Wheat Bran Modifications for Enhanced Nutrition and Functionality in Selected Food Products
Abstract
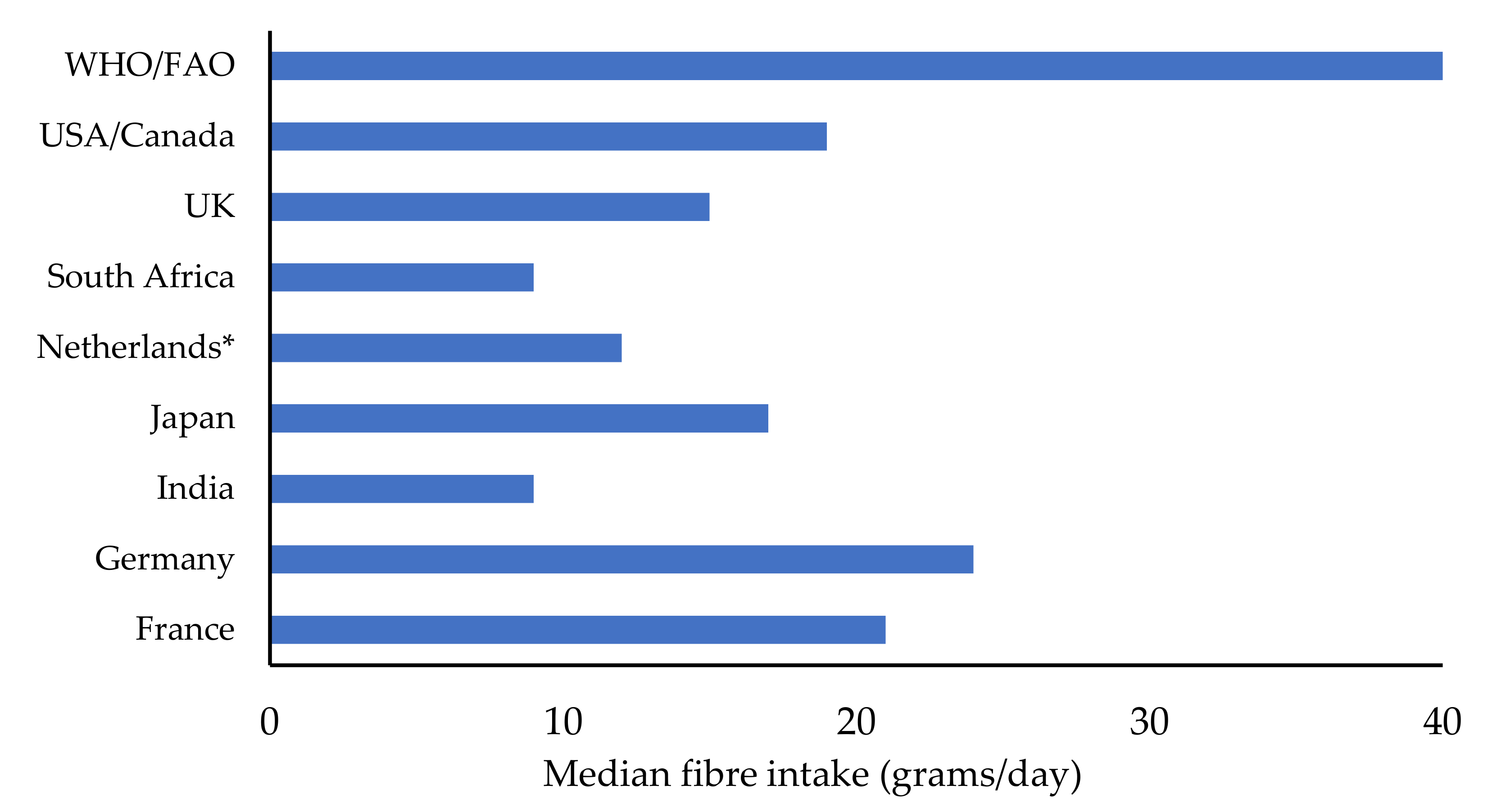
:1. Introduction
2. Wheat Bran Modifications/Pre-Treatments
2.1. Mechanical Treatment
2.2. Thermal Treatment
2.2.1. Dry Heat Treatment
2.2.2. Wet Heat Treatment
Autoclaving
Extrusion
Steam Treatment
2.3. Bioprocessing
2.3.1. Fermentation
2.3.2. Enzymatic Modification
2.3.3. Dephytinization Effects of Bioprocessing
2.4. Combination of Pre-Treatment Methods
2.4.1. Enzymatic and Fermentation Treatment
2.4.2. Thermomechanical and Bioprocessing
3. Modified Wheat Bran as a Functional Ingredient in Selected Food Products
3.1. Bread
3.2. Cookies and Cakes
3.3. Noodles and Pasta
3.4. Fried Dough
3.5. Gluten-Free Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition, and the prevention of chronic diseases: Process, product, and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). Outcome of the public consultation on the draft opinion of the scientific panel on dietetic products, nutrition, and allergies (NDA) on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1508–1569. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B. FAO/WHO scientific update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61, S132–S137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.M. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Longoria-García, S.; Cruz-Hernández, M.A.; Flores-Verástegui, M.I.M.; Contreas-Esquivel, J.C.; Montañez-Sáenz, J.C.; Belmares-Cerda, R.E. Potential functional bakery products as delivery systems for prebiotics and probiotics health enhancers. J. Food Sci. Technol. 2018, 55, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Roye, C.; Henrion, M.; Chanvrier, H.; De Roeck, K.; De Bondt, Y.; Liberloo, I.; King, R.; Courtin, C.M. Extrusion-Cooking Modifies Physicochemical and Nutrition-Related Properties of Wheat Bran. Foods 2020, 9, 738. [Google Scholar] [CrossRef]
- Maćkowiak, K.; Torlińska-Walkowiak, N.; Torlińska, B. Dietary fibre as an important constituent of the diet. Adv. Hyg. Exp. Med. 2016, 70, 104–109. Available online: http://www.phmd.pl/fulltxt.php?ICID=1195842 (accessed on 14 April 2021). [CrossRef]
- Holscher, H.D. Dietary fibre and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Shetty, P.S. Nutrition transition in India. Public Health Nutr. 2002, 5, 175–182. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Dietary fiber intake and risk of first stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1360–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef]
- Van de Vijver, L.P.L.; Van den Bosch, L.M.C.; Van den Brandt, P.A.; Goldbohm, R.A. Whole-grain consumption, dietary fibre intake and body mass index in the Netherlands cohort study. Eur. J. Clin. Nutr. 2009, 63, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekgala, M.D.; Mchiza, Z.J.; Parker, W.-A.; Monyeki, K.D. Dietary Fiber Intake and Metabolic Syndrome Risk Factors among Young South African Adults. Nutrients 2018, 10, 504. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Liu, S.X.; Vaughn, S.F. Effect of corn bran as dietary fiber addition on baking and sensory quality. Biocatal. Agric. Biotechnol. 2012, 1, 348–352. [Google Scholar] [CrossRef]
- Bauer, J.L.; Harbaum-Piayda, B.; Stöckmann, H.; Schwarz, K. Antioxidant activities of corn fiber and wheat bran and derived extracts. LWT Food Sci. Technol. 2013, 50, 132–138. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice Bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Zhou, Z.K.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2019, 59, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.D.R.L.; Rodrigues, P.S.; de las Mercedes Salas-Mellado, M.; de Mederios Burkert, J.F.; Badiale-Furlong, E. Defatted rice bran as a potential raw material to improve the nutritional and functional quality of cakes. Plant. Food Hum. Nutr. 2021, 76, 46–52. [Google Scholar] [CrossRef]
- Gómez, M.; Jiménez, S.; Ruiz, E.; Oliete, B. Effect of extruded wheat bran on dough rheology and bread quality. LWT Food Sci. Technol. 2011, 44, 2231–2237. [Google Scholar] [CrossRef]
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Menis-Henrique, M.E.C.; Scarton, M.; Piran, M.V.F.; Clerici, M.T.P.S. Cereal fibre: Extrusion modifications for food industry. Curr. Opin. Food Sci. 2020, 33, 141–148. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.J.; Dornez, E.; Verspreet, J.; Delcour, J.A.; Courtin, C.M. Wheat (Triticum aestivum L.) bran in bread making: A critical review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Özkaya, B.; Baumgartner, B.; Özkaya, H. Effects of concentrated and dephytinized wheat bran and rice bran addition on bread properties. J. Texture Stud. 2018, 49, 84–93. [Google Scholar] [CrossRef]
- Deroover, L.; Tie, Y.; Verspreet, J.; Courtin, C.M.; Verbeke, K. Modifying wheat bran to improve its health benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 1104–1122. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Ma, S.; Li, L.; Zheng, X.; Wang, X. Impact of wheat bran dietary fibre on gluten and gluten-starch microstructure formation in dough. Food Hydrocoll. 2019, 95, 292–297. [Google Scholar] [CrossRef]
- Brewer, L.R.; Kubola, J.; Siriamornpun, S.; Herald, T.J.; Shi, Y.C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014, 152, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Servi, S.; Özkaya, H.; Colakoglu, A.S. Dephytinization of wheat bran by fermentation with bakers’ yeast, incubation with barley malt flour and autoclaving at different pH levels. J. Cereal Sci. 2008, 48, 471–476. [Google Scholar] [CrossRef]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Duman, B. Dephytinization of wheat and rice brans by hydrothermal autoclaving process and the evaluation of consequences for dietary fibre content, antioxidant activity and phenolics. Innov. Food Sci. Emerg. Technol. 2017, 39, 209–215. [Google Scholar] [CrossRef]
- Zhao, H.M.; Guo, X.N.; Zhu, K.X. Impact of solid-state fermentation on nutritional, physical and flavour properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, M.; Ricci, A.; Calani, L.; Bresciani, L.; Neviani, E.; Dall’Asta, C.; Lazzi, C.; Galaverna, G. Solid state lactic acid fermentation: A strategy to improve wheat bran functionality. LWT 2020, 118, 108668. [Google Scholar] [CrossRef]
- Lauková, M.; Karovičová, J.; Minarovičová, L.; Kohajdová, Z. Effect of thermal stabilization on physico-chemical parameters and functional properties of wheat bran. Potravin. Slovak. J. Food Sci. 2020, 14, 170–177. [Google Scholar] [CrossRef]
- Arcila, J.A.; Weier, S.A.; Rose, D.J. Changes in dietary fibre fractions and gut microbial fermentation properties of wheat bran after extrusion and bread making. Food Res. Int. 2015, 74, 217–223. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Majzoobi, M.; Pashangeh, S.; Farahnaky, A.; Eskandari, M.H.; Jamalian, J. Effect of particle size reduction, hydrothermal and fermentation treatments on phytic acid content and some physicochemical properties of wheat bran. J. Food Sci. Technol. 2014, 51, 2755–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Wang, L.; Li, Z. Superheated steam treatment on wheat bran: Enzyme inactivation and nutritional attributes retention. LWT Food Sci. Technol. 2018, 91, 446–452. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Mattila, O.; Sozer, N.; Poutanen, K.; Koksel, H.; Nordlund, E. Effect of steam explosion on enzymatic hydrolysis and baking quality of wheat bran. J. Cereal Sci. 2017, 78, 25–32. [Google Scholar] [CrossRef]
- Kong, F.; Wang, L.; Gao, H.; Chen, H. Process of steam explosion assisted superfine grinding on particle size, chemical composition, and physico-chemical properties of wheat bran powder. Powder Technol. 2020, 371, 154–160. [Google Scholar] [CrossRef]
- Kong, F.; Wang, L.; Chen, H.; Zhao, X. Improving storage property of wheat bran by steam explosion. Int. J. Food Sci. Technol. 2021, 56, 287–292. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Dar, B.N.; Singh, B. Optimization of process for reduction of antinutritional factors in edible cereal brans. Food Sci. Technol. Int. 2012, 18, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Demuth, T.; Betschart, J.; Nyström, L. Structural modifications to water-soluble wheat bran arabinoxylan through milling and extrusion. Carbohydr. Polym. 2020, 240, 116328. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Arlotti, G.; Bonifati, A.M.; Napolitano, A.; Vitale, D.; Fogliano, V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res. Int. 2005, 38, 1167–1173. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Curiel, J.A.; Poutanen, K.; Katina, K. Effect of bioprocessing and particle size on the nutritional properties of wheat bran fractions. Innov. Food Sci. Emerg. Technol. 2014, 25, 19–27. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Cătoi, A.-F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Katina, K.; Juvonen, R.; Laitila, A.; Flander, L.; Nordlund, E.; Kariluoto, S.; Piironen, V.; Poutanen, K. Fermented wheat bran as a functional ingredient in baking. Cereal Chem. 2012, 89, 126–134. [Google Scholar] [CrossRef]
- Messia, M.C.; Reale, A.; Maiuro, L.; Candigliota, T.; Sorrentino, E.; Marconi, E. Effects of pre-fermented wheat bran on dough and bread characteristics. J. Cereal Sci. 2016, 69, 138–144. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Liao, A.-M.; Thakur, K.; Huang, J.-H.; Zhang, J.-G.; Wei, Z.-J. Modification of wheat bran insoluble dietary fiber with carboxymethylation, complex enzymatic hydrolysis and ultrafine comminution. Food Chem. 2019, 297, 124983. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; et al. Combination of Extrusion and Fermentation with Lactobacillus plantarum and L. uvarum Strains for Improving the Safety Characteristics of Wheat Bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, X.; Zhang, Z.; Zhou, T.; Gao, R.; Li, Y.; Ding, X. Effect of β-endoxylanase and α-arabinofuranosidase enzymatic hydrolysis on nutritional and technological properties of wheat brans. Food Chem. 2020, 302, 125332. [Google Scholar] [CrossRef]
- Noort, M.W.J.; Van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre-protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Onipe, O.O.; Beswa, D.; Jideani, A.I.O. Effect of size reduction on colour, hydration, and rheological properties of wheat bran. Food Sci. Technol. 2017, 37, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.M.M.; Noort, M.W.J.; Rouau, X. Dry fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Van Craeyveld, V.; Holopainen, U.; Selinheimo, E.; Poutanen, K.; Delcour, J.A.; Courtin, C.M. Extensive dry ball milling of wheat and rye bran leads to in situ production of arabinoxylan oligosaccharides through nanoscale fragmentation. J. Agric. Food Chem. 2009, 57, 8467–8473. [Google Scholar] [CrossRef]
- Bondt, Y.D.; Hermans, W.; Moldenaers, P.; Courtin, C.M. Selective modification of wheat bran affects its impact on gluten-starch dough rheology, microstructure and bread volume. Food Hydrocoll. 2020, 113, 106348. [Google Scholar] [CrossRef]
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; De Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods—A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Onipe, O.O.; Beswa, D.; Jideani, V.A.; Jideani, A.I.O. Development of a low-fat, high-fibre snack: Effect of bran particle sizes and processing conditions. Heliyon 2019, 5, e01364. [Google Scholar] [CrossRef] [Green Version]
- Onipe, O.O.; Beswa, D.; Jideani, A.I.O. In Vitro Starch Digestibility and Glycaemic Index of Fried Dough and Batter Enriched with Wheat and Oat Bran. Foods 2020, 9, 1374. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Wang, N.; Zhou, Y. Effects of superfine grinding of bran on the properties of dough and qualities of steamed bread. J. Cereal Sci. 2018, 81, 76–82. [Google Scholar] [CrossRef]
- Jin, X.; Lin, S.; Gao, J.; Wang, Y.; Ying, J.; Dong, Z.; Zhou, W. Effect of coarse and superfine-ground wheat brans on the microstructure and quality attributes of dried white noodle. Food Bioprocess Technol. 2021, 14, 1089–1100. [Google Scholar] [CrossRef]
- Jacobs, P.J.; Hemdane, S.; Delcour, J.A.; Courtin, C.M. Dry heat treatment affects wheat bran surface properties and hydration kinetics. Food Chem. 2016, 203, 513–520. [Google Scholar] [CrossRef]
- Nandeesh, K.; Jyotsna, R.; Venkateswara Rao, G. Effect of differently treated wheat bran on rheology, microstructure, and quality characteristics of soft dough biscuits. J. Food Process. Preserv. 2011, 35, 179–200. [Google Scholar] [CrossRef]
- Lebesi, D.M.; Tzia, C. Effect of the addition of different dietary fiber and edible cereal bran sources on the baking and sensory characteristics of cupcakes. Food Bioprocess. Technol. 2011, 4, 710–722. [Google Scholar] [CrossRef]
- Nikmaram, N.; Leong, S.Y.; Koubaa, M.; Zhu, Z.; Barba, F.J.; Greiner, R.; Roohinejad, S. Effect of extrusion on the anti-nutritional factors of food products: An overview. Food Control. 2017, 79, 62–73. [Google Scholar] [CrossRef]
- Zhang, H.W.; Yang, M.D.; Fan, X.F. Study on modification of dietary fibre from wheat bran. Adv. Mater. Res. 2011, 183, 1268–1272. [Google Scholar] [CrossRef]
- Prückler, M.; Lorenz, C.; Endo, A.; Kraler, M.; Durrschmid, K.; Hendriks, K.; Soares da Silva, F.; Auterith, E.; Kneifel, W.; Michlmayr, H. Comparison of homo- and heterofermentative lactic acid bacteria for implementation of fermented wheat bran in bread. Food Microbiol. 2015, 49, 21–219. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Masatcioglu, M.T.; Köksel, H. Effect of extrusion treatment on enzymatic hydrolysis of wheat bran. J. Cereal Sci. 2020, 93, 102941. [Google Scholar] [CrossRef]
- Gualberto, D.; Bergman, C.J.; Kazemzadeh, M.; Weber, C.W. Effect of extrusion processing on the soluble and insoluble fibre, and phytic acid contents of cereal brans. Plant. Food Hum. Nutr. 1997, 51, 187–198. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Singh, B.; Dar, B.N. Effect of extrusion variables (temperature, moisture) on the antinutrient components of cereal brans. J. Food Sci. Technol. 2015, 52, 1670–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Pérez, F.; Salazar-García, M.G.; Romero-Baranzini, A.L.; Islas-Rubio, A.R.; Ramírez-Wong, B. Estimated glycemic index and dietary fiber content of cookies elaborated with extruded wheat bran. Plant. Food Hum. Nutr. 2013, 68, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Gao, Q.; Hadiatullah, H.; Zhang, J.; Zhang, A.; Yao, Y. Effect of wheat bran steam explosion pretreatment on flavors of nonenzymatic browning products. LWT 2021, 135, 110026. [Google Scholar] [CrossRef]
- Coda, R.; Katina, K.; Rizzello, C.G. Bran bioprocessing for enhanced functional properties. Curr. Opin. Food Sci. 2015, 1, 50–55. [Google Scholar] [CrossRef]
- Savolainen, O.I.; Coda, R.; Suomi, K.; Katina, K.; Juvonen, R.; Hanhineva, K.; Poutanen, K. The role of oxygen in the liquid fermentation of wheat bran. Food Chem. 2014, 153, 424–431. [Google Scholar] [CrossRef]
- Arte, E.; Rizzello, C.G.; Verni, M.; Nordlund, E.; Katina, K.; Coda, R. Impact of enzymatic and microbial bioprocessing on protein modification and nutritional properties of wheat bran. J. Agric. Food Chem. 2015, 63, 8685–8693. [Google Scholar] [CrossRef]
- Katina, K.; Salmenkallio-Marttila, M.; Partanen, R.; Forssell, P.; Autio, K. Effects of sourdough and enzymes on staling of high-fibre wheat bread. LWT Food Sci. Technol. 2006, 39, 479–491. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Joye, I.J.; Lamberts, L.; Brijs, K.; Delcour, J.A. In situ production of gaminobutyric acid in breakfast cereals. Food Chem. 2011, 129, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Manini, F.; Brasca, M.; Plumed-Ferrer, C.; Morandi, S.; Erba, D.; Casiraghi, M.C. Study of the chemical changes and evolution of microbiota during sourdough-like fermentation of wheat bran. Cereal Chem. 2014, 91, 342–349. [Google Scholar] [CrossRef]
- Nordlund, E.; Katina, K.; Aura, A.M.; Poutanen, K. Changes in bran structure by bioprocessing with enzymes and yeast modifies the in vitro digestibility and fermentability of bran protein and dietary fibre complex. J. Cereal Sci. 2013, 58, 200–208. [Google Scholar] [CrossRef]
- Coda, R.; Kärki, I.; Nordlund, E.; Heiniö, R.L.; Poutanen, K.; Katina, K. Influence of particle size on bioprocess induced changes on technological functionality of wheat bran. Food Microbiol. 2014, 37, 69–77. [Google Scholar] [CrossRef]
- Rezaei, S.; Najafi, M.A.; Haddadi, T. Effect of fermentation process, wheat bran size and replacement level on some characteristics of wheat bran, dough, and high-fibre Tafton bread. J. Cereal Sci. 2019, 85, 56–61. [Google Scholar] [CrossRef]
- Ferri, M.; Happel, A.; Zanaroli, G.; Bertolini, M.; Chiesa, S.; Commisso, M.; Tassoni, A. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol. 2020, 56, 38–45. [Google Scholar] [CrossRef]
- Hartikainen, K.; Poutanen, K.; Katina, K. Influence of bioprocessed wheat bran on the physical and chemical properties of dough and wheat bread texture. Cereal Chem. 2014, 91, 115–123. [Google Scholar] [CrossRef]
- Lauková, M.; Karovičová, J.; Minarovičová, L.; Kohajdová, Z. Wheat bran stabilization and its effect on cookies quality. Potravin. Slovak. J. Food Sci. 2019, 13, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Gómez, M.; Moraleja, A.; Oliete, B.; Ruiz, E.; Caballero, P.A. Effect of fibre size on the quality of fibre-enriched layer cakes. LWT Food Sci. Technol. 2010, 43, 33–38. [Google Scholar] [CrossRef]
- Sudha, M.L.; Ramasarma, P.R.; Venkateswara Rao, G. Wheat bran stabilization and its use in the preparation of high-fiber pasta. Food Sci. Tech. Int. 2011, 17, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Fei, M.J.; Shi, C.L.; Tian, J.C.; Sun, C.L.; Zhang, H.; Dong, H.X. Effect of particle size and addition level of wheat bran on quality of dry white Chinese noodles. J. Cereal Sci. 2011, 53, 217–224. [Google Scholar] [CrossRef]
- Kim, B.K.; Chun, Y.G.; Cho, A.R.; Park, D.J. Reduction in fat uptake of doughnut by microparticulated wheat bran. Int. J. Food Sci. Nutr. 2012, 63, 987–995. [Google Scholar] [CrossRef]
- Scherf, K.A.; Wieser, H.; Koehler, P. Novel approaches for enzymatic gluten degradation to create high-quality gluten-free products. Food Res. Int. 2018, 110, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.W.A.P.K.T. Degradation of Gluten in Wheat Bran and Bread Drink by Means of a Proline-Specific Peptidase. J. Nutr. Food Sci. 2014, 4, 293. [Google Scholar] [CrossRef] [Green Version]
- Tanasković, S.J.; Šekuljica, N.; Jovanović, J.; Gazikalović, I.; Grbavčić, S.; Đorđević, N.; Sekulić, M.V.; Hao, J.; Luković, N.; Knežević-Jugović, Z. Upgrading of valuable food component contents and anti-nutritional factors depletion by solid-state fermentation: A way to valorize wheat bran for nutrition. J. Cereal Sci. 2021, 99, 103159. [Google Scholar] [CrossRef]
| Fibre Component | Description | Food Sources |
|---|---|---|
| Cellulose | Polysaccharides comprising up to 10,000 closely packed glucose units arranged linearly. | Grains, vegetables, fruit, nuts, cereal bran. |
| Hemicellulose | Polysaccharides containing sugars other than glucose. | Cereal grains, vegetables, fruit, legumes (for example peas, beans, chickpeas, lentils) and nuts. |
| Lignin | A non-carbohydrate component associated with plant walls. | Foods with a woody component, for example, celery and the outer layers of cereal grains. |
| Beta-glucans | Glucose polymers that (unlike cellulose) have a branched structure | Mainly found in cell wall of oats and barley. |
| Pectins | A non-starch polysaccharide common to all cell walls. | Fruits and vegetables, legumes, nuts, and potatoes. |
| Gums and mucilages | Non-starch polysaccharides are thick gel-forming fibres that help hold plant cell walls together. | Gums: seeds and seaweed extracts; Mucilages: pysillium seeds. Gums and mucillages are used as gelling agents, thickeners, stabilisers, and emulsifying agents. |
| Resistant starch | Starch and the products of starch digestion that are not absorbed by the small intestine. | Legumes, potatoes, cereal grains |
| Oligosaccharides | Short-chain carbohydrates of 3–9 monomers. These include fructo-oligosaccharides and galacto-oligosaccharides. | Onions, chicory, Jerusalem artichokes |
| Micro components (waxes, cutin and suberin) | Micro components of the plant structures. | Cereal grains |
| Disease | Number of Studies in Meta-Analysis | Findings | Reference |
|---|---|---|---|
| Cardiovascular disease | 10 | Inverse association—RR of 0.91 (95% CI 0.88–0.94) for each 7 g/day increase at p < 0.001 | [11] |
| Coronary events | 12 | Inverse association—RR 0.91 (95% CI 0.87–0.94) for each 7 g/day increase at p < 0.001 | [11] |
| Stroke | 7 | Inverse association with incidence of haemorrhagic plus ischemic stroke RR 0.93 (95% CI 0.88, 0.98) for each 7 g/day increase at p = 0.002 | [12] |
| Colorectal cancer | 8 | Inverse association with the incidence of colorectal cancer RR = 0.88 (95% CI 0.83–0.97) for each 10 g/day at increase; p < 0.05. | [13] |
| Type 2 diabetes | 10 | Inverse association RR 0.87 (95% CI 0.90, 0.97) for each 7 g/day increase; p = 0.001 | [14] |
| Modification Type | Impact on Functionality | Effect on Nutritional Properties | Reference |
|---|---|---|---|
| Thermomechanical Treatment | |||
| Milling (900, 750, 500 and 355 µm) | ND | Bound total phenolic content (TPC) and total flavonoid increased by 1.5-fold, total anthocyanin by 2-fold. Zeaxanthin and beta carotene increased in medium bran and lutein in fine bran fraction. Milling did not affect the DPPH content of wheat bran (WB). | [29] |
| Autoclaving (121 °C for 0.5–2 h pH: 3.5–6.2) | ND | At native pH (6–6.2) no change in phytic acid (PA) occurred. Maximum reduction * (96%) at pH 3.5 and 2 h autoclaving was reported. | [30] |
| Autoclaving (121 °C for 0.5–1.5 h, pH: 3.5–6.6) | ND | A 96% decrease * of PA at pH 4 and 1 h processing time. Significant increase in insoluble dietary fibre (IDF) and soluble dietary fibre (SDF). Autoclaving increased the bound and total TPC of wheat bran. | [31] |
| Autoclaving conditions (121 °C for 20–21 min) | Increase in water retention capacity (WRC) and water holding capacity (WHC). No change in microstructure. | Reduction in TPC, PA and IDF. An increase in alkylresorcinol, water-extractable arabinoxylan (WEAX) was observed. | [32,33] |
| Microwave (800 W, 2 min) and hot air oven, (150 °C for 20 min) | Water absorption capacity and swelling capacity (SC) markedly increased in both treatments by up to 11%. Hot air treatment increased bran lightness. | Both methods increased protein and total dietary fibre content. A decrease in moisture and PA content was also observed | [34] |
| Extrusion (temperature: 80 and 120℃, screw speed: 120 and 250 rpm) | ND | Extrusion increased WEAX content, SDF content. Fermentable carbohydrates and short-chain fatty acid (SCFA) content were higher in extruded bran. | [35] |
| Extrusion (temperature:140 °C, screw speed: 150 rpm, 45% moisture) + size reduction (830, 380, 250 and 180 µm) | The surface of extruded bran was full of holes and had an irregular surface structure. WHC < ORC and SC increased with extrusion and size reduction | SDF of extruded WB increased by 70% *. Antioxidant properties increased as dosage (mg/mL) increased. | [36] |
| Extrusion (temperature: 120 and 145 °C, moisture: 23, 27 and 33% screw speed: 310 rpm) | A greater extent of degradation of pericarp and aleurone layer of WB was caused by very high shear than low shear extrusion using light microscopy. | A 1.8-fold and 3.5-fold increase in WEAX and free ferulic acid. PA content decreased by 19% * andA small increase in SCFA was reported after 48 h fermentation. | [6] |
| Milling (420, 280, 170 and 90 µm) Hydrothermal (acetate buffer (pH 4.8) at 55 °C, 60 min and incubation at 5 5°C for 24 h) Yeast fermentation (8 h at 30 °C) | Reduced WHC and swelling power and increase in water solubility index of fermented and hydrothermal bran. Size reduction increased L * values of WB. | 34, 57 and 76% reduction * in PA content in milled, fermented, and hydrothermal WB. Hydrothermal and fermentation treatments increased the total dietary fibre (TDF), SDF and reduced the IDF content of WB. Mineral contents reduced with all treatments. | [37] |
| Super-heated steam (15.0 m3/h, 170 °C for 20 min) Hot air processing in an electro-thermostatic blast oven (170 °C for 20 min) | ND | Superheated steam was more efficient in enzyme inactivation, enhancement of non-starch nutrients, reduction of peroxide value, higher soluble phenolic content, and better sensory profile than hot air treatment. | [38] |
| Milling + Steam explosion (120–160 °C for 5–10 min) | Lightness values of WB treated with steam explosion decreased. Severe disruption of bran cell wall by grinding and steam explosion was reported. | Milling and steam explosion alone and in combination increased AX solubilisation in fine bran. Loaf volume, SDF increased, and PA content reduced in breads with pre-treated WB. | [39] |
| Steam explosion (0.8 MPa, 170 °C, 5 min) + grinding (425–75 µm) | Steam explosion and milling increased WB porosity, WHC and SC. | Fat, starch, protein, SDF, TPC, total flavonoids and DPPH contents increased with steam explosion and size reduction. | [40] |
| Steam explosion (0.3, 0.5 & 0.8 MPa, at 170 °C, for 5 min) | Lipase and peroxidase activity reduced and shelf life increased. | Protein, and lipid content remain unchanged. SDF, TPC, TFC and DPPH values increased at maximum steam (0.8 MPa). | [41] |
| Microwave (2450 MHz at 1.5–2.5 min) Hot air oven (100 & 110 °C, 15, 20 & 25 min) Steaming (100, 110 & 115 °C, 15, 20 at 25 min) | All treatments increased bulk density and darkened the bran samples. | Microwave treatment at 2.5 min caused a significant reduction of PA, polyphenols, saponins, trypsin inhibitors and toxicants | [42] |
| Milling (ultra-centrifugal mill-500 µm) + Extrusion | Structural modification of WEAX was more distinct in extruded bran. | Milling increased WEAX content (26% *) and reduced molecular weight of WB. No significant change in TPC, but 38% * increase in free TPC of milled bran. | [43] |
| Milling + Extrusion | About 1.5-fold increase in WHC and IDF content of bran fractions and a decrease in SDF content after extrusion process. | Antioxidant capacity increased as the particle sizes of the milled bran reduced up to 180µm. | [44] |
| Bioprocessing (Fermentation and Enzymatic Treatments) | |||
| Fermentation at 2–8 h with Saccharomyces. cerevisiae (3–9%). | ND | A reduction (≤96%) in phytic acid content with an increase in fermentation time and yeast concentration. | [30] |
| Lactobacillus brevis and Kazachstania exigua (20 °C for24 h) + enzymes (xylanase, endoglucanase and β-glucanase) | Partial degradation of bran cell wall. | A sixfold increase * in WEAX in fermented bran and up to 11.5-fold increase * when fermentation was combined with enzymes. A 50% increase * in peptide content was observed in bioprocessed bran compared to native bran | [45] |
| Fermentation with L. rhamnosus (37 °C for 24–48 h) | ND | Free TPC and WEAX increased significantly. Caffeic acid was notable in fermented bran. A reduction in phytic acid (PA) content was observed. | [33] |
| Fermentation with S. cerevisiae (30 °C for 6 h) | Increase in water absorption capacity of WB. | An 86% decrease * of PA at pH 4 and 1 h processing time. TDF of bran was not affected by fermentation. | [26] |
| Fermentation with S. cerevisiae at (30 °C for 6 days) | ND | A significant increase in the TPC, DPPH, antioxidant activity of WB was observed on day 3 of fermentation. | [46] |
| Spontaneous and yeast fermentation (20 & 32 °C for 20 h) | ND | Significant increase (≥40% *) in folates, free ferulic acid and soluble AX in yeast-fermented bran. Acidification of bran slurries at maximum fermentation temperature. | [47] |
| Fermentation with L. brevis (28 °C for 16 h) | An increase in gas retention of dough and bread volume was observed with the inclusion of fermented WB. Significant reduction in bread staling compared to bread with unfermented WB. Improved viscoelasticity of dough | There was a two- and four-fold increase * in WEAX and SDF of fermented WB compared to native bran. | [48] |
| Enzymatic treatment (cellulase and xylanase) | WRC increased by 16%. Enzymatic treatment improved oil holding and swelling capacity. Glucose adsorption capacity improved by 1.4-fold. Loose structure, wall structure damaged/degradation of wall PS. | A twofold increase of TPC, and antioxidant properties of enzyme-treated WB compared to the control sample. | [49] |
| Treatment with Lactobacillus bulgaricus, Streptococcus thermophiles and Saccharomyces cerevisiae (alone and in combination)–37 °C for 24 h & 48 h | The WHC and WRC improved significantly in fermented WB. Partial degradation of aleurone cells. | Five-fold increase in WEAX content, 60% increase in phenolic lipids, 2-fold increase in SDF, 23–27% reduction in PA. | [32] |
| Extrusion (115 and 130 °C; screw speeds: 16, 20, and 25 rpm) + fermentation (L. plantarum and L. uvarum) | ND | Combination of both treatments lowered mycotoxin content by 80.6% * and increased biogenic amines by 42.9% * of bran samples. Fructose content increased by 15% * after fermentation. | [50] |
| Enzymatic treatment (β-endoxylanase and α-L-arabinofuranosidase) | WRC and fat binding capacity increased in single and combined enzyme-treated WB. Improved porosity of enzyme-treated bran dough | TPC and DPPH content increased in single and combined enzyme-treated WB. pH reduced in WB treated with xylanase and combined enzymes. | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onipe, O.O.; Ramashia, S.E.; Jideani, A.I.O. Wheat Bran Modifications for Enhanced Nutrition and Functionality in Selected Food Products. Molecules 2021, 26, 3918. https://doi.org/10.3390/molecules26133918
Onipe OO, Ramashia SE, Jideani AIO. Wheat Bran Modifications for Enhanced Nutrition and Functionality in Selected Food Products. Molecules. 2021; 26(13):3918. https://doi.org/10.3390/molecules26133918
Chicago/Turabian StyleOnipe, Oluwatoyin O., Shonisani E. Ramashia, and Afam I. O. Jideani. 2021. "Wheat Bran Modifications for Enhanced Nutrition and Functionality in Selected Food Products" Molecules 26, no. 13: 3918. https://doi.org/10.3390/molecules26133918
APA StyleOnipe, O. O., Ramashia, S. E., & Jideani, A. I. O. (2021). Wheat Bran Modifications for Enhanced Nutrition and Functionality in Selected Food Products. Molecules, 26(13), 3918. https://doi.org/10.3390/molecules26133918






