A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. AGE Inhibited Cells Proliferation and Caused Cells Death
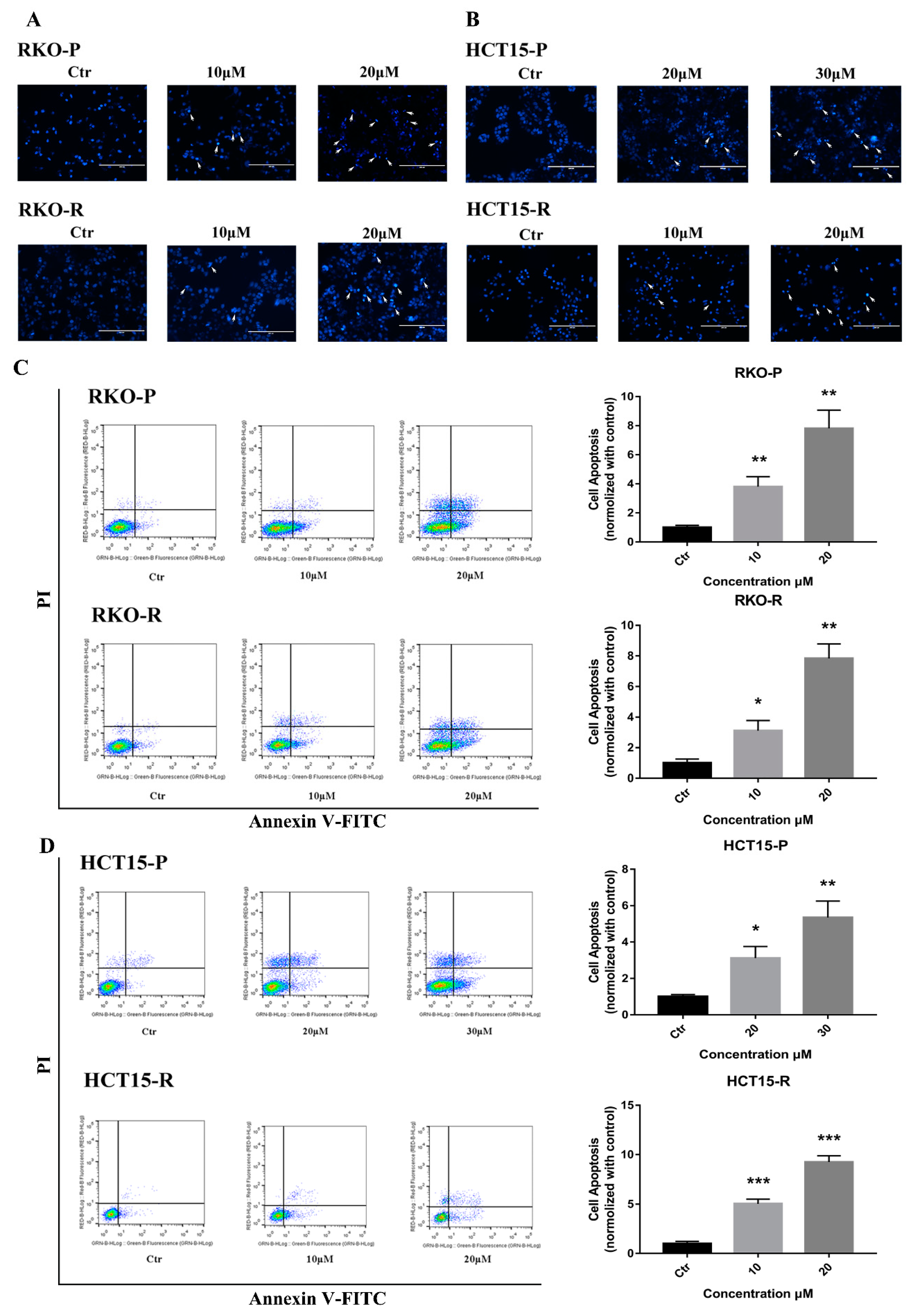
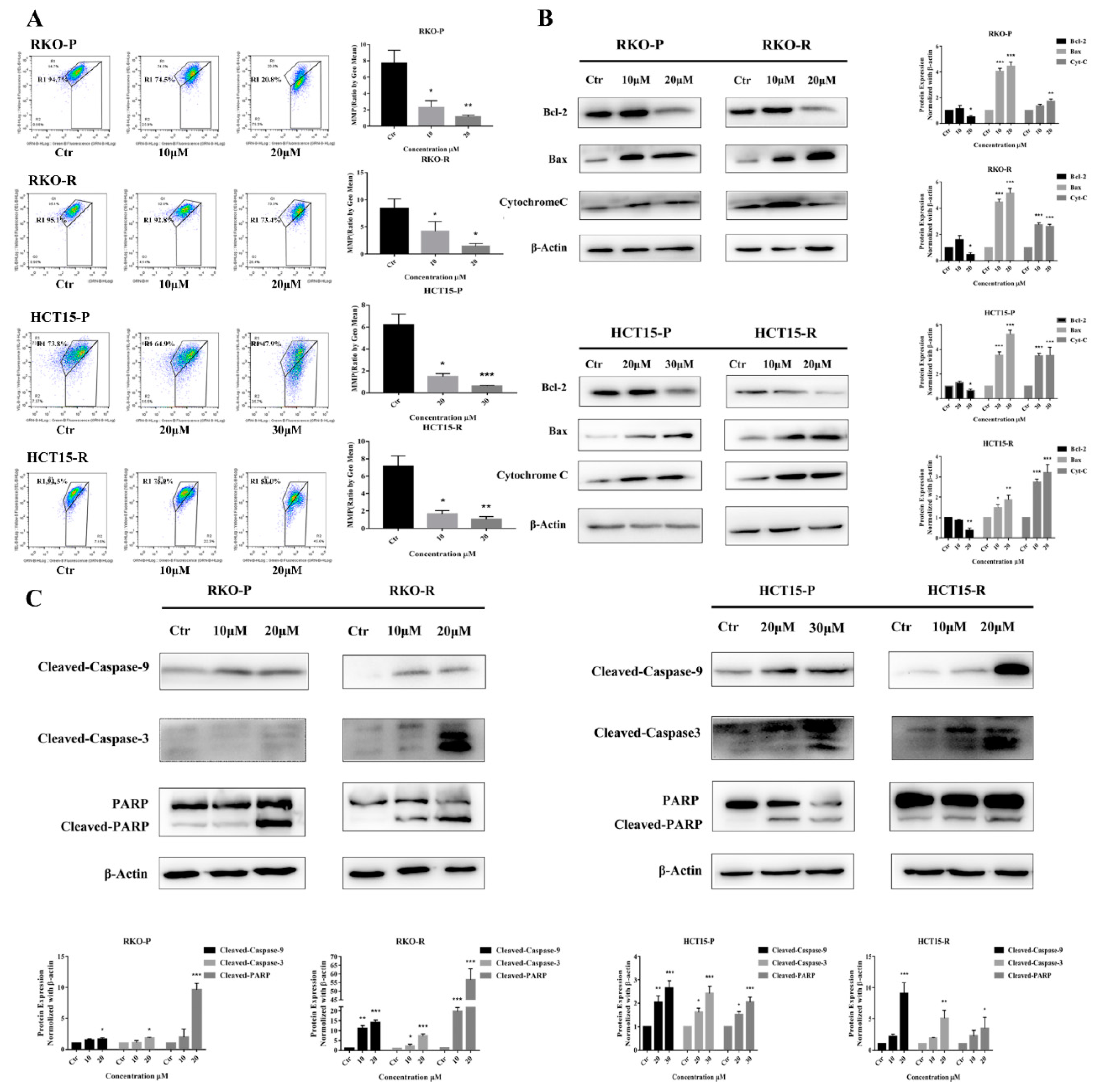
2.2. AGE Initiated Cells Intracellular Apoptotic Pathway
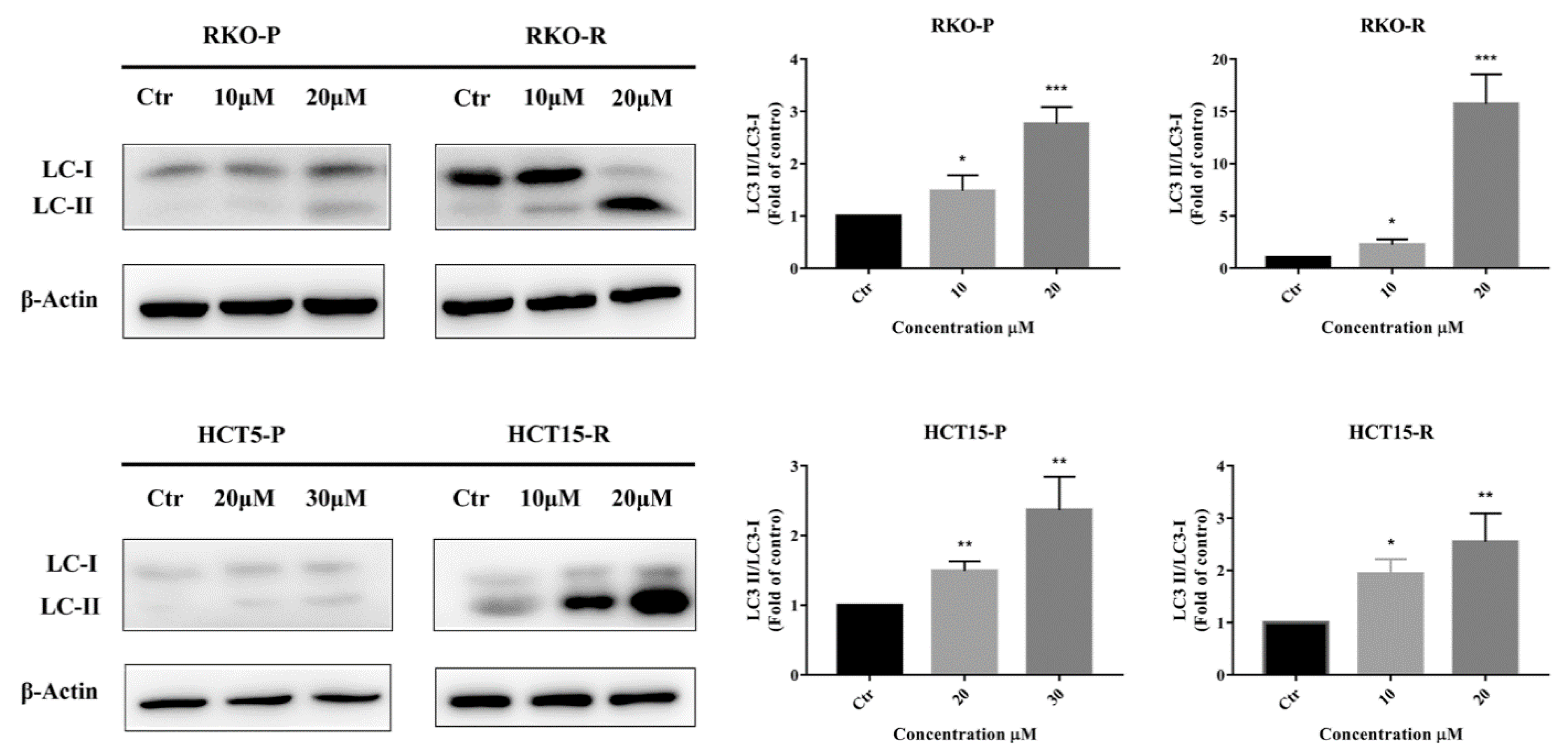
2.3. AGE Elicited Autophagy
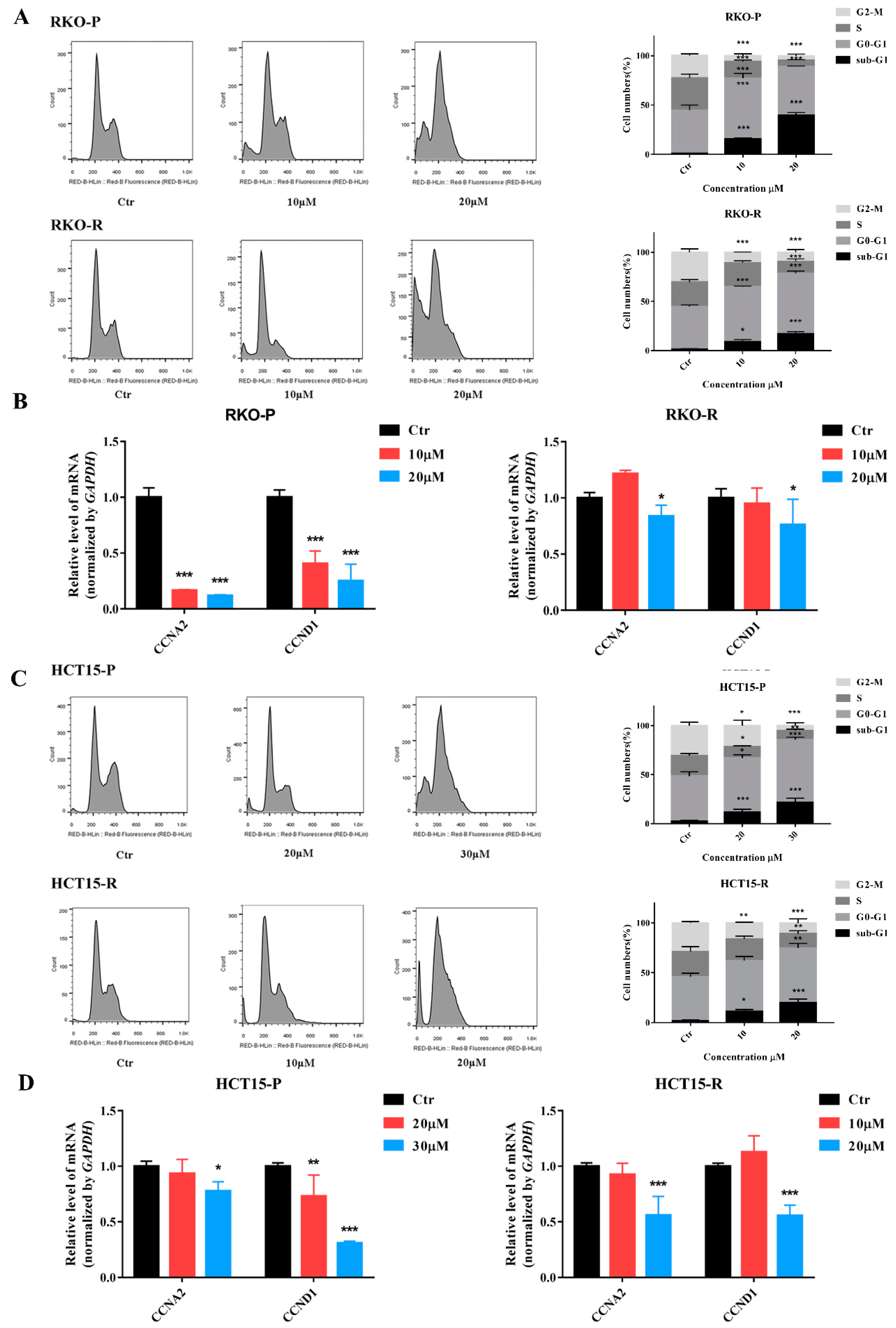
2.4. AGE Arrested Cell Cycle at G0-G1 Phase
2.5. AGE Blocked Wnt/β-Catenin Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Lines and CULTURE
4.2. Chemicals and Reagents
4.3. Cell Viability Assay
4.4. Cell Colony Formation Assay
4.5. Hoechst 33258 Staining Assay
4.6. Flow Cytometry Analysis of Apoptosis and Cell Cycle
4.7. Mitochondrial Membrane Potential (MMP) Measurement
4.8. Western Blot Analysis
4.9. Reverse Transcription Quantitative PCR Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pract. 2015, 211, 557–569. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef] [PubMed]
- Su, X.D.; Guo, R.H.; Li, H.X.; Ma, J.Y.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Anti-allergic inflammatory components from Sanguisorba officinalis L. Bioorg. Med. Chem. Lett. 2018, 28, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Graja, I.; Balicki, S.; Ziewiecki, R.; Matulova, M.; Capek, P.; Gancarz, R. Polyphenolic-polysaccharide conjugates of Sanguisorba officinalis L. with anticoagulant activity mediated mainly by heparin cofactor II. Int. J. Biol. Macromol. 2016, 93, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; He, X.; Zhang, Q.; Wei, X.; Huang, L.; Fang, J.C.; Wang, X.; Zhao, M.; Bai, Y.; Zheng, X. Traditional Uses, Chemical Constituents and Biological Activities of Plants from the Genus sanguisorba L. Am. J. Chin. Med. 2017, 45, 199–224. [Google Scholar] [CrossRef]
- Liu, M.P.; Liao, M.; Dai, C.; Chen, J.F.; Yang, C.J.; Liu, M.; Chen, Z.G.; Yao, M.C. Sanguisorba officinalis L. synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway. Sci. Rep. 2016, 6, 34245. [Google Scholar] [CrossRef]
- Wu, C.; Yao, M.; Li, W.; Cui, B.; Dong, H.; Ren, Y.; Yang, C.; Gan, C. Simultaneous Determination and Pharmacokinetics Study of Six Triterpenes in Rat Plasma by UHPLC-MS/MS after Oral Administration of Sanguisorba officinalis L. Extract. Molecules 2018, 23, 2980. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Munoz-Pinedo, C.; Martin, S.J. Autosis: A new addition to the cell death Tower of Babel. Cell Death Dis. 2014, 5, e1319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suwala, A.K.; Koch, K.; Rios, D.H.; Aretz, P.; Uhlmann, C.; Ogorek, I.; Felsberg, J.; Reifenberger, G.; Kohrer, K.; Deenen, R.; et al. Inhibition of Wnt/beta-catenin signaling downregulates expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to reduce resistance against temozolomide in glioblastoma in vitro. Oncotarget 2018, 9, 22703–22716. [Google Scholar] [CrossRef]
- Chikazawa, N.; Tanaka, H.; Tasaka, T.; Nakamura, M.; Tanaka, M.; Onishi, H.; Katano, M. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010, 30, 2041–2048. [Google Scholar] [PubMed]
- Panda, M.; Biswal, B.K. Cell signaling and cancer: A mechanistic insight into drug resistance. Mol. Biol. Rep. 2019, 46, 5645–5659. [Google Scholar] [CrossRef] [PubMed]
- Ewen, M.E.; Lamb, J. The activities of cyclin D1 that drive tumorigenesis. Trends Mol. Med. 2004, 10, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.M. Cyclin A2 transcriptional regulation: Modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem. Pharm. 2000, 60, 1179–1184. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Colorectal cancer and medicinal plants: Principle findings from recent studies. Biomed. Pharm. 2018, 107, 408–423. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharm. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Y.; Song, F.; Ding, Y. Chinese herbal medicine promote tissue differentiation in colorectal cancer by activating HSD11B2. Arch. Biochem. Biophys. 2020, 695, 108644. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Inn, K.S.; Jang, Y.P.; Lee, K.T.; Lee, J.H. Phytotherapeutic Activities of Sanguisorba officinalis and its Chemical Constituents: A Review. Am. J. Chin. Med. 2018, 46, 299–318. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, Z.; Zhou, L.; Zhang, H.Y.; Chen, Y.; Tang, Y. Repurposing Ziyuglycoside II Against Colorectal Cancer via Orchestrating Apoptosis and Autophagy. Front. Pharmacol. 2020, 11, 576547. [Google Scholar] [CrossRef]
- Hong, Z.; Tang, P.; Liu, B.; Ran, C.; Yuan, C.; Zhang, Y.; Lu, Y.; Duan, X.; Yang, Y.; Wu, H. Ferroptosis-related Genes for Overall Survival Prediction in Patients with Colorectal Cancer can be Inhibited by Gallic acid. Int. J. Biol. Sci. 2021, 17, 942–956. [Google Scholar] [CrossRef]
- Bai, C.; Sun, Y.; Pan, X.; Yang, J.; Li, X.; Wu, A.; Qin, D.; Cao, S.; Zou, W.; Wu, J. Antitumor Effects of Trimethylellagic Acid Isolated From Sanguisorba officinalis L. on Colorectal Cancer via Angiogenesis Inhibition and Apoptosis Induction. Front. Pharmacol. 2019, 10, 1646. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.J.; Wang, L.Q.; Wu, J.; Dai, C.; Yuan, Y.M.; Li, G.Q.; Yao, M.C. A tannin compound from Sanguisorba officinalis blocks Wnt/beta-catenin signaling pathway and induces apoptosis of colorectal cancer cells. Chin. Med. 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Wei, J.; Dang, S.; Yu, X.; Ding, L.; Shang, C.; Zhang, H.; Zhang, Z.; Chen, H.; et al. Ellagic acid induces cell cycle arrest and apoptosis via the TGFbeta1/Smad3 signaling pathway in human colon cancer HCT116 cells. Oncol. Rep. 2020, 44, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Bo, W.; Zhou, Y.; Dang, S.; Wei, J.; Li, X.; Liu, M. Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. Int. J. Oncol. 2017, 50, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.D.; Xu, J.H.; Yu, T.; Li, J.Y.; Zhao, L.N.; Ouyang, H.; Luo, S.; Lu, X.J.; Huang, C.Z.; Lan, Q.S.; et al. Knockdown of linc-POU3F3 suppresses the proliferation, apoptosis, and migration resistance of colorectal cancer. Oncotarget 2016, 7, 961–975. [Google Scholar] [CrossRef]
- Wang, L.; Hu, T.; Shen, J.; Zhang, L.; Chan, R.L.; Lu, L.; Li, M.; Cho, C.H.; Wu, W.K. Dihydrotanshinone I induced apoptosis and autophagy through caspase dependent pathway in colon cancer. Phytomedicine 2015, 22, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.R.; Shi, Z.Q.; Zhu, H.P.; Gu, L.H.; Wang, X.F.; Yang, Y. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget 2017, 8, 62759–62768. [Google Scholar] [CrossRef]
- Albayrak, G.; Konac, E.; Dikmen, A.U.; Bilen, C.Y. Memantine induces apoptosis and inhibits cell cycle progression in LNCaP prostate cancer cells. Hum. Exp. Toxicol. 2018, 37, 953–958. [Google Scholar] [CrossRef]
- Dai, J.; Wei, R.J.; Li, R.; Feng, J.B.; Yu, Y.L.; Liu, P.S. A study of CCND1 with epithelial ovarian cancer cell proliferation and apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4230–4235. [Google Scholar]
- Katoh, M.; Katoh, M. Molecular genetics and targeted therapy of WNT-related human diseases (Review). Int. J. Mol. Med. 2017, 40, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhao, J.; Fan, D.; Wang, Z.; Zhao, T.; Li, Y.; Zhao, Y.; Adelson, D.; Hao, H. Alkaloids from nux vomica suppresses colon cancer cell growth through Wnt/beta-catenin signaling pathway. Phytother. Res. 2019, 33, 1570–1578. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Brabletz, T.; Fearon, E.; Willis, A.L.; Hu, C.Y.; Li, X.Y.; Weiss, S.J. Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc. Natl. Acad. Sci. USA 2012, 109, 11312–11317. [Google Scholar] [CrossRef]
- Ahn, V.E.; Chu, M.L.; Choi, H.J.; Tran, D.; Abo, A.; Weis, W.I. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev. Cell. 2011, 21, 862–873. [Google Scholar] [CrossRef]
- Kukcinaviciute, E.; Jonusiene, V.; Sasnauskiene, A.; Dabkeviciene, D.; Eidenaite, E.; Laurinavicius, A. Significance of Notch and Wnt signaling for chemoresistance of colorectal cancer cells HCT116. J. Cell. Biochem. 2018, 119, 5913–5920. [Google Scholar] [CrossRef]
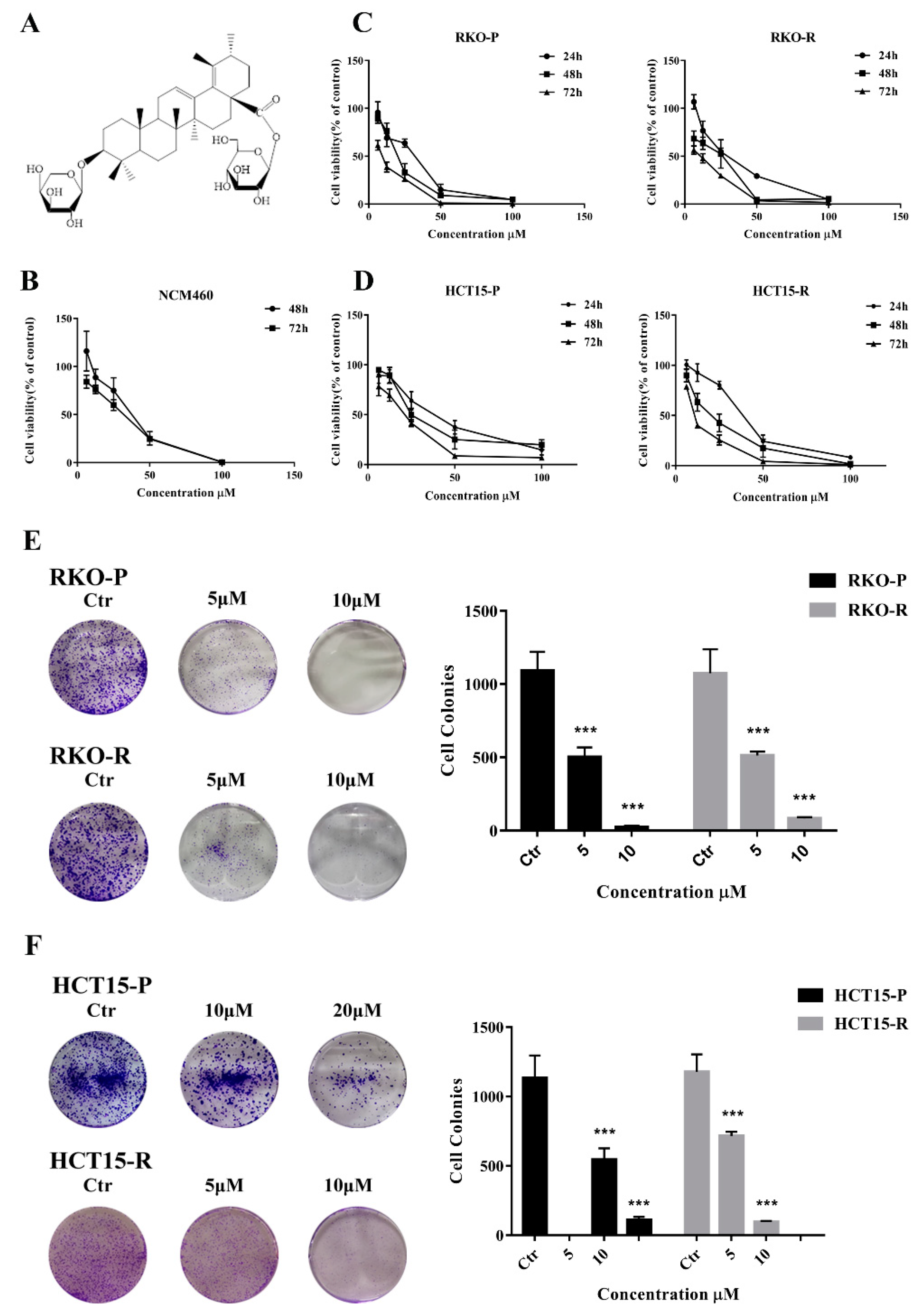

| Cell | IC50 Value (μM) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| RKO-P | 28.96 ± 0.64 | 18.74 ± 1.92 | 9.15 ± 0.99 |
| RKO-R | 25.12 ± 0.46 | 14.62 ± 1.89 | 6.49 ± 1.23 |
| HCT15-P | 38.96 ± 1.23 | 29.82 ± 2.83 | 17.10 ± 2.29 |
| HCT15-R | 30.26 ± 1.59 | 18.93 ± 1.35 | 10.99 ± 1.42 |
| NCM460 | NA | 34.15 ± 2.65 | 28.18 ± 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Peng, C.; Shen, X.; Yuan, Y.; Zhang, W.; Yang, C.; Yao, M. A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells. Molecules 2021, 26, 3843. https://doi.org/10.3390/molecules26133843
Zhang W, Peng C, Shen X, Yuan Y, Zhang W, Yang C, Yao M. A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells. Molecules. 2021; 26(13):3843. https://doi.org/10.3390/molecules26133843
Chicago/Turabian StyleZhang, Weijia, Chang Peng, Xue Shen, Yuemei Yuan, Wei Zhang, Chunjuan Yang, and Meicun Yao. 2021. "A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells" Molecules 26, no. 13: 3843. https://doi.org/10.3390/molecules26133843
APA StyleZhang, W., Peng, C., Shen, X., Yuan, Y., Zhang, W., Yang, C., & Yao, M. (2021). A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells. Molecules, 26(13), 3843. https://doi.org/10.3390/molecules26133843






