How Does Diet Influence Our Lives? Evaluating the Relationship between Isotopic Signatures and Mortality Patterns in Italian Roman Imperial and Medieval Periods
Abstract
1. Introduction
1.1. Dietary Pattern in Imperial Rome
1.2. Dietary Patterns during the Middle Ages
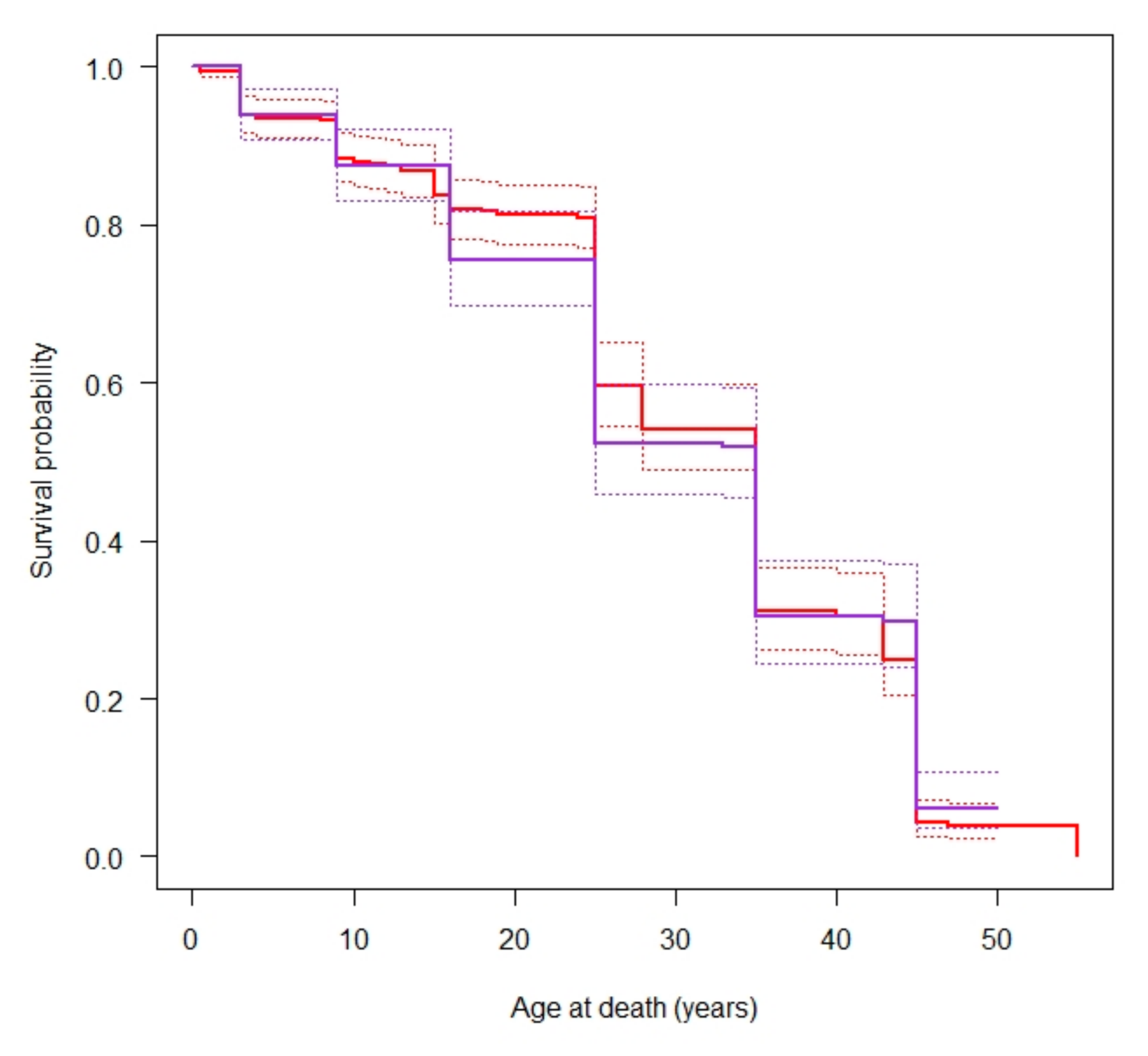
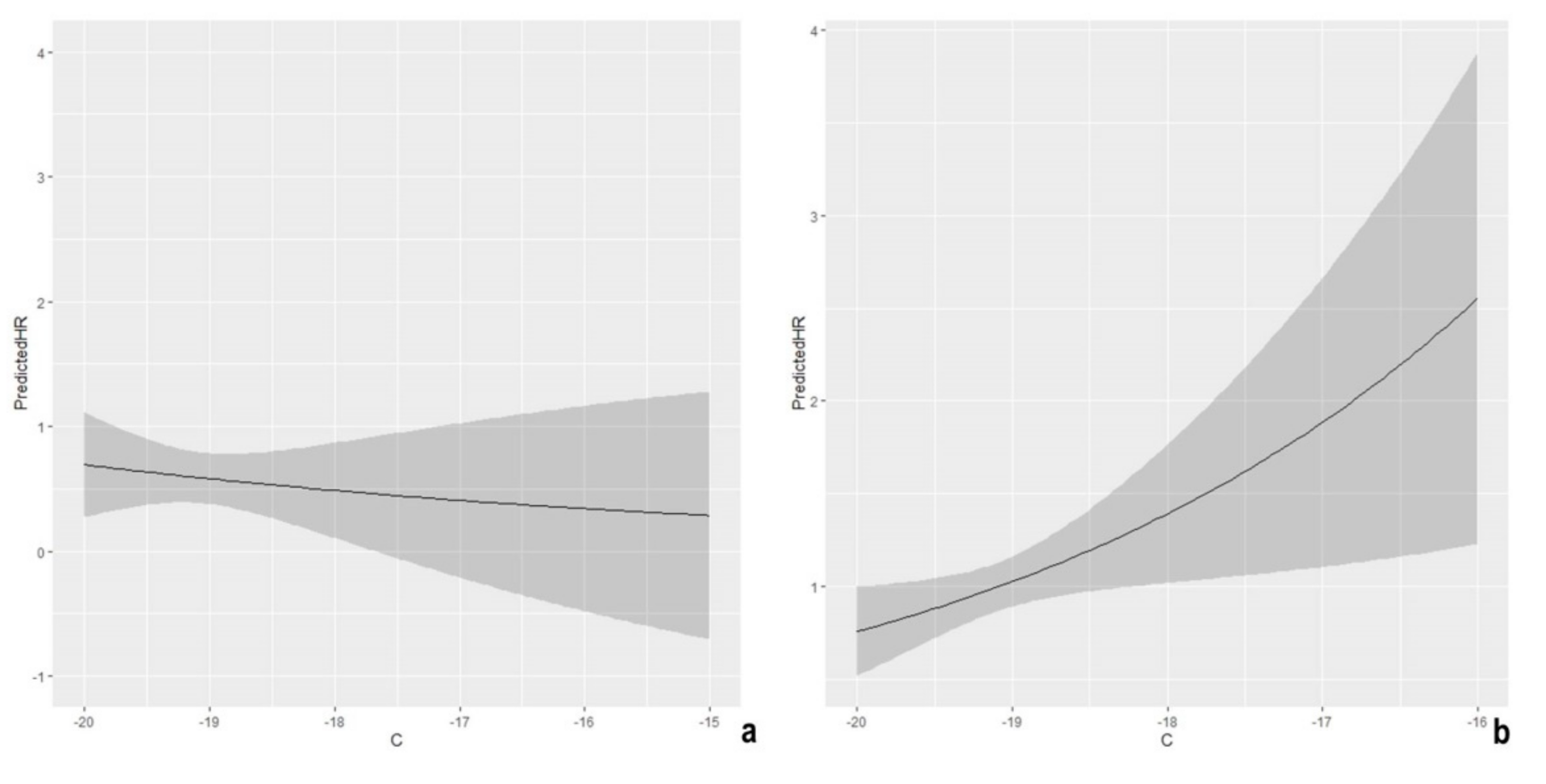
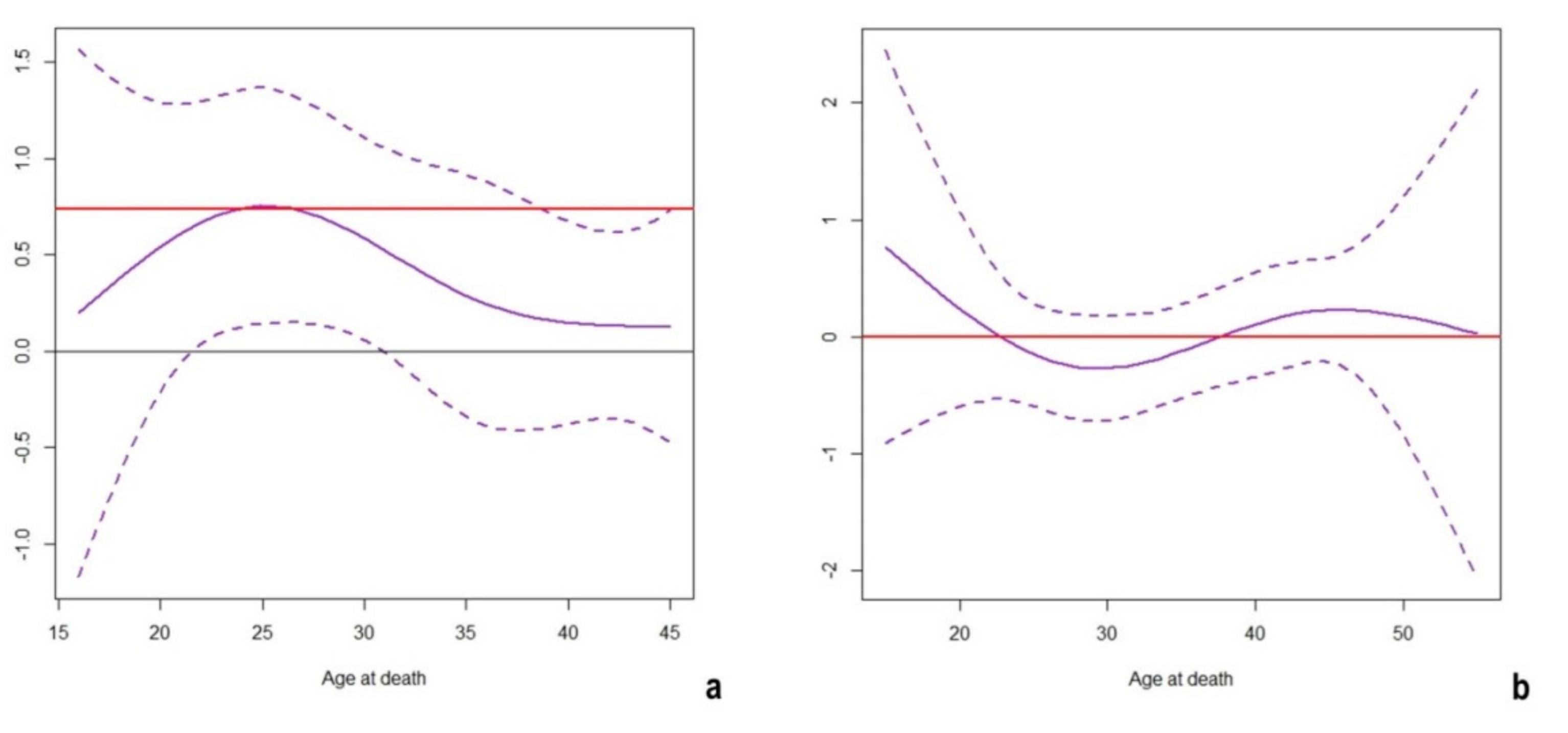
2. Results
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Makarewicz, C.A.; Sealy, J. Dietary reconstruction, mobility, and the analysis of ancient skeletal tissues: Expanding the prospects of stable isotope research in archaeology. J. Archaeol. Sci. 2015, 56, 146–158. [Google Scholar] [CrossRef]
- Schoeninger, M.J. Diet reconstruction and ecology using stable isotope ratios. In A Companion to Biological Anthropology; Larsen, C.S., Ed.; Wiley-Blackwell: Chichester, UK, 2011; pp. 445–464. [Google Scholar]
- Sehrawat, J.S.; Kaur, J. Role of stable isotope analyses in reconstructing past life-histories and the provenancing human skeletal remains: A review. Anthr. Rev. 2017, 80, 243–258. [Google Scholar] [CrossRef][Green Version]
- Curto, A.; Mahoney, P.; Maurer, A.-F.; Barrocas-Dias, C.; Fernandes, T.; Fahy, G.E. Diet and disease in Tomar, Portugal: Comparing stable carbon and nitrogen isotope ratios between skeletons with and without signs of infectious disease. J. Archaeol. Sci. 2019, 105, 59–69. [Google Scholar] [CrossRef]
- Redfern, R.C.; Dewitte, S.N.; Beaumont, J.; Millard, A.R.; Hamlin, C. A new method for investigating the relationship between diet and mortality: Hazard analysis using dietary isotopes. Ann. Hum. Biol. 2019, 46, 378–387. [Google Scholar] [CrossRef]
- Reitsema, L.J.; Holder, S. Stable Isotope Analysis and the Study of Human Stress, Disease, and Nutrition. Bioarchaeol. Int. 2018, 2, 63–74. [Google Scholar] [CrossRef]
- Scorrano, G.; Brilli, M.; Martinez-Labarga, C.; Giustini, F.; Pacciani, E.; Chilleri, F.; Scaldaferri, F.; Gasbarrini, A.; Gasbarrini, G.; Rickards, O. Palaeodiet reconstruction in a woman with probable celiac disease: A stable isotope analysis of bone remains from the archaeological site of Cosa (Italy). Am. J. Phys. Anthr. 2014, 154, 349–356. [Google Scholar] [CrossRef]
- Scorrano, G. The Stable Isotope Method in Human Paleopathology and Nutritional Stress Analysis. Archaeol. Anthr. Open Access 2018, 1, 1–3. [Google Scholar] [CrossRef]
- Lightfoot, E.; Šlaus, M.; O’Connell, T. Changing cultures, changing cuisines: Cultural transitions and dietary change in iron age, roman, and early medieval Croatia. Am. J. Phys. Anthr. 2012, 148, 543–556. [Google Scholar] [CrossRef]
- Müldner, G. Investigating Medieval Diet and Society by Stable Isotope Analysis of Human Bone. In Reflections: 50 Years of Medieval Archaeology, 1957–2007; Gilchrist, R., Reynolds, A., Eds.; Routledge: London, UK, 2009; pp. 327–346. [Google Scholar]
- Scorrano, G.; Baldoni, M.; Brilli, M.; Rolfo, M.F.; Fornaciari, G.; Rickards, O.; Martínez-Labarga, C. Effect of Neolithic transition on an Italian community: Mora Cavorso (Jenne, Rome). Archaeol. Anthr. Sci. 2019, 11, 1443–1459. [Google Scholar] [CrossRef]
- Zazpe, I.; Sanchez-Tainta, A.; Toledo, E.; Villegas, A.S.; Martinez-Gonzalez, M.A. Dietary Patterns and Total Mortality in a Mediterranean Cohort: The SUN Project. J. Acad. Nutr. Diet. 2014, 114, 37–47. [Google Scholar] [CrossRef]
- Biesbroek, S.; Verschuren, W.M.M.; Boer, J.M.A.; Van De Kamp, M.E.; Van Der Schouw, Y.T.; Geelen, A.; Looman, M.; Temme, E.H.M. Does a better adherence to dietary guidelines reduce mortality risk and environmental impact in the Dutch sub-cohort of the European Prospective Investigation into Cancer and Nutrition? Br. J. Nutr. 2017, 118, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.; Villegas, A.S.; DE Irala, J.; Marti, A.; Martínez, J. Mediterranean Diet and Stroke: Objectives and Design of the SUN Project. Nutr. Neurosci. 2002, 5, 65–73. [Google Scholar] [CrossRef]
- Hedges, R.E.; Stevens, R.E.; Richards, M. Bone as a stable isotope archive for local climatic information. Quat. Sci. Rev. 2004, 23, 959–965. [Google Scholar] [CrossRef]
- Killgrove, K.; Tykot, R. Food for Rome: A stable isotope investigation of diet in the Imperial period (1st–3rd centuries AD). J. Anthr. Archaeol. 2013, 32, 28–38. [Google Scholar] [CrossRef]
- Killgrove, K.; Tykot, R.H. Diet and collapse: A stable isotope study of Imperial-era Gabii (1st–3rd centuries AD). J. Archaeol. Sci. Rep. 2018, 19, 1041–1049. [Google Scholar] [CrossRef]
- Tykot, R.H. Stable isotope and diet: You are what you eat. In Proceedings of the International School of Physiscs “Enrico Fermi” Course CLIV; Martini, M., Milazzo, M., Piacentini, M., Eds.; IOS Press: Amsterdam, The Netherlands, 2004; pp. 433–444. [Google Scholar]
- Bocherens, H.; Drucker, D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. Int. J. Osteoarchaeol. 2003, 13, 46–53. [Google Scholar] [CrossRef]
- Bogaard, A.; Heaton, T.H.E.; Poulton, P.; Merbach, I. The impact of manuring on nitrogen isotope ratios in cereals: Archaeological implications for reconstruction of diet and crop management practices. J. Archaeol. Sci. 2007, 34, 335–343. [Google Scholar] [CrossRef]
- Doppler, T.; Gerling, C.; Heyd, V.; Knipper, C.; Kuhn, T.; Lehmann, M.F.; Pike, A.W.G.; Schibler, J. Landscape opening and herding strategies: Carbon isotope analyses of herbivore bone collagen from the Neolithic and Bronze Age lakeshore site of Zurich-Mozartstrasse, Switzerland. Quat. Int. 2017, 436, 18–28. [Google Scholar] [CrossRef]
- Fraser, R.A.; Bogaard, A.; Heaton, T.; Charles, M.; Jones, G.; Christensen, B.T.; Halstead, P.; Merbach, I.; Poulton, P.R.; Sparkes, D.; et al. Manuring and stable nitrogen isotope ratios in cereals and pulses: Towards a new archaeobotanical approach to the inference of land use and dietary practices. J. Arcaheol. Sci. 2011, 38, 2790–2804. [Google Scholar] [CrossRef]
- Gron, K.J.; Gröcke, D.R.; Larsson, M.; Sørensen, L.; Larsson, L.; Rowley-Conwy, P.; Church, M.J. Nitrogen isotope evidence for manuring of early Neolithic Funnel Beaker Culture cereals from Stensborg, Sweden. J. Archaeol. Sci. Rep. 2017, 14, 575–579. [Google Scholar] [CrossRef]
- Göhring, A.; Mauder, M.; Vohberger, M.; Nehlich, O.; von Carnap-Bornheim, C.; Hilberg, V.; Kröger, P.; Grupe, G. Palaeobiodiversity research based on stable isotopes: Correction of the sea spray effect on bone carbonate δ13C and δ18O by Gaussian Mixture Model clustering. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 490, 673–686. [Google Scholar] [CrossRef]
- Hedges, R.E.; Reynard, L.M. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 2007, 34, 1240–1251. [Google Scholar] [CrossRef]
- Larsson, M.; Bergman, J.; Lagerås, P. Manuring practices in the first millennium AD in southern Sweden inferred from isotopic analysis of crop remains. PLoS ONE 2019, 14, e0215578. [Google Scholar] [CrossRef]
- Makarewicz, C.A. Winter pasturing practices and variable fodder provisioning detected in nitrogen (δ15N) and carbon (δ13C) isotopes in sheep dentinal collagen. J. Archaeol. Sci. 2014, 41, 502–510. [Google Scholar] [CrossRef]
- Nehlich, O.; Fuller, B.T.; Marquez-Grant, N.; Richards, M.P. Investigation of diachronic dietary patterns on the islands of Ibiza and Formentera, Spain: Evidence from sulphur stable isotope ratio analysis. Am. J. Phys. Anthr. 2012, 149, 115–124. [Google Scholar] [CrossRef]
- Schulting, R.J.; Vaiglova, P.; Crozier, R.; Reimer, P.J. Further isotopic evidence for seaweed-eating sheep from Neolithic Orkney. J. Archaeol. Sci. Rep. 2017, 11, 463–470. [Google Scholar] [CrossRef]
- Szpak, P. Complexities of nitrogen isotope biogeochemistry in plant-soil systems: Implications for the study of ancient agricultural and animal management practices. Front. Plant Sci. 2014, 5, 288. [Google Scholar] [CrossRef]
- Reitsema, L.J. Beyond diet reconstruction: Stable isotope applications to human physiology, health, and nutrition. Am. J. Hum. Biol. 2013, 25, 445–456. [Google Scholar] [CrossRef]
- Beaumont, J.; Montgomery, J. The Great Irish Famine: Identifying Starvation in the Tissues of Victims Using Stable Isotope Analysis of Bone and Incremental Dentine Collagen. PLoS ONE 2016, 11, e0160065. [Google Scholar] [CrossRef]
- Crowder, K.; Montgomery, J.; Gröcke, D.; Kori, F. Childhood “stress” and stable isotope life-historied in Transylvania. Int. J. Osteoarchaeol. 2019, 29, 644–653. [Google Scholar]
- Fuller, B.T.; Fuller, J.L.; Sage, N.E.; Harris, D.A.; O’Connell, T.C.; Hedges, R.E.M. Nitrogen balance and δ15N: Why you’re not what you eat during nutritional stress. Rapid Commun. Mass Spectrom. 2005, 19, 2497–2506. [Google Scholar] [CrossRef]
- Garland, C.J.; Reitsema, L.J.; Larsen, C.S.; Thomas, D.H. Early Life Stress at Mission Santa Catalina de Guale: An Integrative Analysis of Enamel Defects and Dentin Incremental Isotope Variation in Malnutrition. Bioarchaeol. Int. 2018, 2, 75–94. [Google Scholar] [CrossRef]
- Katzenberg, M.A.; Lovell, N.C. Stable isotope variation in pathological bone. Int. J. Osteoarchaeol. 1999, 9, 316–324. [Google Scholar] [CrossRef]
- Mekota, A.M.; Grupe, G.; Ufer, S.; Cuntz, U. Serian analysis of stable nitrogen and carbon isotopes in hair: Monitoring star-vation and recovery phases of patiens suffering from anorexia nervosa. Rapid Commun. Mass Spectrom. 2006, 20, 1604–1610. [Google Scholar] [CrossRef]
- Toyne, M.J.; Turner, B.L. Linking isotope analysis and paleopathology: An Andean perspective. Int. J. Paleopathol. 2020, 29, 117–127. [Google Scholar] [CrossRef]
- Baldoni, M.; Nardi, A.; Muldner, G.; Lelli, R.; Gnes, M.; Ferraresi, F.; Meloni, V.; Cerino, P.; Greco, S.; Manenti, G.; et al. Archaeo-biological reconstruction of the Italian medieval population of Colonna (8th–10th centuries CE). J. Archaeol. Sci. Rep. 2016, 10, 483–494. [Google Scholar] [CrossRef]
- Baldoni, M.; Scorrano, G.; Gismondi, A.; D’Agostino, A.; Alexander, M.; Gaspari, L.; Vallelonga, F.; Canini, A.; Rickards, O.; Martínez-Labarga, C. Who were the miners of Allumiere? A multidisciplinary approach to reconstruct the osteobiography of an Italian worker community. PLoS ONE 2018, 13, e0205362. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, M.; Scorrano, G.; Alexander, M.; Stasolla, F.; Marsella, L.; Rickards, O.; Martínez-Labarga, C. The medieval population of Leopoli-Cencelle (Viterbo, Latium): Dietary reconstruction through stable isotope analysis from bone proteins. J. Archaeol. Sci. Rep. 2019, 24, 92–101. [Google Scholar] [CrossRef]
- De Angelis, F.; Varano, S.; Battistini, A.; Di Giannantonio, S.; Ricci, P.; Lubritto, C.; Facchin, G.; Brancazi, L.; Santangeli-Valenzani, R.; Catalano, P.; et al. Food at the heart of the Empire: Dietary reconstruction for Imperial Rome inhabitants. Archaeol. Anthr. Sci. 2020, 12, 1–21. [Google Scholar] [CrossRef]
- Gismondi, A.; Baldoni, M.; Gnes, M.; Scorrano, G.; D’Agostino, A.; Di Marco, G.; Calabria, G.; Petrucci, M.; Müldner, G.; Von Tersch, M.; et al. A multidisciplinary approach for investigating dietary and medicinal habits of the Medieval population of Santa Severa (7th–15th centuries, Rome, Italy). PLoS ONE 2020, 15, e0227433. [Google Scholar] [CrossRef] [PubMed]
- Pescucci, L.; Battistini, A.; De Angelis, F.; Catalano, P. Vivere al centro di Roma nell’VIII secolo D.C. Indicazioni antropologiche. Boll. Archeol. On-Line 2013, 4, 113–138. [Google Scholar]
- Varano, S.; De Angelis, F.; Battistini, A.; Brancazi, L.; Pantano, W.; Ricci, P.; Romboni, M.; Catalano, P.; Gazzaniga, V.; Lubritto, C.; et al. The edge of the Empire: Diet characterization of medieval Rome through stable isotope analysis. Archaeol. Anthr. Sci. 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Purcell, N. The Way We Used to Eat: Diet, Community, and History at Rome. Am. J. Philol. 2003, 124, 329–358. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.M.; Hill, S. Food in the Ancient World; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Yardley, J.C. The symposium in Roman elegy. In Dining in a Classical Context; Slater, W.J., Ed.; University of Michigan: Ann Arbor, MI, USA, 1991; pp. 149–155. [Google Scholar]
- Delgado, A.M.; Vaz Almeida, M.D.; Parisi, S. Chemistry of the Mediterranean Diet; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Garnsey, P. Food and Society in Classical Antiquity; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- De Ligt, L. The economy: Agrarian change during the second century. In A companion to the Roman Republic; Rosenstein, N., Morstein-Marx, R., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 590–605. [Google Scholar]
- Spurr, M.S. The cultivation of millet in Roman Italy. Pap. Br. Sch. Rome 1983, 51, 1–15. [Google Scholar] [CrossRef]
- Prowse, T.L. Isotopic and Dental Evidence for Diet from the Necropolis of Isola Sacra (1st–3rd centuries AD), Italy. Ph.D. Dissertation, McMaster University, Hamilton, ON, CA, 2001. [Google Scholar]
- Kron, G. Comparative perspectives on nutrition and social inequality in the Roman World. In Diet and Nutrition in the Roman World; Erdkamp, P., Holleran, C., Eds.; Routledge: London, UK, 2002; pp. 156–174. [Google Scholar]
- MacKinnon, M.R. Production and Consumption of Animals in Roman Italy: Integrating the Zooarchaeological and teXtual Evidence; Journal of Roman Archaeology. Supplementary series, no. 54; Journal of Roman Archaeology: Portsmouth, RI, USA, 2004. [Google Scholar]
- Brothwell, D.; Brothwell, P. Food in Antiquity: A Survey of the Diet of Early Peoples; Johns Hopkins University Press: Baltimore, MD, USA, 1998. [Google Scholar]
- Marzano, A. Fish and fishing in the Roman World. J. Marit. Archaeol. 2018, 13, 437–447. [Google Scholar] [CrossRef]
- Rowan, E. Bioarchaeological preservation and non-elite diet in the Bay of Naples: An analysis of the food remains from the Cardo V sewer at Roman site of Herculaneum. Environ. Arcaheol. 2017, 22, 318–336. [Google Scholar] [CrossRef]
- Gismondi, A.; D’Agostino, A.; Di Marco, G.; Martínez-Labarga, C.; Leonini, V.; Rickards, O.; Canini, A. Back to the roots: Dental calculus analysis of the first documented case of coeliac disease. Archaeol. Anthr. Sci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Gismondi, A.; D’Agostino, A.; Di Marco, G.; Scuderi, F.; De Angelis, F.; Rickards, O.; Catalano, P.; Canini, A. Archaeobotanical record from dental calculus of a Roman individual affected by bilateral temporo-mandibular joint ankylosis. Quat. Int. 2020. [Google Scholar] [CrossRef]
- King, A. Diet in the Roman world: A regional inter-site comparison of the mammal bones. J. Rom. Archaeol. 1999, 12, 168–202. [Google Scholar] [CrossRef]
- Cool, H.E.M. Eating and Drinking in Roman Britain; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Prowse, T.L.; Schwarcz, H.P.; Saunders, S.R.; Macchiarelli, R.; Bondioli, L. Isotopic paleodiet studies of skeletons from the Imperial Roman-age cemetery of Isola Sacra, Rome, Italy. J. Archaeol. Sci. 2004, 31, 259–272. [Google Scholar] [CrossRef]
- Prowse, T.L.; Schwarcz, H.P.; Saunders, S.R.; Macchiarelli, R.; Bondioli, L. Isotopic evidence for age-related variation in diet from Isola Sacra, Italy. Am. J. Phys. Anthr. 2005, 128, 2–13. [Google Scholar] [CrossRef]
- Prowse, T.L.; Saunders, S.R.; Schwarcz, H.P.; Garnsey, P.; Macchiarelli, R.; Bondioli, L. Isotopic and dental evidence for infant and young child feeding practices in an imperial Roman skeletal sample. Am. J. Phys. Anthr. 2008, 137, 294–308. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Ballantyne, R.; Hamilton-Dyer, S.; Margaritis, E.; Oxford, S.; Pantano, W.; Millett, M.; Keay, S.J. Living and dying at the Portus Romae. Antiquity 2019, 93, 719–734. [Google Scholar] [CrossRef]
- Rutgers, L.V.; van Strydonc, M.; Boudin, M. Stable isotope data from the early Christian catacombs of ancient Rome: New in-sights into the dietary habits of Rome’s early Christians. J. Archaeol. Sci. 2009, 36, 1127–1134. [Google Scholar] [CrossRef]
- Salesse, K. Archéo-Biogéchimie Isotopique, Reconstitutions des Régimes Alimentaires et des Chémas de Mobilité, et Interactions Bioculturelles. Les Sépultures Plurielles de la Catacombe des Saints Pierre-Et Marcellin (Rome, Ier-Iie S. Ap. J.-C.): Les Sépultures Plurielles de la Région X de la Catacombe des Saints Pierre-Et-Marcellin (Rome, Ier-Iiie S. Ap. J.-C.). Archéologie et Préhistoire; Université de Bordeaux: Bordeaux, France, 2015. [Google Scholar]
- Salesse, K.; Dufour, E.; Lebon, M.; Wurster, C.; Castex, D.; Bruzek, J.; Zazzo, A. Variability of bone preservation in a confined environment: The case of the catacomb of Sts Peter and Marcellinius (Rome, Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 43–54. [Google Scholar] [CrossRef]
- Killgrove, K.; Montgomery, J. All roads lead to Rome: Exploring human migration to the eternal city through biochemistry of skeletons from two Imperial-Era cemeteries (1st–3rd c AD). PLoS ONE 2016, 11, e0147585. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Veltre, V.; Varano, S.; Romboni, M.; Renzi, S.; Zingale, S.; Ricci, P.; Caldarini, C.; Di Giannantonio, S.; Lubritto, C.; et al. Dietary and Weaning Habits of the Roman Community of Quarto Cappello del Prete (Rome, 1st–3rd Century CE). Environ. Archaeol. 2020, 1–15. [Google Scholar] [CrossRef]
- Montanari, M. Medioevo vicino, Medioevo lontano. In Gusti del Medioevo. I Prodotti, la Cucina, la Tavola; Montanari, M., Ed.; Editori Laterza: Rome, Italy; Bari, Italy, 2012; pp. 3–13. [Google Scholar]
- Alexander, M.M.; Gerrard, C.M.; Gutiérrez, A.; Millard, A.M. Diet, Society, and Economy in Late Medieval Spain: Stable Isotope Evidence from Muslims and Christians from Gandía, Valencia. Am. J. Phys. Anthropol. 2015, 156, 263–273. [Google Scholar] [CrossRef]
- Alexander, M.M.; Gutiérrez, A.; Millard, A.R.; Richards, M.P.; Gerrard, C.M. Economic and socio-cultural consequences of changing political rule on human and faunal diets in medieval Valencia (c. fifth–fifteenth century AD) as evidenced by stable isotopes. Archaeol. Anthr. Sci. 2019, 11, 3875–3893. [Google Scholar] [CrossRef]
- Ciaffi, R.; Lelli, R.; Müldner, G.; Stantcheva, K.; Fischetti, A.; Ghini, G.; Craig, O.; Milano, F.; Rickards, O.; Arcudi, G.; et al. Palaeobiology of the medieval population of Albano (Rome, Italy): A combined morphological and bio-molecular approach. Int. J. Osteoarchaeol. 2015, 25, 477–488. [Google Scholar] [CrossRef]
- Paladin, A.; Moghaddam, N.; Stawinoga, A.E.; Siebke, I.; DePellegrin, V.; Tecchiati, U.; Lösch, S.; Zink, A. Early medieval Italian Alps: Reconstructing diet and mobility in the valleys. Archaeol. Anthr. Sci. 2020, 12, 1–20. [Google Scholar] [CrossRef]
- Rolandsen, G.L.; Arthur, P.; Alexander, M. A tale of two villages: Isotopic insight into diet, economy, cultural diversity and agrarian communities in medieval (11th–15th century CE) Apulia, Southern Italy. J. Archaeol. Sci. Rep. 2019, 28, 102009. [Google Scholar] [CrossRef]
- Duby, G. Le Origini dell’Economia Europea. Guerrieri e Contadini nel Medioevo; Editori Laterza: Rome/Bari, Italy, 1975; pp. 22–32. [Google Scholar]
- Montanari, M. Barbari e Romani. In Alimentazione e Cultura nel Medioevo; Montanari, M., Ed.; Editori Laterza: Rome, Italy; Bari, Italy, 1988; pp. 13–22. [Google Scholar]
- Belcastro, G.; Rastelli, E.; Mariotti, V.; Consiglio, C.; Facchini, F.; Bonfiglioli, B. Continuity or Discontinuity of the Life-Style in Central Italy during the Roman Imperial Age-Early Middle Ages Transition: Diet, Health, and Behavior. Am. J. Phys. Anthropol. 2007, 132, 381–394. [Google Scholar] [CrossRef]
- Montanari, M. Desiderio di carne. In Gusti del Medioevo. I Prodotti, la Cucina, la Tavola; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 2012; pp. 69–79. [Google Scholar]
- Alaica, A.K.; Schalburg-Clayton, J.; Dalton, A.; Kranioti, E.; Graziani Echávarri, G.; Pickard, C. Variability along the frontier: Stable carbon and nitrogen isotope ratio analysis of human remains from the Late Roman-Early Byzantine cemetery site of Joan Planells, Ibiza, Spain. Archaeol. Anthropol. Sci. 2019, 11, 3783–3796. [Google Scholar] [CrossRef]
- López-Costas, O.; Müldner, G. Fringes of the empire: Diet and cultural change at the Roman to post-Roman transition in NW Iberia. Am. J. Phys. Anthropol. 2016, 16, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, M.A.; Goude, G.; Manzi, G. Isotopic evidence of diet variation at the transition between classical and post-classical times in Central Italy. J. Archaeol. Sci. Rep. 2018, 21, 496–503. [Google Scholar] [CrossRef]
- Gismondi, A.; D’Agostino, A.; Canuti, L.; Di Marco, G.; Martínez-Labarga, C.; Angle, M.; Rickards, O.; Canini, A. Dental calculus reveals dietary habits and medicinal plant use in the Early Medieval Italian population of Colonna. J. Archaeol. Sci. Rep. 2018, 20, 556–564. [Google Scholar]
- Iacumin, P.; Galli, E.; Cavalli, F.; Cecere, L. C4-Consumers in Southern Europe: The Case of Friuli V.G. (NE-Italy) During Early and Central Middle Ages. Am. J. Phys. Anthropol. 2014, 154, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Reitsema, L.J.; Vercellotti, G. Stable Isotope Evidence for Sex- and Status- Based Variations in Diet and Life History at Medieval Trino Vercellese, Italy. Am. J. Phys. Anthropol. 2012, 148, 589–600. [Google Scholar] [CrossRef]
- Montanari, M. A ciascuno il suo. In La fame e l’abbondanza. Storia dell’Alimentazione in Europa; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 1993; pp. 87–121. [Google Scholar]
- Montanari, M. Campagne Medievali. Strutture Produttive, Rapporti di Lavoro, Sistemi Alimentari; Piccola Biblioteca Einaudi: Torino, Italy, 1984; p. 130. [Google Scholar]
- Montanari, M. Modelli di civiltà: Il consumo di cereali. In Alimentazione e Cultura nel Medioevo; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 1988; pp. 124–146. [Google Scholar]
- Montanari, M. La svolta. In La Fame e l’Abbondanza. Storia dell’Alimentazione in Europa; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 1993; pp. 51–85. [Google Scholar]
- Montanari, M. Profumo di civiltà: Il pane. In Gusti del Medioevo. I Prodotti, la Cucina, la Tavola; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 2012; pp. 59–68. [Google Scholar]
- Montanari, M. L’Europa e il mondo. In La Fame e l’Abbondanza. Storia dell’Alimentazione in Europa; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 1993; pp. 123–159. [Google Scholar]
- Montanari, M. Diete monastiche. In Alimentazione e Cultura nel Medioevo; Montanari, M., Ed.; Editori Laterza: Rome/Bari, Italy, 1988; pp. 63–104. [Google Scholar]
- Barrett, J.H.; Richards, M.P. Identity, gender, religion and economy: New isotope and radiocarbon evidence for marine resource intensification in early historic Orkney, Scotland, UK. Eur. J. Archaeol. 2004, 7, 249–271. [Google Scholar] [CrossRef]
- Lanconelli, A. Gli statuta pescivendulorum Urbis (1405). Note sul commerco di pesca a Roma fra XIV e XV secolo. Arch. Soc. Romana Stor. Patria 1985, 108, 84–131. [Google Scholar]
- Lanconelli, A. I Lavori alla Peschiera del Marta. Contributo alla Storia della Pesca nel Lazio Bassomedievale, Scritti in Memoria di Giuseppe Marchetti Longhi; Istituto di Storia e di Arte del Lazio Medidionale: Anagni, Italy, 1990. [Google Scholar]
- Müldner, G.; Richards, M.P. Fast or feast: Reconstructing diet in later medieval England by stable isotope analysis. J. Archaeol. Sci. 2005, 32, 39–48. [Google Scholar] [CrossRef]
- Müldner, G.; Richards, M.P. Stable isotopic evidence for 1500 years of human diet at the city of York, UK. Am. J. Phys. Anthropol. 2007, 133, 682–697. [Google Scholar] [CrossRef]
- Nada Patrone, A.M.N. Il Cibo del Ricco ed il Cibo del Povero: Contrinuto alla Storia Qualitativa dell’Alimentazione; l’Area Pedemontana Negli Ultimi Secoli del Medio Evo; Centro Studi Piemontesi: Torino, Italy, 1981. [Google Scholar]
- Reitsema, L.J.; Crews, D.E.; Polcyn, M. Preliminary ecidence for medieval Polish diet from carbon and nitrogen stable isotopes. J. Archaeol. Sci. 2010, 37, 1413–1423. [Google Scholar] [CrossRef]
- Salamon, M.; Coppa, A.; McCormick, M.; Rubini, M.; Vargui, R.; Tuross, N. The consilience of historical and isotopic approaches in recontructing the medieval Mediterranean diet. J. Archaeol. Sci. 2008, 35, 1667–1672. [Google Scholar] [CrossRef]
- Sundman, E.A. Masculinities and diet: An analysis of skeletal material from the Dominican priory in Medieval Västerås, Sweden. Nor. Arcahaeol. Rev. 2018, 51, 95–111. [Google Scholar] [CrossRef]
- Woolgar, C. Take this penance now, and afterwards the fare will improve: Seafood and late medieval diet. In England’s Sea Fisheries: The Commercial Sea Fisheries of England and Wales Since 1300; Starkey, D.J., Reid, C., Ashcroft, N., Eds.; Chatham: London, UK, 2000; pp. 36–44. [Google Scholar]
- Zug Tucci, H. Il Mondo Medievale dei Esci tra Realtà e Immaginazione. L’Uomo di Fronte al Mondo Animale nell’Alto Medioevo; Centro Italiano di Studi sull’Alto Medioevo: Spoleto, Italy, 1985. [Google Scholar]
- Southern, P. The third century: The nature of the problem. The Roman Empire from Severus to Constantine, Routledge: Abingdon, UK, 2015; 1–17. [Google Scholar]
- Waters-Rist, A.L.; Katzenberg, M.A. The effect of growth on stable nitrogen isotope ratios in subadult bone collagen. Int. J. Osteoarchaeol. 2010, 20, 172–191. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2020, 1–25. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nat. Cell Biol. 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Keusch, G.T. The History of Nutrition: Malnutrition, Infection and Immunity. J. Nutr. 2003, 133, 336S–340S. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Antonio, M.L.; Gao, Z.; Moots, H.M.; Lucci, M.; Candilio, F.; Sawyer, S.; Oberreiter, V.; Calderon, D.; Devitofranceschi, K.; Aikens, R.C.; et al. Ancient Rome: A genetic crossroads of Europe and the Mediterranean. Science 2019, 366, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Jongman, W.M.; Jacobs, J.P.; Goldewijk, G.M.K. Health and wealth in the Roman Empire. Econ. Hum. Biol. 2019, 34, 138–150. [Google Scholar] [CrossRef]
- De Angelis, F.; Veltre, V.; Romboni, M.; Di Corcia, T.; Scano, G.; Martínez-Labarga, C.; Catalano, P.; Rickards, O. Ancient genomes from a rural site in Imperial Rome (1st–3rd cent. CE): A genetic junction in the Roman Empire. Ann. Hum. Biol. 2021. [Google Scholar] [CrossRef]
- Caldarini, C.; Zavaroni, F.; Benassi, V. Indicatori scheletrici di lavoro: Marcatori muscolo-scheletrici, artropatie e traumi. J. Hist. Med. 2015, 27, 905–967. [Google Scholar]
- Mosticone, R.; Pescucci, L.; Porreca, F. Le condizioni di vita: Indicatori di stress aspecifici e affezioni dento-alveolari. J. Hist. Med. 2015, 27, 969–1042. [Google Scholar]
- Wickham, C. Framing the Early Middle Ages: Europe and the Mediterranean, 400–800; Oxford University Press: Oxford, UK, 2005; p. 2. [Google Scholar]
- Barbehenn, R.; Chen, Z.; Karowe, D.N.; Spickard, A. C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2. Glob. Chang. Biol. 2004, 10, 1565–1575. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Naumann, N.; Fitzgerald, G.J.; Seneweera, S. Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions. Agric. Ecosyst. Environ. 2014, 185, 24–33. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Fitzgerals, G.J.; Myers, S.; Walker, C.; Strangoulis, J.; Seneweera, S. Wheat grain quality under increasing atmospheric CO2 concentrations in a semi-arid cropping system. J. Cereal Sci. 2012, 56, 684–690. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.; Fitzgerald, G.; Seneweera, S. Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chem. 2012, 133, 1307–1311. [Google Scholar] [CrossRef]
- Jobe, T.O.; Rahimzadeh Karvansara, P.; Zenzen, I.; Kopriva, S. Ensuring nutritious food under elevated CO2 Conditions: A care for improved C4 crops. Front. Plant Sci. 2020, 11, 1267. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142, Correction in Nature 2019, 574, E14. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Ujiie, K.; Ishimaru, K.; Hirotsu, N.; Nagasaka, S.; Miyakoshi, Y.; Ota, M.; Tokida, T.; Sakai, H.; Usui, Y.; Ono, K.; et al. How elevated CO2 affects our nutrition in rice, and how we can deal with it. PLoS ONE 2019, 14, e0212840. [Google Scholar] [CrossRef]
- Butz, D.E.; Casperson, S.L.; Whigham, L.D. The emerging role of carbon isotope ratio determination in health research and medical diagnostics. J. Anal. At. Spectrom. 2014, 29, 594–598. [Google Scholar] [CrossRef]
- DeWitte, S.N. Sex and frailty. In Exploring Sex and Gender in Bioarchaeology; Agarwal, S.C., Wesp, J.K., Eds.; University of Mexico Press: Albuquerque, Mexico, 2017; pp. 189–220. [Google Scholar]
- Roberts, C.A. Human Remains in Archaeology: A Handbook; Council for British Archaeology: York, UK, 2009. [Google Scholar]
- Wells, C. Ancient obstetric hazards and female mortality. Bull. N. Y. Acad. Med. 1975, 51, 1235–1249. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Wang, P.; Han, Y.; Ma, J.; Troy, F.A., II; Wang, B. Evaluation of dietary intake of lactating women in China and its potential impact on the health of mothers and infants. BMC Women Health 2012, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Engidaw, M.T.; Gebremariam, A.D.; Tiruneh, S.A.; Asnakew, D.T.; Abate, B.A. Chronic Energy Deficiency and its Associated Factors Among Lactating Women in Debre Tabor General Hospital, Northcentral Ethiopia. J. Fam. Med. Health Care 2019, 5, 1. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am. J. Clin. Nutr. 2004, 80, 1752S–1758S. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, C.O.; Meindl, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronological metamorphosis of the auricular surface of the ilium: A new method for the determination of adult skeletal age at death. Am. J. Phys. Anthr. 1985, 68, 15–28. [Google Scholar] [CrossRef]
- Brooks, S.; Suchey, J.M. Skeletal age determination based on the os pubis: A comparison of the Acsádi-Nemeskéri and Suchey-Brooks methods. Hum. Evol. 1990, 5, 227–238. [Google Scholar] [CrossRef]
- Işcan, M.Y.; Loth, S.R.; Wright, R.K. Metamorphosis at the sternal rib end: A new method to estimate age at death in white males. Am. J. Phys. Anthr. 1984, 65, 147–156. [Google Scholar] [CrossRef]
- Işcan, M.Y.; Loth, S.R.; Wright, R.K. Age estimation from the rib by phase analysis: White females. J. Forensic Sci. 1985, 30, 853–863. [Google Scholar] [PubMed]
- Meindl, R.S.; Lovejoy, C.O. Ectocranial suture closure: A revised method for the determination of skeletal age at death based on the lateral-anterior sutures. Am. J. Phys. Anthr. 1985, 68, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Brothwell, D.R. Digging up Bones: The Excavation, Treatment, and Study of Human Skeletal Remains; Cornell University Press: Ithaca, NY, USA, 1981. [Google Scholar]
- Lovejoy, C.O. Dental wear in the Libben population: Its functional pattern and role in the determination of adult skeletal age at death. Am. J. Phys. Anthropol. 1985, 68, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ubelaker, D.H. Human Skeletal Remains: Excavation, Analysis, Interpretation, 2nd ed.; Taraxacum Washington: Washington, WA, USA, 1989. [Google Scholar]
- Fazekas, I.G.; Kósa, F. Forensic Fetal Osteology; Akadémiai Kiadó: Budapest, Hungary, 1978. [Google Scholar]
- Stloukal, M.; Hanáková, H. Die Länge der Längsknochen altslawischer Bevölkerungen-Unter besonderer Berücksichtigung von Wachstumsfragen. Homo 1978, 29, 53–69. [Google Scholar]
- Scheuer, L.; Black, S. Developmental Juvenile Osteology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Scheuer, L.; Black, S. Juvenile Osteology; Academic Press: London, UK, 2004. [Google Scholar]
- Acsádi, G.; Nemeskéri, J. History of Human Life Span and Mortality; Akadémiai Kiadó: Budapest, Hungary, 1970. [Google Scholar]
- Ferembach, D.; Schwidetzky, I.; Stloukal, M. Recommandations pour determiner l’âge et le sexe sur le squelette. Bull. Mém. Soc. Anthropol. Paris 1979, 6, 7–45. [Google Scholar] [CrossRef]
- Phenice, T.W. A newly developed visual method of sexing the os pubis. Am. J. Phys. Anthropol. 1969, 30, 297–302. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Series B Stat. Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Cox, D.R. Partial Likelihood. Biometrika 1975, 62, 269–276. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M.; Fleming, T.R. Martingale-Based Residuals for Survival Models. Biometrika 1990, 77, 147–160. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- Hall, P.; Opsomer, D. Theory for Penalised Spline Regression. Biometrika 2005, 92, 105–118. [Google Scholar] [CrossRef]


| Roman Imperial Period | |||||
|---|---|---|---|---|---|
| Variable | Parameter Estimate | Hazard Ratio (HR) | 95% Hazard Ratio Confidence Limits | p | |
| Lower | Upper | ||||
| Sex | 0.74 | 2.09 | 1.28 | 3.42 | 0.0032 |
| (Female vs. Male) | |||||
| δ13C (x increasing unit) | −0.18 | 0.84 | 0.53 | 1.32 | 0.4440 |
| Linear effect | |||||
| δ15N (x unit) | −2.93 | 0.0199 | |||
| Linear effect | |||||
| δ15N (x unit) | 0.14 | 0.0235 | |||
| Quadratic effect | |||||
| Medieval Period | |||||
| Variable | Parameter Estimate | Hazard Ratio (HR) | 95% Hazard Ratio Confidence Limits | p | |
| Lower | Upper | ||||
| Sex | −0.26 | 1.00 | 0.7 | 1.43 | 0.9875 |
| (Female vs. Male) | |||||
| δ13C (x increasing unit) | 0.30 | 1.36 | 1.04 | 1.77 | 0.0248 |
| Linear effect | |||||
| δ15N (x increasing unit) | −0.26 | 0.77 | 0.67 | 0.89 | 0.0005 |
| Linear effect | |||||
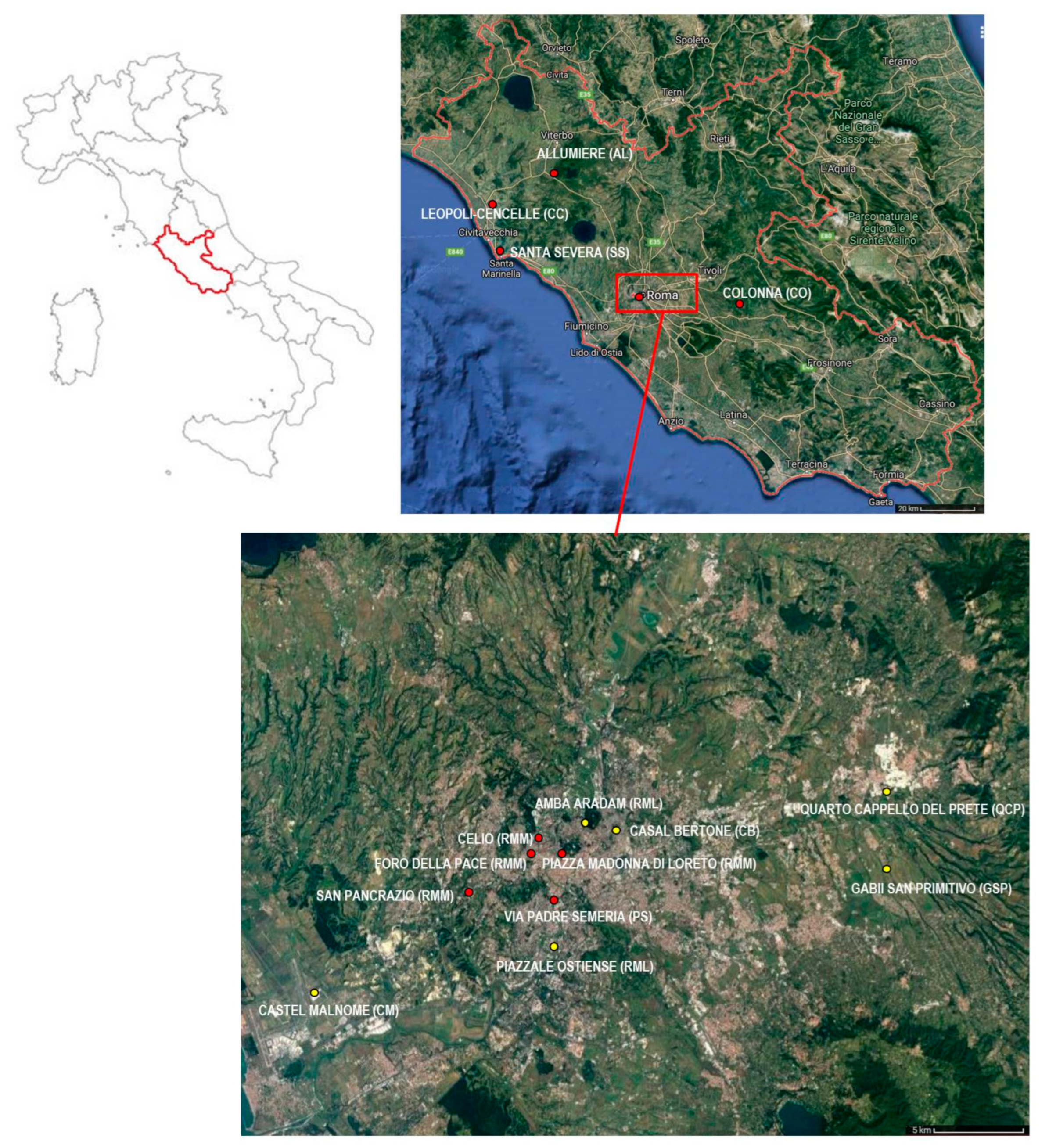
| Roman Imperial Samples (1st–5th Centuries CE) | |||
| Archeological Sites | Chronology (Centuries) | Abbreviations and Grouping | References |
| Castel Malnome | 1st–3rd centuries CE | CM | [42] |
| Via Padre Semeria | 1st–3rd centuries CE | PS | [42] |
| Quarto Cappello del Prete | 1st–3rd centuries CE | QCP | [42] |
| Casal Bertone Necropolis | 1st–3rd centuries CE | CBN | [42] |
| Casal Bertone Mausoleum | 1st–3rd centuries CE | CBM | [42] |
| Casal Bertone Area Q | 1st–3rd centuries CE | CBQ | [42] |
| Piazzale Ostiense | 4th–5th centuries CE | RML | [45] |
| Amba Aradam | 5th century CE | RML | [45] |
| Medieval Samples (8th–16th Centuries CE) | |||
| Archeological Sites | Chronology (Centuries) | Abbreviations and Grouping | References |
| Colonna | 8th–10th centuries CE | CO | [39] |
| Santa Severa | 7th–15th centuries CE | SS | [43] |
| Allumiere | 15th–16th centuries CE | AL | [40] |
| Cencelle | 12th–15th centuries CE | CC | [41] |
| Piazza Madonna di Loreto | 8th century CE | RMM | [44] |
| San Pancrazio | 7th–8th centuries CE | RMM | [45] |
| Celio I | 6th–9th centuries CE | RMM | [45] |
| Celio II | 10th–11th centuries CE | RMM | [45] |
| Foro della Pace | 10th–11th centuries CE | RMM | [45] |
| Gabii San Primitivo | 10th–11th centuries CE | GSP | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldoni, M.; Nardi, A.; De Angelis, F.; Rickards, O.; Martínez-Labarga, C. How Does Diet Influence Our Lives? Evaluating the Relationship between Isotopic Signatures and Mortality Patterns in Italian Roman Imperial and Medieval Periods. Molecules 2021, 26, 3895. https://doi.org/10.3390/molecules26133895
Baldoni M, Nardi A, De Angelis F, Rickards O, Martínez-Labarga C. How Does Diet Influence Our Lives? Evaluating the Relationship between Isotopic Signatures and Mortality Patterns in Italian Roman Imperial and Medieval Periods. Molecules. 2021; 26(13):3895. https://doi.org/10.3390/molecules26133895
Chicago/Turabian StyleBaldoni, Marica, Alessandra Nardi, Flavio De Angelis, Olga Rickards, and Cristina Martínez-Labarga. 2021. "How Does Diet Influence Our Lives? Evaluating the Relationship between Isotopic Signatures and Mortality Patterns in Italian Roman Imperial and Medieval Periods" Molecules 26, no. 13: 3895. https://doi.org/10.3390/molecules26133895
APA StyleBaldoni, M., Nardi, A., De Angelis, F., Rickards, O., & Martínez-Labarga, C. (2021). How Does Diet Influence Our Lives? Evaluating the Relationship between Isotopic Signatures and Mortality Patterns in Italian Roman Imperial and Medieval Periods. Molecules, 26(13), 3895. https://doi.org/10.3390/molecules26133895






