273–280. [Google Scholar] [PubMed]
- DEBRA International. Wound healing in epidermolysis bullosa clinical research review and priority-setting consensus meeting. In Proceedings of the British Association of Dermatologists, Fitzroy Square, London, UK, 5–6 December 2016. Available online: http://www.debra-international.org/fileadmin/media_data/4_DEBRA_International/5_Research/Research_events/DEBRA_Wound_Healing_Meeting_Report.pdf (accessed on 18 May 2021).
- Uitto, J.; Bruckner-Tuderman, L.; McGrath, J.A.; Riedl, R.; Robinson, C. EB2017—Progress in Epidermolysis Bullosa Research toward Treatment and Cure. J. Investig. Dermatol. 2018, 138, 1010–1016. [Google Scholar] [CrossRef]
- Nyström, A.; Bernasconi, R.; Bornert, O. Therapies for genetic extracellular matrix diseases of the skin. Matrix Biol. 2018, 71–72, 330–347. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Injury- and inflammation-driven skin fibrosis: The paradigm of epidermolysis bullosa. Matrix Biol. 2018, 68–69, 547–560. [Google Scholar] [CrossRef]
- Woelfle, U.; Laszczyk, M.N.; Kraus, M.; Leuner, K.; Kersten, A.; Simon-Haarhaus, B.; Scheffler, A.; Martin, S.F.; Müller, W.E.; Nashan, D.; et al. Triterpenes promote keratinocyte differentiation in vitro, ex vivo and in vivo: A role for the transient receptor potential canonical (subtype) 6. J. Investig. Dermatol. 2010, 130, 113–123. [Google Scholar] [CrossRef]
- Schwieger-Briel, A.; Kiritsi, D.; Schempp, C.; Has, C.; Schumann, H. Betulin-Based Oleogel to Improve Wound Healing in Dystrophic Epidermolysis Bullosa: A Prospective Controlled Proof-of-Concept Study. Dermatol. Res. Pr. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef]
- Beserra, F.P.; Xue, M.; Maia, G.L.D.A.; Rozza, A.L.; Pellizzon, C.H.; Jackson, C.J. Lupeol, a Pentacyclic Triterpene, Promotes Migration, Wound Closure, and Contractile Effect In Vitro: Possible Involvement of PI3K/Akt and p38/ERK/MAPK Pathways. Molecules 2018, 23, 2819. [Google Scholar] [CrossRef]
- Pereira Beserra, F.; Sérgio Gushiken, L.F.; Vieira, A.J.; Augusto Bérgamo, D.; Luísa Bérgamo, P.; Oliveira de Souza, M.; Al-berto Hussni, C.; Kiomi Takahira, R.; Henrique Nóbrega, R.; Monteiro Martinez, E.R.; et al. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-κB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef] [PubMed]
- Agra, L.C.; Ferro, J.N.S.; Barbosa, F.T.; Barreto, E. Triterpenes with healing activity: A systematic review. J. Dermatol. Treat. 2015, 26, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Ski. Res. Technol. 2006, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig ear skin ex vivo as a model for in vivo dermatopharmacoki-netic studies in man. Pharm Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Şoica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.; Gǎluşcan, A.; Cinta-Pinzaru, S.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Central J. 2012, 6, 137. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M. Antitumor and Antiviral Activity of Pentacyclic Triterpenes. Mini Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as Potentially Cytotoxic Compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef]
- Battineni, J.K.; Koneti, P.K.; Bakshi, V.; Boggula, N. Triterpenoids: A review. Int. J. Pharm. Pharm. Sci. 2018, 3, 91–96. [Google Scholar]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2016, 34, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Rosca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macasoi, I.; Olariu, T.; Dehelean, C.; Cretu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Eciency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kashiwada, Y.; Chen, C.H.; Qian, K.; Susan, L.; Natschke, M.; Lee, K.H.; Takaishi, Y. Conjugates of Betulin Deriva-tives with AZT as Potent Anti-HIV Agents. Bioorg Med. Chem. 2010, 18, 6451–6469. [Google Scholar] [CrossRef] [PubMed]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic acid as a potent and complex antitumor phyto-chemical: A minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef]
- Blaževski, J.; Petković, F.; Momčilović, M.; Paschke, R.; Kaluđerović, G.N.; Mostarica Stojković, M.; Miljković, D. Betulinic acid regulates generation of neuroinflammatory mediators responsible for tissue destruction in multiple sclerosis in vitro. Acta Pharmacol. Sin. 2013, 34, 424–431. [Google Scholar] [CrossRef]
- Weckesser, S.; Schumann, H.; Laszczyk, M.; Müller, M.; Schempp, C.; Weckesser, S.; Schumann, H.; Laszczyk, M.; Müller, M.; Schempp, C. Topical Treatment of Necrotising Herpes Zoster with Betulin from Birch Bark. Forsch. Komplement. Res. Comp. Med. 2010, 17, 271–273. [Google Scholar] [CrossRef]
- Huyke, C.; Reuter, J.; Rödig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic ker-atoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J. Dtsch. Dermatol. Ges. 2009, 7, 128–133. [Google Scholar]
- Aburahma, M.H.; Badr-Eldin, S.M. Compritol 888 ATO: A multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin. Drug Deliv. 2014, 11, 1865–1883. [Google Scholar] [CrossRef]
- Guidoni, M.; Figueira, M.M.; Ribeiro, G.P.; Lenz, D.; Grizotto, P.A.; de Melo Costa Pereira, T.; Scherer, R.; Bogusz, S., Jr.; Fronza, M. Development and evaluation of a vegetable oil blend formulation for cutaneous wound healing. Arch. Dermatol. Res. 2019, 311, 443–452. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Behera, B.; Rafanan, R.R.; Bhattacharya, C.; Pal, K.; Banerjee, I.; Rousseau, D. Organo-gels as Matrices for Con-trolled Drug Delivery: A Review on the Current State. Soft Mater. 2014, 12, 47–72. [Google Scholar] [CrossRef]
- Co, E.D.; Marangoni, A.G. Oleogel: An Introduction. In Edible Oleogels. Structure and Health Implications, 2nd, ed.; Marangoni, A.G., Garti, N., Eds.; AOCS Press: Urbana, IL, USA, 2018; pp. 1–29. [Google Scholar]
- Council of Europe. European Pharmacopeia, 10th ed.; Suppl. 10.2. Gycerol Dibehenate: 2767; EDQM Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Devi, R.; Agarwal, S. Some Multifunctional Lipid Excipients and Their Pharmaceutical Applications. Int. J. Pharm. Pharm. Sci. 2019, 11, 1–7. [Google Scholar] [CrossRef]
- Badea, M.; FlorOian, L.; Seculin, T.; Moga, M.; Marculescu, A. Romanian Pharmacopoeia, 10th ed.; Medical Publishing House: Bucharest, Romania, 1993; p. 952. [Google Scholar]
- Grysko, M.; Daniels, R. Evaluation of the mechanism of gelation of an oleogel based on a triterpene extract from the outer bark of birch. Die Pharm. 2013, 68, 572–577. [Google Scholar]
- Steinbrenner, I.; Houdek, P.; Pollok, S.; Brandner, J.M.; Daniels, R. Influence of the Oil Phase and Topical Formulation on the Wound Healing Ability of a Birch Bark Dry Extract. PLoS ONE 2016, 11, e0155582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Muktar, B.; Bello, I.; Sallau, M. Isolation, characterization and antimicrobial study of lupeol acetate from the root bark of Fig-Mulberry Sycamore (Ficus sycomorus LINN). J. Appl. Sci. Environ. Manag. 2018, 22, 1129. [Google Scholar] [CrossRef]
- Cinta-Pinzaru, S.; A Dehelean, C.; Soica, C.; Culea, M.; Borcan, F. Evaluation and differentiation of the Betulaceae birch bark species and their bioactive triterpene content using analytical FT-vibrational spectroscopy and GC-MS. Chem. Central J. 2012, 6, 67. [Google Scholar] [CrossRef]
- Fǎlǎmaş, A.; Pînzaru, S.C.; Dehelean, C.A.; Peev, C.I.; Soica, C. Betulin and its natural resource as potential anticancer drug candidate seen by FT-Raman and FT-IR spectroscopy. J. Raman Spectrosc. 2011, 42, 97–107. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells. Antioxidants 2021, 10, 85. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Arrifin Mohd, N.H.; Hasham, R. Assesment of non-invasive techniques and herbal based products on dermatological physi-ology and intercellular lipid properties. Helyon 2020, 6, e03955. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopeia, 10th ed.; Suppl. 10.2. Potentiometric determination of pH: 24; EDQM Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- Färber, A.; Daniels, R. Ex vivo Skin Permeation of Betulin from Water-in-Oil Foams. Ski. Pharmacol. Physiol. 2016, 29, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Günther, A.; Makuch, E.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Pełech, R.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Betulin and Its Derivatives. Molecules 2021, 26, 3435. [Google Scholar] [CrossRef] [PubMed]
- Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High Concen-trations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients 2020, 12, 3600. [Google Scholar] [CrossRef]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Murillo, G.J.; Betancourt, J.E.; Oliver, P.; Damiana, T. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Ciurlea, S.; Bojin, M.F.; Csanyi, E.; Ionescu, D.; Borcan, F.; Gălușcan, A.; Dehelean, C.A. Evaluation of Skin Parameters Changes in Chemical and Photochemical Initiated Tumors on SKH1 Mice. Fiziologia 2011, 3, 18–22. [Google Scholar]


| Formulation | Organoleptic Parameters | pH | |||
|---|---|---|---|---|---|
| Appearance | Color | Odor | Opacity | ||
| Blank OG | smooth-oily | yellowish-white | odorless | opaque | 7.803 ± 0.025 |
| Bet OG | smooth-oily | yellowish-white | odorless | opaque | 7.117 ± 0.005 |
| Lup OG | smooth-oily | yellowish-white | odorless | opaque | 7.750 ± 0.01 |
| Formulation | Blank OG | Bet OG | Lup OG |
|---|---|---|---|
| Viscosity (Pa·s) | 0.478 | 0.681 | 0.442 |
| Thixotropy (Pa/s) | 1314 | 2451 | 901.1 |
| Parameters of Herschel–Bulkley model K (Pa·sn) n (dimensionless) | 4.428 0.503 | 5.55 0.535 | 1.882 0.679 |
| R (flow curve) R (viscosity curve) | 0.9107 0.9907 | 0.9005 0.9734 | 0.9621 0.9811 |
| Formulation | Firmness (g) | Spreadability (g.s) |
|---|---|---|
| Blank OG | 273.80 ± 0.824 | 165.09 ± 0.603 |
| Bet OG | 30.53 ± 0.258 | 18.23 ± 0.397 |
| Lup OG | 20.37 ± 0.147 | 9.02 ± 0.105 |
| Formulation | Permeation Parameters | Release Parameter | Drug Retention in the Skin (μg/cm2 of Skin) | |
|---|---|---|---|---|
| Js (μg/cm2/h) | tL (h) | k (μg/cm2/h1/2) | ||
| Bet OG | 13.38 ± 1.05 | - | 69.60 ± 2.38 | 580.62 ± 9.31 |
| Lup OG | 15.57 ± 1.18 | 5.1 | 99.76 ± 4.51 | 539.27 ± 8.18 |
| SDS 1% | DMSO 0.5% | H2O | Blank OG | Bet OG | Lup OG | |
|---|---|---|---|---|---|---|
| IS | 19.52 | 8.36 | 1.27 | 3.61 | 0.67 | 0.46 |
| tH | 27 s | 52 | 300 | 300 | 300 | 300 |
| tL | 19 s | 168 | 300 | 287 | 300 | 296 |
| tC | 22 s | 264 | 260 | 192 | 280 | 290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pârvănescu, R.D.; Watz, C.-G.; Moacă, E.-A.; Vlaia, L.; Marcovici, I.; Macașoi, I.G.; Borcan, F.; Olariu, I.; Coneac, G.; Drăghici, G.-A.; et al. Oleogel Formulations for the Topical Delivery of Betulin and Lupeol in Skin Injuries—Preparation, Physicochemical Characterization, and Pharmaco-Toxicological Evaluation. Molecules 2021, 26, 4174. https://doi.org/10.3390/molecules26144174
Pârvănescu RD, Watz C-G, Moacă E-A, Vlaia L, Marcovici I, Macașoi IG, Borcan F, Olariu I, Coneac G, Drăghici G-A, et al. Oleogel Formulations for the Topical Delivery of Betulin and Lupeol in Skin Injuries—Preparation, Physicochemical Characterization, and Pharmaco-Toxicological Evaluation. Molecules. 2021; 26(14):4174. https://doi.org/10.3390/molecules26144174
Chicago/Turabian StylePârvănescu (Pană), Ramona Daniela, Claudia-Geanina Watz, Elena-Alina Moacă, Lavinia Vlaia, Iasmina Marcovici, Ioana Gabriela Macașoi, Florin Borcan, Ioana Olariu, Georgeta Coneac, George-Andrei Drăghici, and et al. 2021. "Oleogel Formulations for the Topical Delivery of Betulin and Lupeol in Skin Injuries—Preparation, Physicochemical Characterization, and Pharmaco-Toxicological Evaluation" Molecules 26, no. 14: 4174. https://doi.org/10.3390/molecules26144174
APA StylePârvănescu, R. D., Watz, C.-G., Moacă, E.-A., Vlaia, L., Marcovici, I., Macașoi, I. G., Borcan, F., Olariu, I., Coneac, G., Drăghici, G.-A., Crăiniceanu, Z., Flondor, D., Enache, A., & Dehelean, C. A. (2021). Oleogel Formulations for the Topical Delivery of Betulin and Lupeol in Skin Injuries—Preparation, Physicochemical Characterization, and Pharmaco-Toxicological Evaluation. Molecules, 26(14), 4174. https://doi.org/10.3390/molecules26144174













