Novel Poly(ester urethane urea)/Polydioxanone Blends: Electrospun Fibrous Meshes and Films
Abstract
:1. Introductions
2. Materials and Methods
2.1. Polyurethane Synthesis
2.2. Scaffolds and Films Preparation
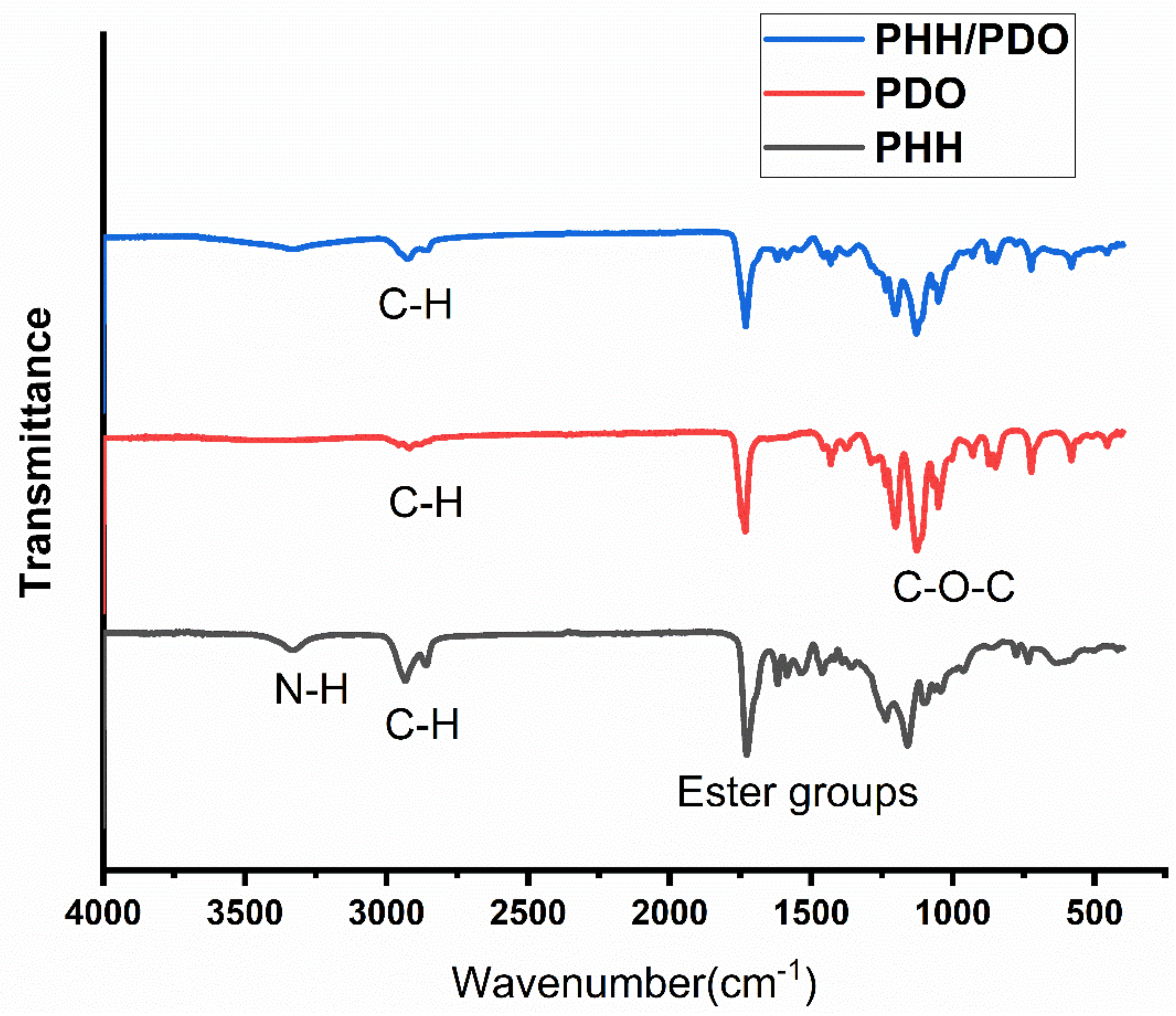
2.3. Structural Morphological and Chemical Characterizations
2.4. Biomechanical Evaluation
3. Results and Discussion
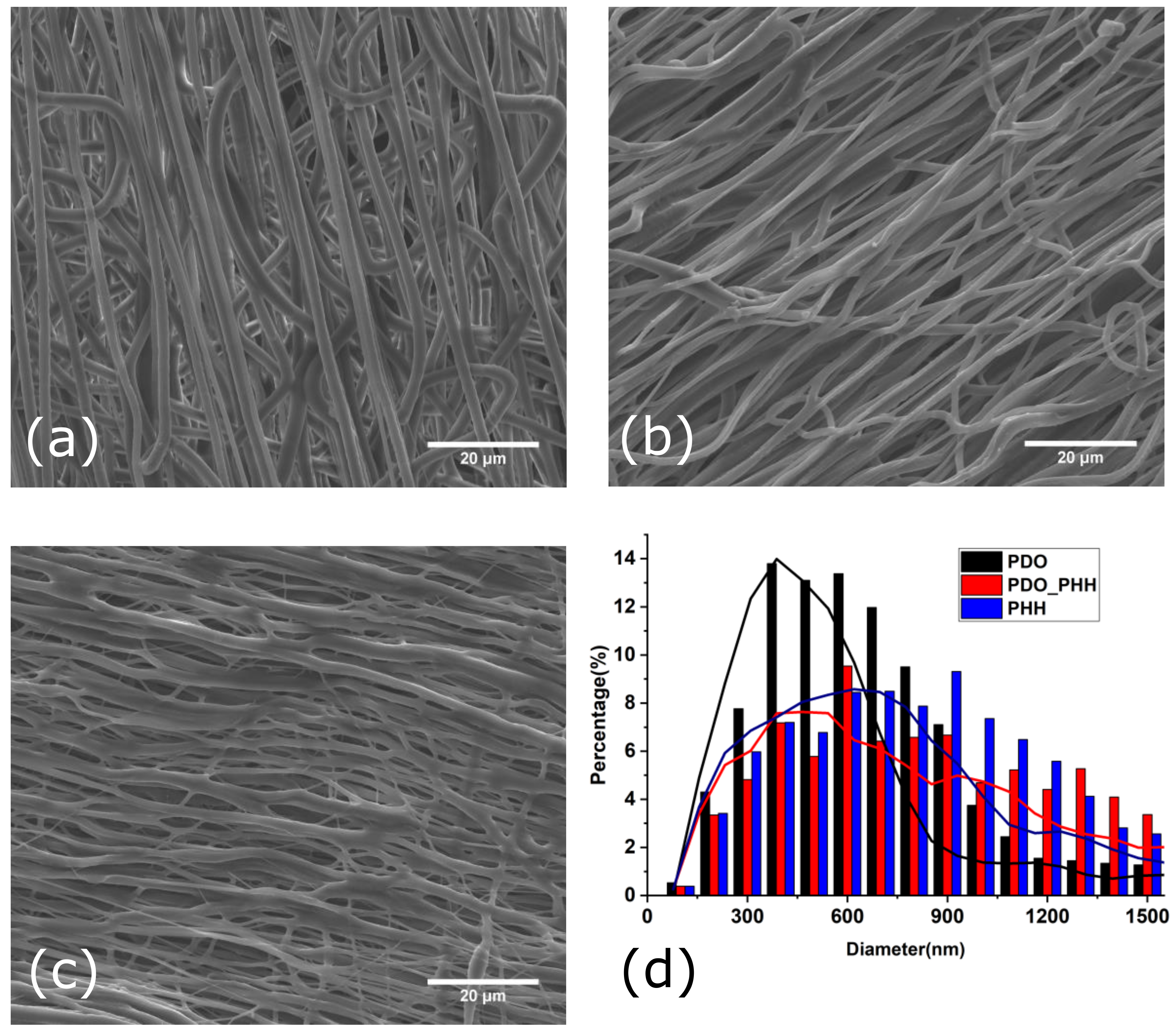
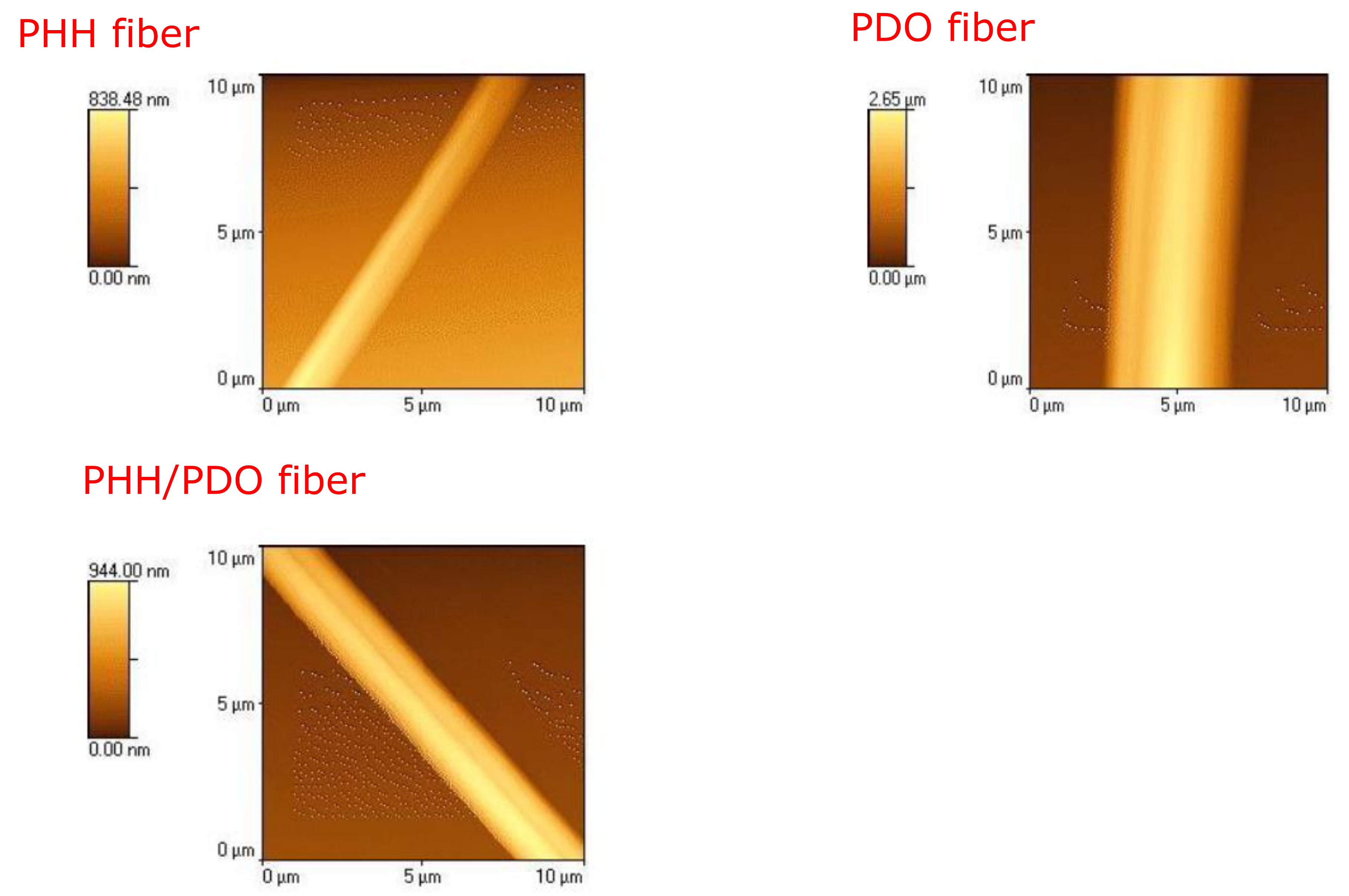
3.1. Structural and Morphological Characterization
3.2. Crystallinity, Porosity, and PBS Uptake by the Electrospun Mats
3.3. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A Novel Carrier for Cell and Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.M. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nat. Cell Biol. 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gelain, F.; Zhao, X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 2005, 15, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Li, B.; Ma, Z.; Wei, H.; Chan, C.; Ramakrishna, S. Biomimetic electrospun nanofibers for tissue regeneration. Biomed. Mater. 2006, 1, R45–R53. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Derrick, D.R.; Vohra, Y.K. Nanostructured Biomaterials for Regenerative Medicine. Available online: http://www.eurekaselect.com/56585/article (accessed on 26 September 2019).
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Sell, S.; Barnes, C.; Smith, M.; McClure, M.; Madurantakam, P.; Grant, J.; McManus, M.; Bowlin, G. Extracellular matrix regenerated: Tissue engineering via electrospun biomimetic nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Lim, S.H.; Mao, H.-Q. Electrospun scaffolds for stem cell engineering. Adv. Drug Deliv. Rev. 2009, 61, 1084–1096. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Yu, D.-G.; Zhu, M.-J.; Zhao, M.; Williams, G.R. Tunable zero-order drug delivery systems created by modified triaxial electrospinning. Chem. Eng. J. 2019, 356, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Burger, C.; Hsiao, B.S.; Chu, B. Nanofibrous Materials and their Applications. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Jian, S.; Zhu, J.; Jiang, S.; Chen, S.; Fang, H.; Song, Y.; Duan, G.; Zhang, Y.; Hou, H. Nanofibers with diameter below one nanometer from electrospinning. RSC Adv. 2018, 8, 4794–4802. [Google Scholar] [CrossRef]
- Vollrath, F. Spider Silk: Thousands of Nano-Filaments and Dollops of Sticky Glue. Curr. Biol. 2006, 16, R925–R927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsdale, T.; Bard, J. Collagen Substrata for Studies on Cell Behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, D.R.; Forsythe, J.S.; Shen, W.; Finkelstein, D.I.; Horne, M. Review Paper: A Review of the Cellular Response on Electrospun Nanofibers for Tissue Engineering. J. Biomater. Appl. 2008, 24, 7–29. [Google Scholar] [CrossRef]
- Stepanyan, R.; Subbotin, A.; Cuperus, L.; Boonen, P.; Dorschu, M.; Oosterlinck, F.; Bulters, M.J.H. Fiber diameter control in electrospinning. Appl. Phys. Lett. 2014, 105, 173105. [Google Scholar] [CrossRef]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer Scaffolds for Biomaterials Applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Pektok, E.; Nottelet, B.; Tille, J.-C.; Gurny, R.; Kalangos, A.; Möller, M.; Walpoth, B.H. Degradation and Healing Characteristics of Small-Diameter Poly(ε-Caprolactone) Vascular Grafts in the Rat Systemic Arterial Circulation. Circulation 2008, 118, 2563–2570. [Google Scholar] [CrossRef] [Green Version]
- Guelcher, S.A. Biodegradable Polyurethanes: Synthesis and Applications in Regenerative Medicine. Tissue Eng. Part B Rev. 2008, 14, 3–17. [Google Scholar] [CrossRef]
- Zdrahala, R.J.; Zdrahala, I.J. Biomedical Applications of Polyurethanes: A Review of Past Promises, Present Realities, and a Vibrant Future. J. Biomater. Appl. 1999, 14, 67–90. [Google Scholar] [CrossRef]
- Skarja, G.A.; Woodhouse, K.A. In vitro degradation and erosion of degradable, segmented polyurethanes containing an amino acid-based chain extender. J. Biomater. Sci. Polym. Ed. 2001, 12, 851–873. [Google Scholar] [CrossRef]
- Saad, B.; Hirt, T.D.; Welti, M.; Uhlschmid, G.K.; Neuenschwander, P.; Suter, U. Development of degradable polyesterurethanes for medical applications:In vitro andin vivo evaluations. J. Biomed. Mater. Res. 1997, 36, 65–74. [Google Scholar] [CrossRef]
- Caracciolo, P.; de Queiroz, A.; Higa, O.; Buffa, F.; Abraham, G. Segmented poly(esterurethane urea)s from novel urea–diol chain extenders: Synthesis, characterization and in vitro biological properties. Acta Biomater. 2008, 4, 976–988. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Buffa, F.; Abraham, G.A. Effect of the hard segment chemistry and structure on the thermal and mechanical properties of novel biomedical segmented poly(esterurethanes). J. Mater. Sci. Mater. Electron. 2008, 20, 145–155. [Google Scholar] [CrossRef]
- Abraham, G.; de Queiroz, A.A.; Román, J.S. Hydrophilic hybrid IPNs of segmented polyurethanes and copolymers of vinylpyrrolidone for applications in medicine. Biomaterials 2001, 22, 1971–1985. [Google Scholar] [CrossRef]
- Smith, M.J.; McClure, M.J.; Sell, S.A.; Barnes, C.P.; Walpoth, B.H.; Simpson, D.G.; Bowlin, G.L. Suture-reinforced electrospun polydioxanone–elastin small-diameter tubes for use in vascular tissue engineering: A feasibility study. Acta Biomater. 2008, 4, 58–66. [Google Scholar] [CrossRef]
- Sell, S.; McClure, M.J.; Barnes, C.P.; Knapp, D.C.; Walpoth, B.H.; Simpson, D.G.; Bowlin, G.L. Electrospun polydioxanone–elastin blends: Potential for bioresorbable vascular grafts. Biomed. Mater. 2006, 1, 72–80. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Im, J.N.; Kim, J.K.; Kim, H.-K.; In, C.H.; Lee, K.Y.; Park, W.H. In vitro and in vivo degradation behaviors of synthetic absorbable bicomponent monofilament suture prepared with poly(p-dioxanone) and its copolymer. Polym. Degrad. Stab. 2007, 92, 667–674. [Google Scholar] [CrossRef]
- Thomas, V.; Zhang, X.; Vohra, Y.K. A biomimetic tubular scaffold with spatially designed nanofibers of protein/PDS® bio-blends. Biotechnol. Bioeng. 2009, 104, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Goonoo, N.; Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur. J. Pharm. Biopharm. 2015, 97, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-J.; Shin, Y.C.; Kim, S.E.; Kwon, I.K.; Lee, J.-H.; Hyon, S.-H.; Han, D.-W.; Kim, B. Aligned laminin core-polydioxanone/collagen shell fiber matrices effective for neuritogenesis. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, M.; Khorrami, M.; Yi, N.; Majd, S.; Abidian, M.R. Electrospinning of highly aligned fibers for drug delivery applications. J. Mater. Chem. B 2018, 7, 224–232. [Google Scholar] [CrossRef]
- Kallem, P.; Yanar, N.; Choi, H. Nanofiber-Based Proton Exchange Membranes: Development of Aligned Electrospun Nanofibers for Polymer Electrolyte Fuel Cell Applications. ACS Sustain. Chem. Eng. 2018, 7, 1808–1825. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Q.; Zhang, Y. Improving Fiber Alignment during Electrospinning; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–147. [Google Scholar]
- Kim, J.I.; Hwang, T.I.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Controlled Design of Aligned and Random Nanofibers for 3D Bi-functionalized Nerve Conduits Fabricated via a Novel Electrospinning Set-up. Sci. Rep. 2016, 6, 23761. [Google Scholar] [CrossRef] [Green Version]
- Sankar, S.; Sharma, C.S.; Rath, S.N.; Ramakrishna, S. Electrospun Fibers for Recruitment and Differentiation of Stem Cells in Regenerative Medicine. Biotechnol. J. 2017, 12, 1700263. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; El-Aassar, M.; Mohamed, I.M.; Kwak, H.-H.; Woo, H.-M.; Abdal-Hay, A. Development of biocompatible tri-layered nanofibers patches with endothelial cells for cardiac tissue engineering. Eur. Polym. J. 2020, 129, 109630. [Google Scholar] [CrossRef]
- Beck, E.C.; Jarrell, D.K.; Lyons, A.C.; Vanderslice, E.J.; VeDepo, M.C.; Jacot, J.G. Assessment of electrospun cardiac patches made with sacrificial particles and polyurethane-polycaprolactone blends. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Thomas, V.; Vohra, Y.K.; Buffa, F.; Abraham, G.A. Electrospinning of novel biodegradable poly(ester urethane)s and poly(ester urethane urea)s for soft tissue-engineering applications. J. Mater. Sci. Mater. Electron. 2009, 20, 2129–2137. [Google Scholar] [CrossRef]
- Thomas, V.; Dean, D.R.; Jose, M.V.; Mathew, B.; Chowdhury, A.S.; Vohra, Y.K. Nanostructured Biocomposite Scaffolds Based on Collagen Coelectrospun with Nanohydroxyapatite. Biomacromolecules 2007, 8, 631–637. [Google Scholar] [CrossRef]
- Thomas, V.; Zhang, X.; Catledge, S.A.; Vohra, Y.K. Functionally graded electrospun scaffolds with tunable mechanical properties for vascular tissue regeneration. Biomed. Mater. 2007, 2, 224–232. [Google Scholar] [CrossRef]
- Jeffries, E.; Allen, R.A.; Gao, J.; Pesce, M.; Wang, Y. Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 2015, 18, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Chavoshnejad, P.; Razavi, M.J. Effect of the Interfiber Bonding on the Mechanical Behavior of Electrospun Fibrous Mats. Sci. Rep. 2020, 10, 7709–7710. [Google Scholar] [CrossRef]
- Li, H.; Zhu, C.; Xue, J.; Ke, Q.; Xia, Y. Enhancing the Mechanical Properties of Electrospun Nanofiber Mats through Controllable Welding at the Cross Points. Macromol. Rapid Commun. 2017, 38, 1600723. [Google Scholar] [CrossRef] [Green Version]
- Ishikiriyama, K.; Pyda, M.; Zhang, G.; Forschner, T.; Grebowicz, J.; Wunderlich, B. Heat capacity of poly-p-dioxanone. J. Macromol. Sci. Part B 1998, 37, 27–44. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-08-091510-4. [Google Scholar]
- Chen, C.-H.; Wei, H.-J.; Lin, W.-W.; Chiu, I.; Hwang, S.-M.; Wang, C.-C.; Lee, W.-Y.; Chang, Y.; Sung, H.-W. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc. Res. 2008, 80, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.L.; Tobita, K.; Merryman, W.D.; Guan, J.; Momoi, N.; Stolz, D.B.; Sacks, M.S.; Keller, B.B.; Wagner, W.R. An Elastic, Biodegradable Cardiac Patch Induces Contractile Smooth Muscle and Improves Cardiac Remodeling and Function in Subacute Myocardial Infarction. J. Am. Coll. Cardiol. 2007, 49, 2292–2300. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.A.; Burugapalli, K.; Neuenschwander, P.; Pandit, A. Structural variants of biodegradable polyesterurethane in vivo evoke a cellular and angiogenic response that is dictated by architecture. Acta Biomater. 2009, 5, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, D.N.; Akins, R.E.; Parrag, I.C.; Woodhouse, K.A.; Rabolt, J.F. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro. Biomaterials 2008, 29, 4783–4791. [Google Scholar] [CrossRef] [Green Version]
- Stankus, J.J.; Soletti, L.; Fujimoto, K.; Hong, Y.; Vorp, D.; Wagner, W.R. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials 2007, 28, 2738–2746. [Google Scholar] [CrossRef] [Green Version]



| Sample | Crystallinity (%) | Porosity (%) | PBS Absorption (%) |
|---|---|---|---|
| ePHH | 11.8 | 66.4 ± 3.8 | 197.1 ± 12.3 |
| ePDO | 57.2 | 79.2 ± 2.7 | 503.3 ± 34.5 |
| ePHH/PDO | 25.6 | 71.7 ± 5.3 | 852.5 ± 17.6 |
| Polymers | UTS (MPa) | Young’s Modulus (Mpa) | Elongation at Break (%) | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| PHH film | 5.3 ± 0.5 | 4.5 ± 0.68 | 10.0 ± 1.5 | 7.8 ± 1.9 | 291.0 ± 44 | 259.0 ± 94 |
| PDO film | 5.6 ± 1.3 | 4.4 ± 0.89 | 44.4 ± 7.8 | 26.7 ± 2.2 | 28.6 ± 8.2 | 13.4 ± 2.3 |
| PHH/PDO film | 5.7 ± 1.1 | 3.2 ± 1.1 | 10.5 ± 1.2 | 23.5 ± 0.1 | 71.4 ± 21.5 | 53.6 ± 37.9 |
| ePHH | 1.1 ± 0.2 | 0.7 ± 0.3 | 1.0 ± 0.1 | 1.7 ± 0.7 | 176.3 ± 13.8 | 90.4 ± 19.2 |
| ePDO | 3.7 ± 0.5 | 2.5 ± 0.2 | 9.5 ± 1.0 | 7.1 ± 0.1 | 139.2 ± 28.1 | 150.0 ± 15.6 |
| ePHH/PDO | 2.2 ± 0.4 | 2.0 ± 0.5 | 4.8 ± 0.7 | 3.9 ± 1.1 | 73.4 ± 15.5 | 150.2 ± 44.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, K.R.; Stanishevskaya, I.; Caracciolo, P.C.; Abraham, G.A.; Thomas, V. Novel Poly(ester urethane urea)/Polydioxanone Blends: Electrospun Fibrous Meshes and Films. Molecules 2021, 26, 3847. https://doi.org/10.3390/molecules26133847
Adhikari KR, Stanishevskaya I, Caracciolo PC, Abraham GA, Thomas V. Novel Poly(ester urethane urea)/Polydioxanone Blends: Electrospun Fibrous Meshes and Films. Molecules. 2021; 26(13):3847. https://doi.org/10.3390/molecules26133847
Chicago/Turabian StyleAdhikari, Kiran R., Inessa Stanishevskaya, Pablo C. Caracciolo, Gustavo A. Abraham, and Vinoy Thomas. 2021. "Novel Poly(ester urethane urea)/Polydioxanone Blends: Electrospun Fibrous Meshes and Films" Molecules 26, no. 13: 3847. https://doi.org/10.3390/molecules26133847
APA StyleAdhikari, K. R., Stanishevskaya, I., Caracciolo, P. C., Abraham, G. A., & Thomas, V. (2021). Novel Poly(ester urethane urea)/Polydioxanone Blends: Electrospun Fibrous Meshes and Films. Molecules, 26(13), 3847. https://doi.org/10.3390/molecules26133847








