Hydrogen-Bonded Cyclic Dimers at Large Compression: The Case of 1H-pyrrolo[3,2-h]quinoline and 2-(2′-pyridyl)pyrrole
Abstract
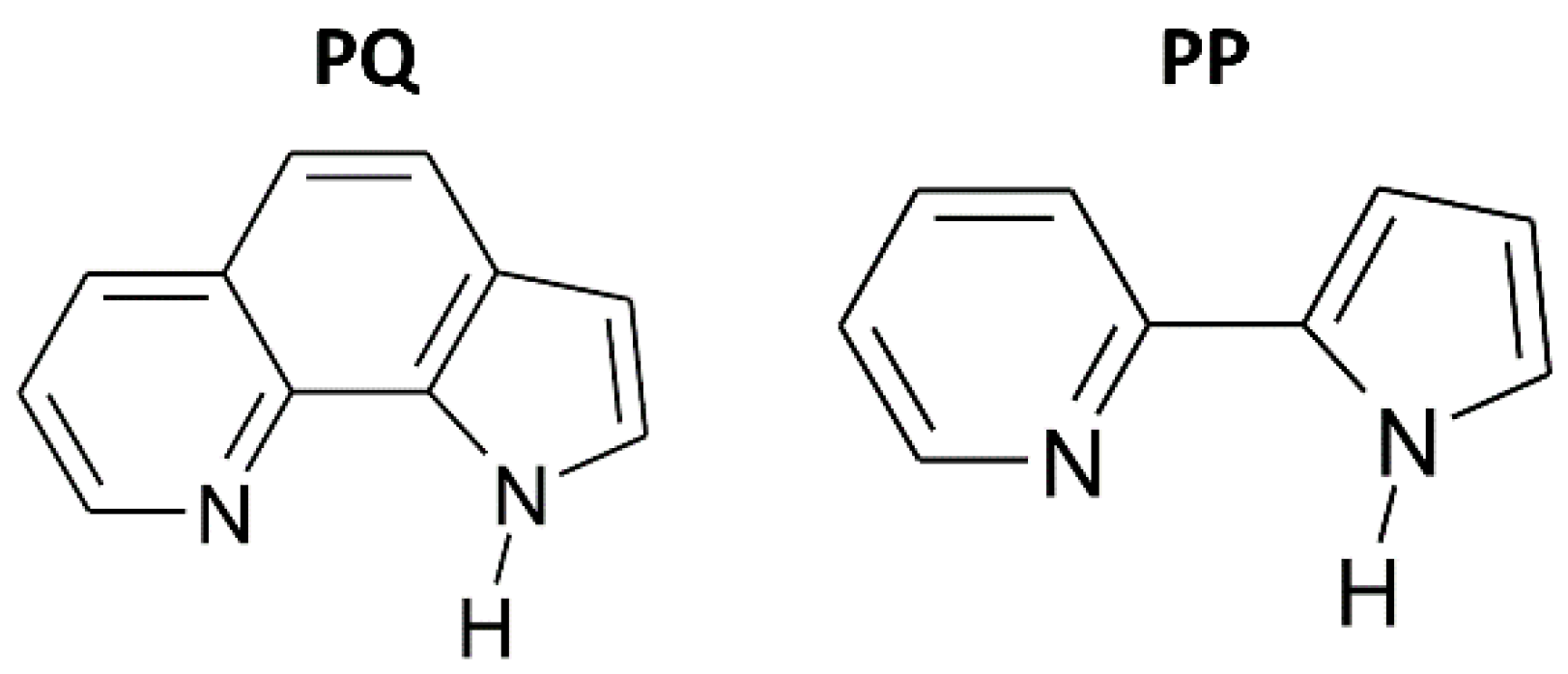
:1. Introduction
2. Results
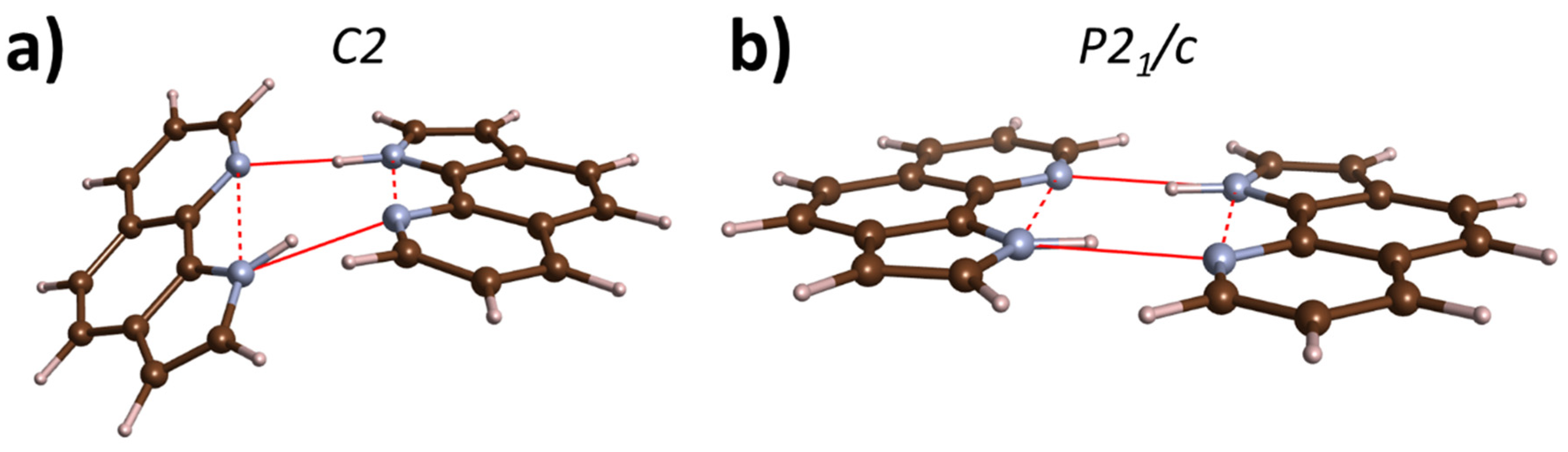
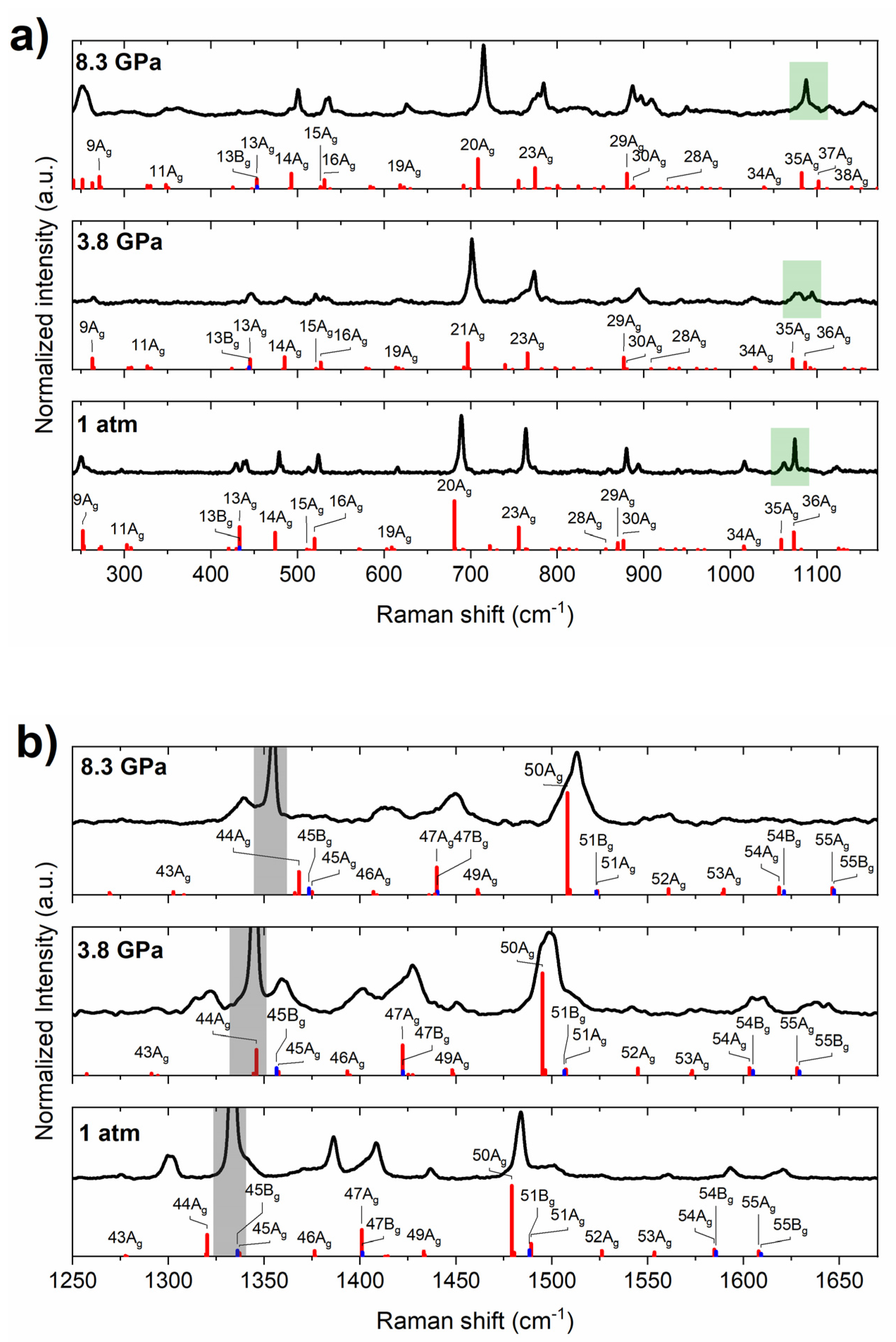
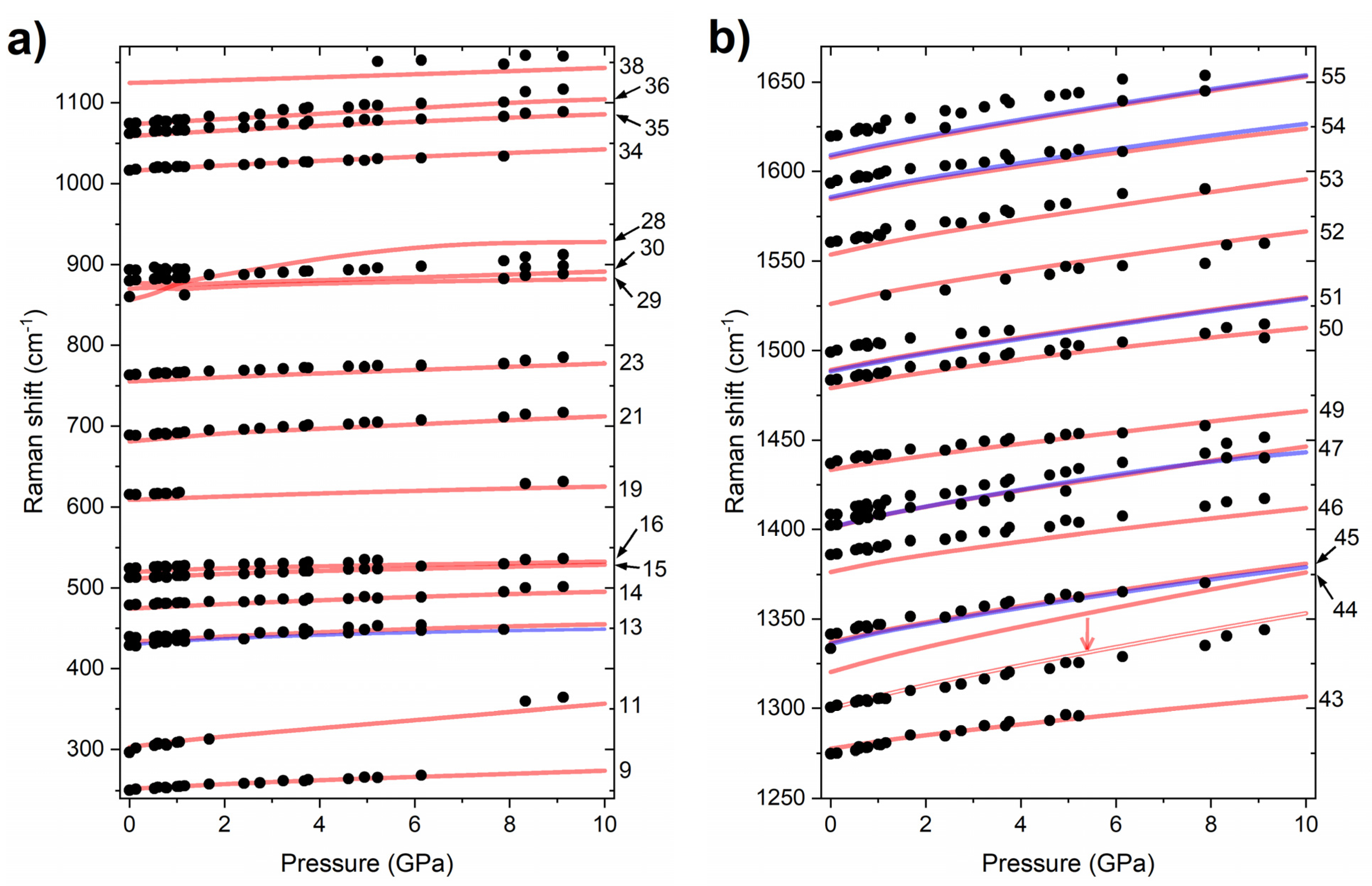
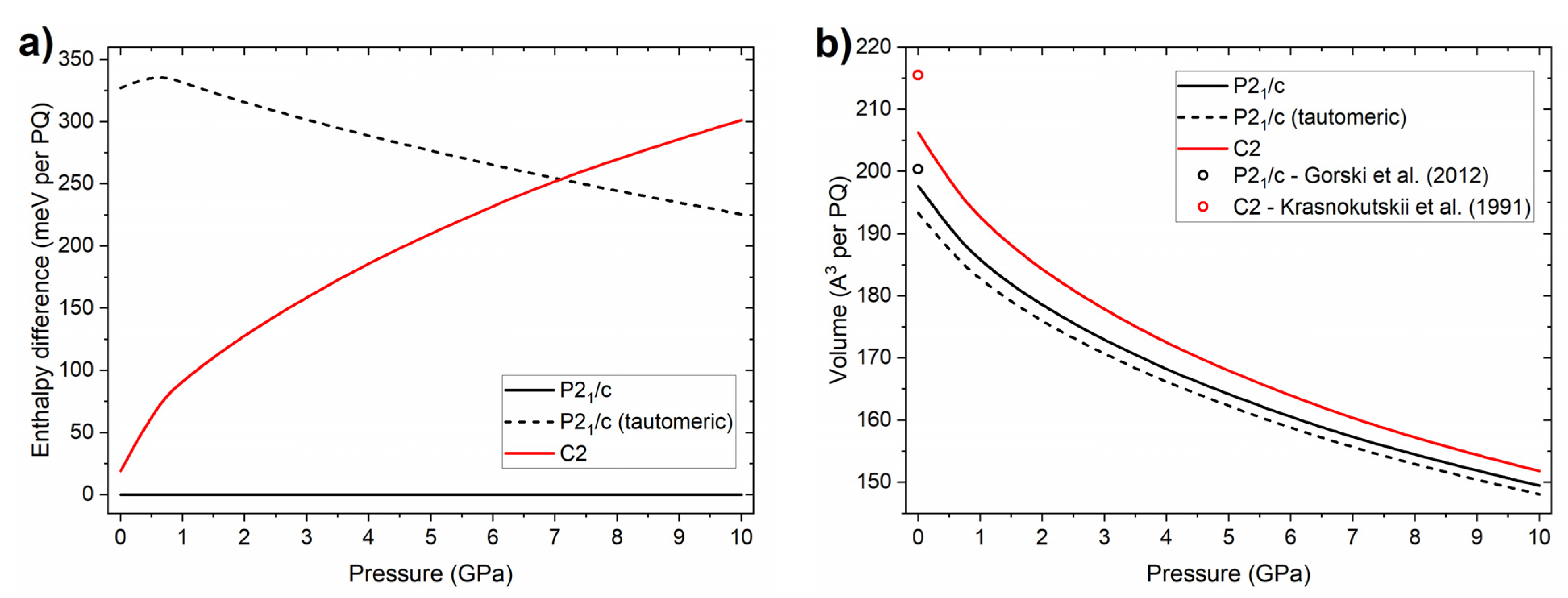
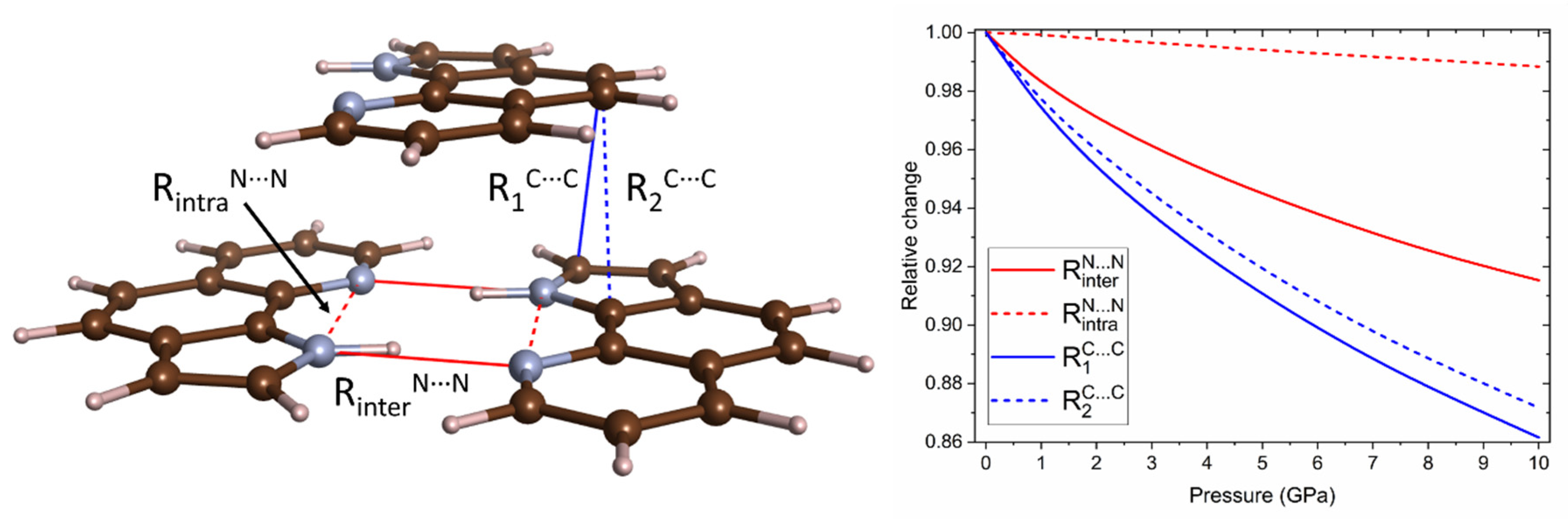
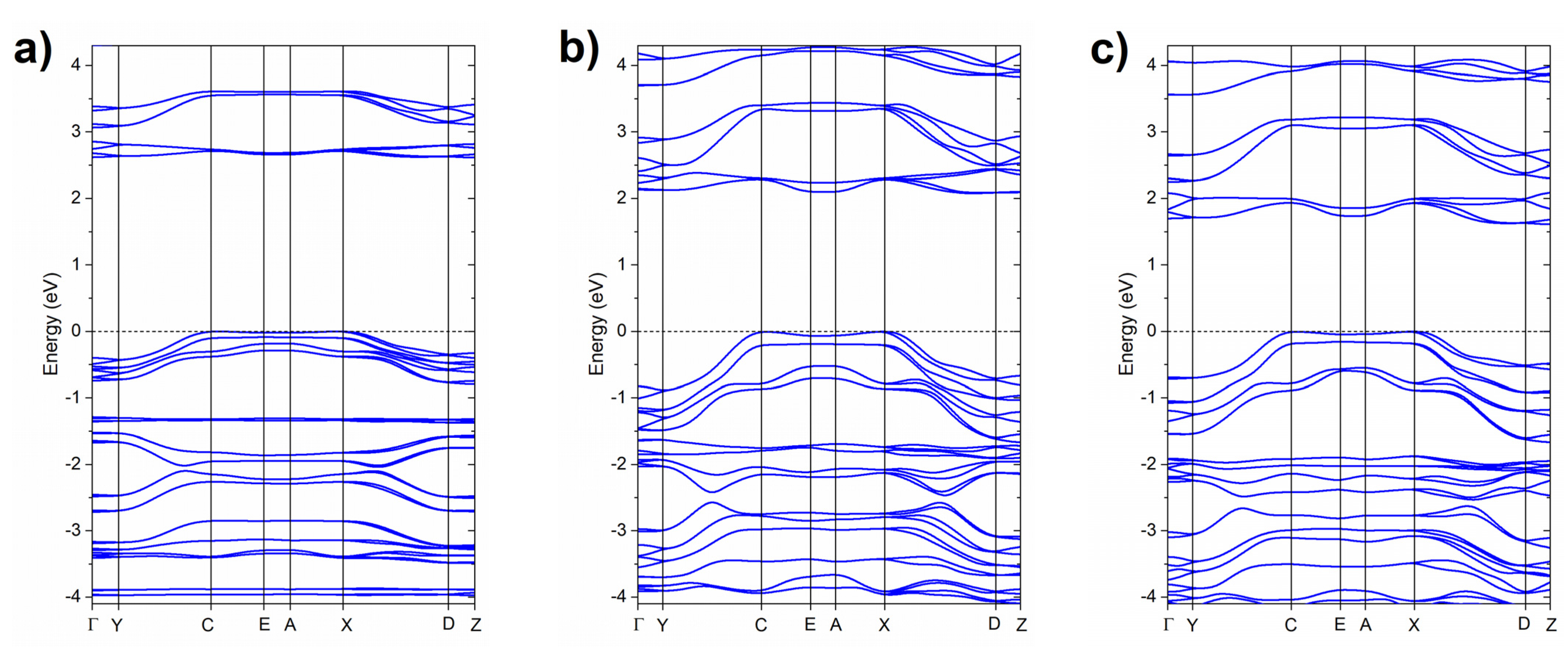
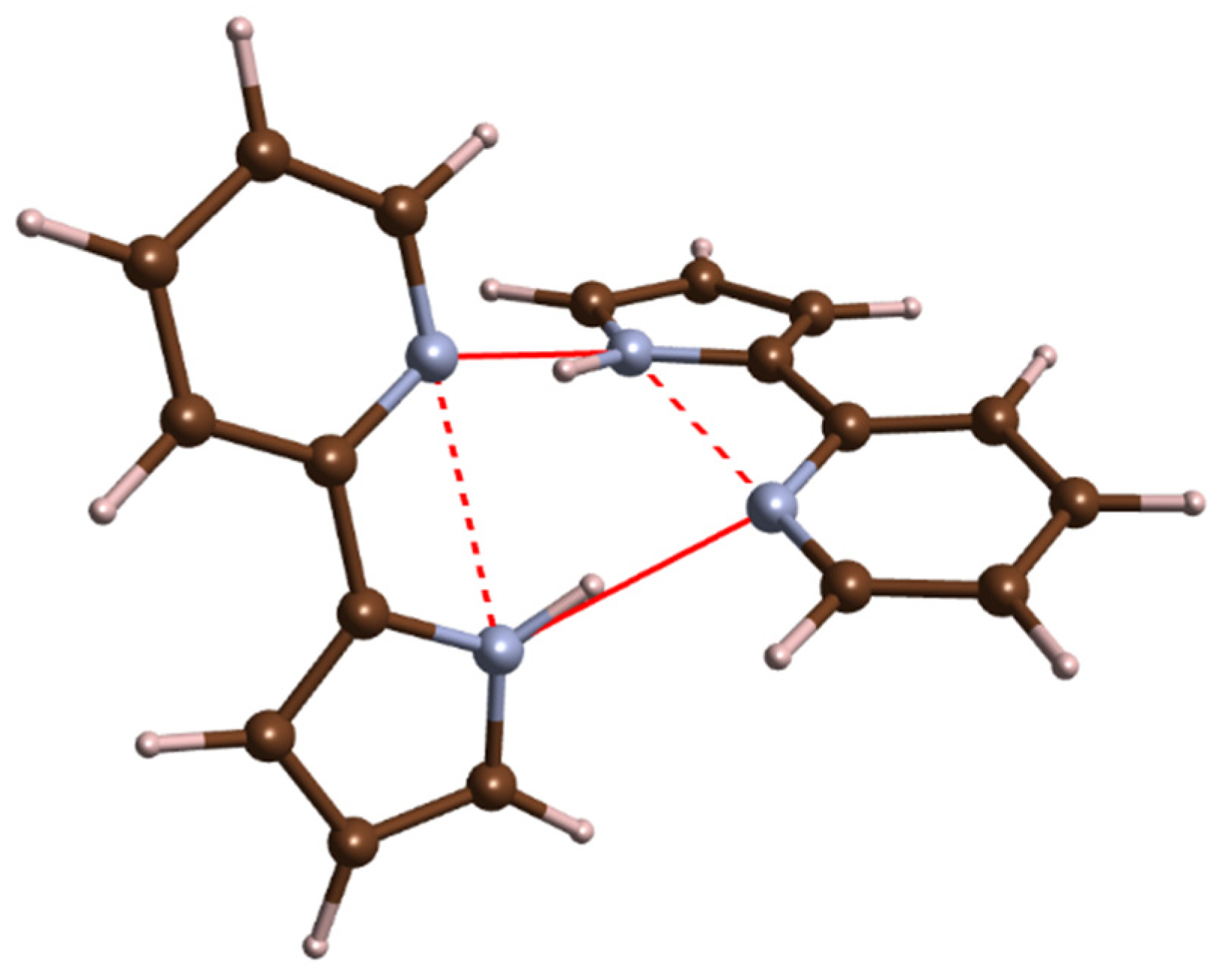
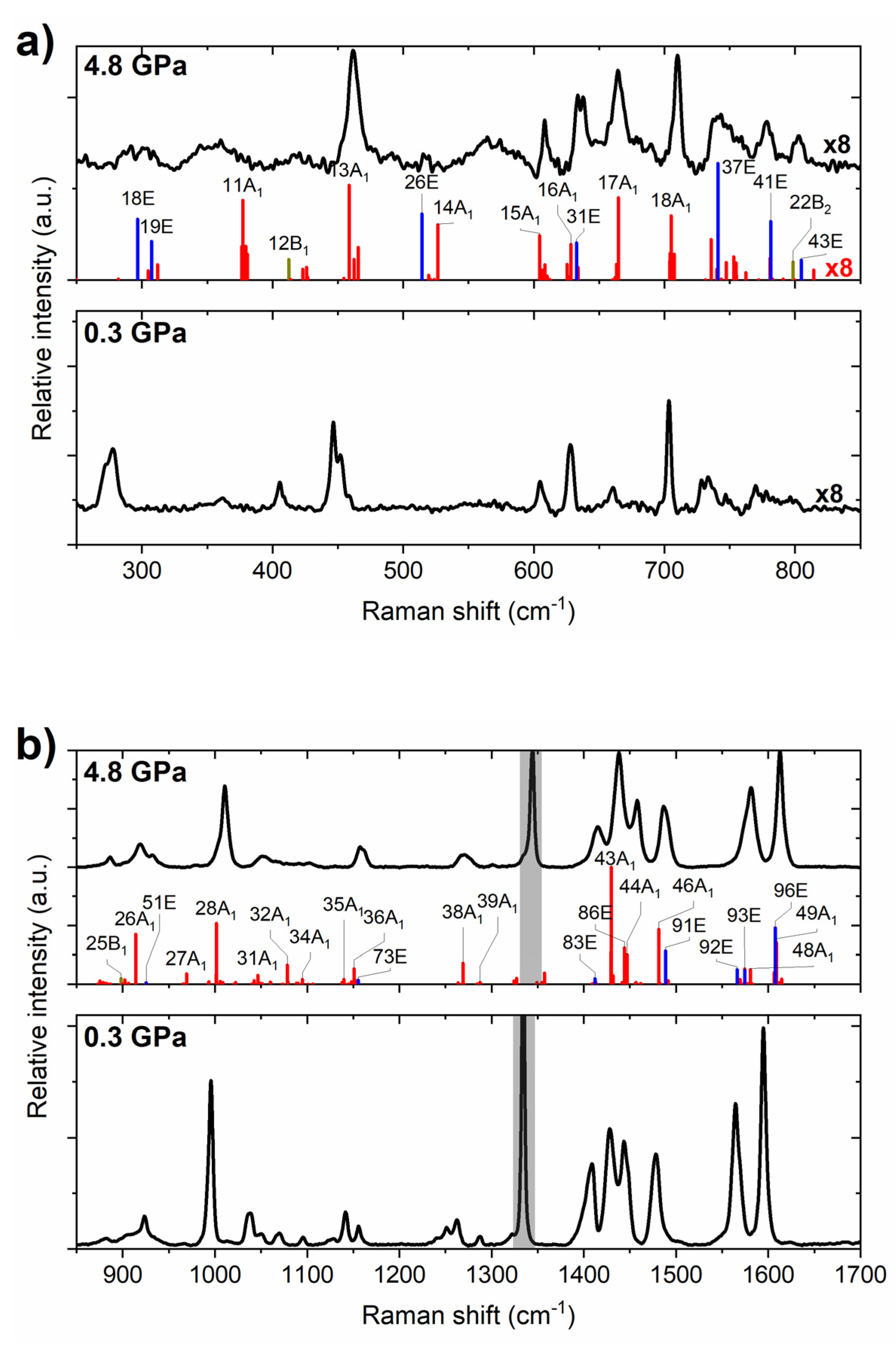
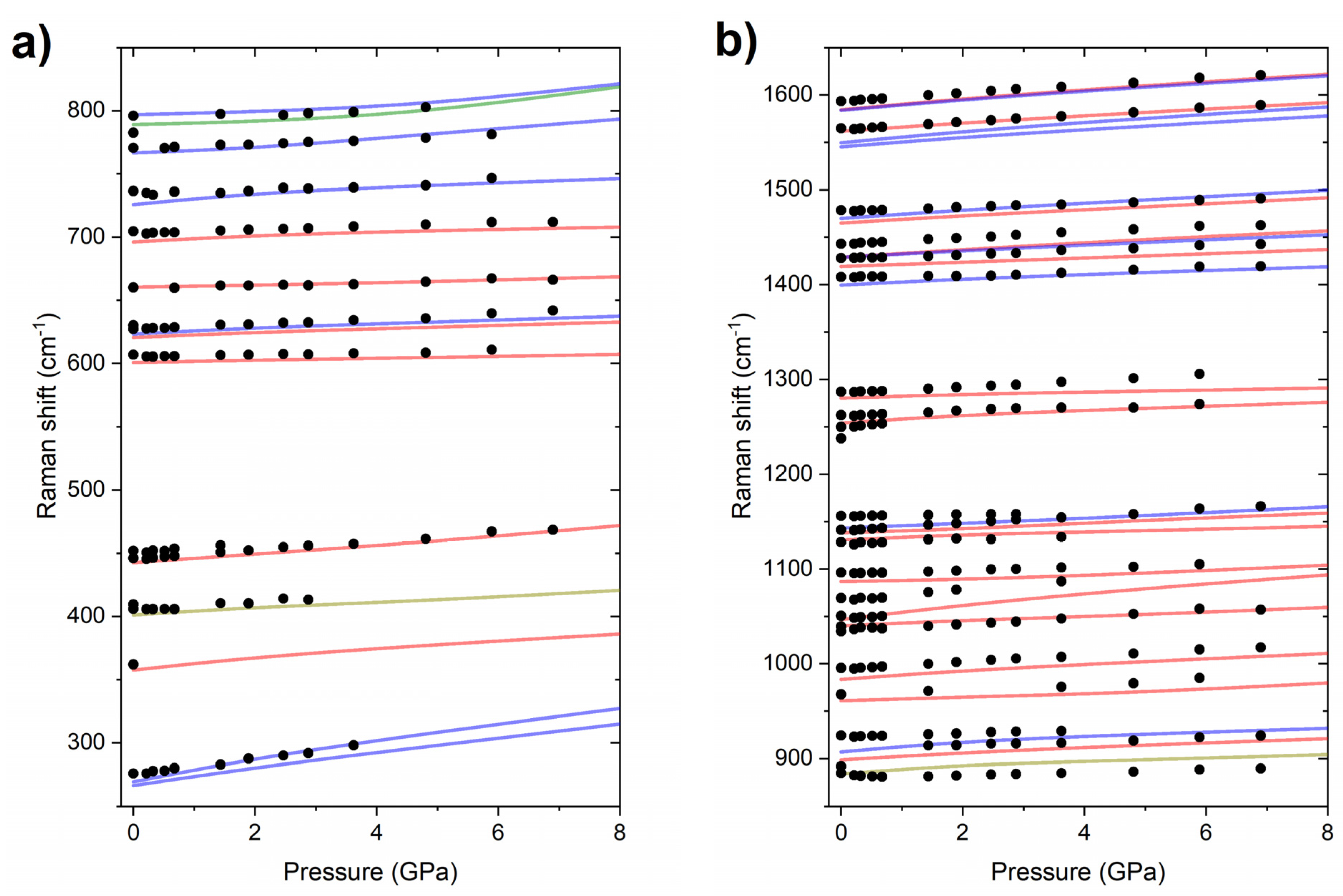
2.1. High-Pressure Behaviour of PQ
2.2. High-Pressure Behaviour of PP
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- May, P.; Batka, D.; Hefter, G.; Konigsberger, E.; Rowland, D. Goodbye to S2– in Aqueous Solution. Chem. Commun. 2018, 1980–1983. [Google Scholar] [CrossRef]
- Mayer, J.M. Proton-Coupled Electron Transfer: A Reaction Chemist’s View. Annu. Rev. Phys. Chem. 2004, 55, 363–390. [Google Scholar] [CrossRef]
- Garczarek, F.; Gerwert, K. Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 2006, 439, 109–112. [Google Scholar] [CrossRef]
- Joshi, H.C.; Antonov, L. Excited-state intramolecular proton transfer: A short introductory review. Molecules 2021, 26, 1475. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Tang, K.-C.; Chou, P.-T. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [CrossRef]
- Waluk, J. Spectroscopy and Tautomerization Studies of Porphycenes. Chem. Rev. 2017, 117, 2447–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Han, K. Unraveling the Detailed Mechanism of Excited-State Proton Transfer. Acc. Chem. Res. 2018, 51, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.; Coutinho Neto, M.; Bartoloni, F.; Homem-de-Mello, P. Density Functional Theory Applied to Excited State Intramolecular Proton Transfer in Imidazole-, Oxazole-, and Thiazole-Based Systems. Molecules 2018, 23, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.E.; Park, S.Y. Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process. Adv. Mater. 2011, 23, 3615–3642. [Google Scholar] [CrossRef]
- Hansen, P.E. A Spectroscopic Overview of Intramolecular Hydrogen Bonds of NH…O,S,N Type. Molecules 2021, 26, 2409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; El-Bayoumi, M.A.; Kasha, M. Excited-State Two-Proton Tautomerism in Hydrogen-Bonded N-Heterocyclic Base Pairs. Proc. Natl. Acad. Sci. USA 1969, 63, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Kyrychenko, A.; Herbich, J.; Izydorzak, M.; Wu, F.; Thummel, R.P.; Waluk, J. Role of Ground State Structure in Photoinduced Tautomerization in Bifunctional Proton Donor−Acceptor Molecules: 1H-Pyrrolo[3,2-h]quinoline and Related Compounds. J. Am. Chem. Soc. 1999, 121, 11179–11188. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Herbich, J.; Wu, F.; Thummel, R.P.; Waluk, J. Solvent-Induced s yn—A nti Rotamerization of 2-(2′-Pyridyl)indole and the Structure of its Alcohol Complexes. J. Am. Chem. Soc. 2000, 122, 2818–2827. [Google Scholar] [CrossRef]
- Yu, W.-S.; Cheng, C.-C.; Cheng, Y.-M.; Wu, P.-C.; Song, Y.-H.; Chi, Y.; Chou, P.-T. Excited-State Intramolecular Proton Transfer in Five-Membered Hydrogen-Bonding Systems: 2-Pyridyl Pyrazoles. J. Am. Chem. Soc. 2003, 125, 10800–10801. [Google Scholar] [CrossRef] [PubMed]
- Waluk, J. Hydrogen-Bonding-Induced Phenomena in Bifunctional Heteroazaaromatics. Acc. Chem. Res. 2003, 36, 832–838. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Waluk, J. Excited-State Proton Transfer through Water Bridges and Structure of Hydrogen-Bonded Complexes in 1H-Pyrrolo[3,2-h]quinoline: Adiabatic Time-Dependent Density Functional Theory Study. J. Phys. Chem. A 2006, 110, 11958–11967. [Google Scholar] [CrossRef] [PubMed]
- Kijak, M.; Nosenko, Y.; Singh, A.; Thummel, R.P.; Waluk, J. Mode-Selective Excited-State Proton Transfer in 2-(2′-Pyridyl)pyrrole Isolated in a Supersonic Jet. J. Am. Chem. Soc. 2007, 129, 2738–2739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hsu, Y.-H.; Chen, Y.-A.; Chen, C.-L.; Lin, T.-C.; Shen, J.-Y.; Chou, P.-T. New six- and seven-membered ring pyrrole–pyridine hydrogen bond systems undergoing excited-state intramolecular proton transfer. Chem. Commun. 2014, 50, 15026–15029. [Google Scholar] [CrossRef]
- Olejniczak, A.; Ostrowska, K.; Katrusiak, A. H-Bond Breaking in High-Pressure Urea. J. Phys. Chem. C 2009, 113, 15761–15767. [Google Scholar] [CrossRef]
- Yan, T.; Li, S.; Wang, K.; Tan, X.; Jiang, Z.; Yang, K.; Liu, B.; Zou, G.; Zou, B. Pressure-Induced Phase Transition in N–H···O Hydrogen-Bonded Molecular Crystal Oxamide. J. Phys. Chem. B 2012, 116, 9796–9802. [Google Scholar] [CrossRef]
- Podsiadło, M.; Olejniczak, A.; Katrusiak, A. Halogen∙∙∙halogen contra C–H∙∙∙halogen interactions. CrystEngComm 2014, 16, 8279–8285. [Google Scholar] [CrossRef] [Green Version]
- Tomkowiak, H.; Katrusiak, A. Compression of Hydrogen-Bonded Layers in Imidazolidine-2-thione. Cryst. Growth Des. 2019, 19, 285–290. [Google Scholar] [CrossRef]
- Katrusiak, A. Lab in a DAC—High-pressure crystal chemistry in a diamond-anvil ceacll. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 918–926. [Google Scholar] [CrossRef] [Green Version]
- Boldyreva, E.V. High Pressure Crystallography: Elucidating the Role of Intermolecular Interactions in Crystals of Organic and Coordination Compounds. In Understanding Intermolecular Interactions in the Solid State: Approaches and Techniques; Chopra, D., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 32–97. [Google Scholar]
- Kurzydłowski, D.; Chumak, T.; Rogoża, J. Phase Stability of Chloroform and Dichloromethane at High Pressure. Crystals 2020, 10, 920. [Google Scholar] [CrossRef]
- Olejniczak, A.; Katrusiak, A.; Podsiadło, M.; Katrusiak, A. Crystal design by CH...N and N...N interactions: High-pressure structures of high-nitrogen-content azido-triazolopyridazines compounds. Acta Crystallogr. B. Struct. Sci. Cryst. Eng. Mater. 2020, 76, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, H.G. Pressure tuning spectroscopy. Acc. Chem. Res. 1986, 19, 329–334. [Google Scholar] [CrossRef]
- Nosenko, Y.; Kyrychenko, A.; Thummel, R.P.; Waluk, J.; Brutschy, B.; Herbich, J. Fluorescence quenching in cyclic hydrogen-bonded complexes of 1H-pyrrolo[3,2-h]quinoline with methanol: Cluster size effect. Phys. Chem. Chem. Phys. 2007, 9, 3276. [Google Scholar] [CrossRef] [PubMed]
- Kungwan, N.; Daengngern, R.; Piansawan, T.; Hannongbua, S.; Barbatti, M. Theoretical study on excited-state intermolecular proton transfer reactions of 1H-pyrrolo[3,2-h]quinoline with water and methanol. Theor. Chem. Acc. 2013, 132, 1397. [Google Scholar] [CrossRef] [Green Version]
- Klots, T.D.; Chirico, R.D.; Steele, W.V. Complete vapor phase assignment for the fundamental vibrations of furan, pyrrole and thiophene. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 765–795. [Google Scholar] [CrossRef]
- Gorski, A.; Gawinkowski, S.; Luboradzki, R.; Tkacz, M.; Thummel, R.P.; Waluk, J. Polymorphism, Hydrogen Bond Properties, and Vibrational Structure of 1H-Pyrrolo[3,2-h]Quinoline Dimers. J. At. Mol. Opt. Phys. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kijak, M.; Nosenko, Y.; Singh, A.; Thummel, R.P.; Brutschy, B.; Waluk, J. Ground and excited state vibrations of 2-(2′-pyridyl)pyrrole. J. Mol. Struct. 2007, 844–845, 286–299. [Google Scholar] [CrossRef]
- Noland, W.E.; Cole, K.P.; Britton, D. Five (1H-pyrrol-2-yl)pyridines. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, 59, o263–o267. [Google Scholar] [CrossRef]
- Krasnokutskii, S.N.; Kurbovskaya, L.N.; Shibanova, T.A.; Shabunova, V.P. Structure of 1H-pyrrolo[3,2-h]quinoline. J. Struct. Chem. 1991, 32, 106–110. [Google Scholar] [CrossRef]
- Gorski, A.; Gawinkowski, S.; Herbich, J.; Krauss, O.; Brutschy, B.; Thummel, R.P.; Waluk, J. 1H-Pyrrolo[3,2-h]quinoline: A Benchmark Molecule for Reliable Calculations of Vibrational Frequencies, IR Intensities, and Raman Activities. J. Phys. Chem. A 2012, 116, 11973–11986. [Google Scholar] [CrossRef]
- Antonov, L. Tautomerism in azo and azomethyne dyes: When and if theory meets experiment. Molecules 2019, 24, 2252. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłoński, M. A Critical Overview of Current Theoretical Methods of Estimating the Energy of Intramolecular Interactions. Molecules 2020, 25, 5512. [Google Scholar] [CrossRef] [PubMed]
- Fabbiani, F.P.A.; Allan, D.R.; Parsons, S.; Pulham, C.R. Exploration of the high-pressure behaviour of polycyclic aromatic hydrocarbons: Naphthalene, phenanthrene and pyrene. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 826–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Li, S.; Wang, K.; Li, W.; Liu, J.; Liu, B.; Zou, G.; Zou, B. Compression studies of face-to-face π-stacking interaction in sodium squarate salts: Na2C4O4 and Na2C4O4•3H2 O. J. Chem. Phys. 2012, 137, 184905. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Duan, D.; Huang, X.; Jin, X.; Yang, X.; Li, S.; Jiang, S.; Huang, Y.; Li, F.; Cui, Q.; et al. Pressure-induced diversity of π-stacking motifs and amorphous polymerization in pyrrole. J. Phys. Chem. C 2014, 118, 12420–12427. [Google Scholar] [CrossRef]
- Kijak, M.; Zielińska, A.; Chamchoumis, C.; Herbich, J.; Thummel, R.P.; Waluk, J. Conformational equilibria and photoinduced tautomerization in 2-(2′-pyridyl)pyrrole. Chem. Phys. Lett. 2004, 400, 279–285. [Google Scholar] [CrossRef]
- Rode, M.F.; Sobolewski, A.L. Photophysics of inter- and intra-molecularly hydrogen-bonded systems: Computational studies on the pyrrole–pyridine complex and 2(2′-pyridyl)pyrrole. Chem. Phys. 2008, 347, 413–421. [Google Scholar] [CrossRef]
- Siu, J.; Baxendale, I.R.; Ley, S.V. Microwave assisted Leimgruber–Batcho reaction for the preparation of indoles, azaindoles and pyrroylquinolines. Org. Biomol. Chem. 2004, 2, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Withall, D.M.; Haynes, S.W.; Challis, G.L. Stereochemistry and Mechanism of Undecylprodigiosin Oxidative Carbocyclization to Streptorubin B by the Rieske Oxygenase RedG. J. Am. Chem. Soc. 2015, 137, 7889–7897. [Google Scholar] [CrossRef] [PubMed]
- Katrusiak, A. High-pressure crystallography. Acta Crystallogr. Sect. A Found. Crystallogr. 2007, 64, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Wang, Y.; Dewaele, A.; Wu, C.; Fratanduono, D.E.; Eggert, J.; Klotz, S.; Dziubek, K.F.; Loubeyre, P.; Fat’yanov, O.V.; et al. Toward an international practical pressure scale: A proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Press. Res. 2020, 40, 299–314. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233. [Google Scholar] [CrossRef] [Green Version]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
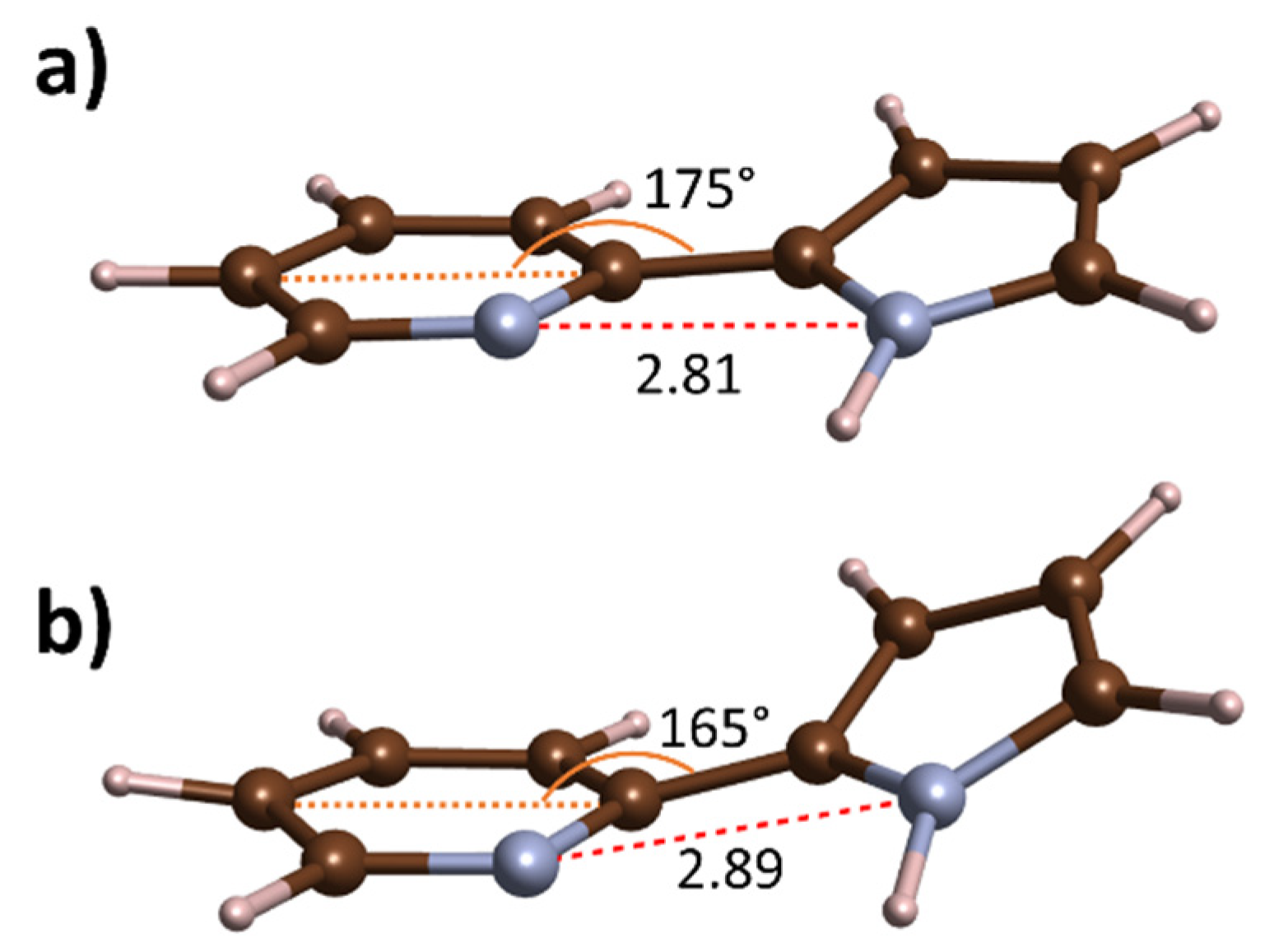
| Contact | P21/c | P21/c (Tautomeric) | |
|---|---|---|---|
| 1 atm | 10 GPa | 10 GPa | |
| 2.96 | 2.71 | 2.61 | |
| 3.02 | 2.98 | 3.01 | |
| 3.35 | 2.89 | 2.89 | |
| 3.30 | 2.88 | 2.88 | |
| 1.04 | 1.05 | 1.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzydłowski, D.; Chumak, T.; Rogoża, J.; Listkowski, A. Hydrogen-Bonded Cyclic Dimers at Large Compression: The Case of 1H-pyrrolo[3,2-h]quinoline and 2-(2′-pyridyl)pyrrole. Molecules 2021, 26, 3802. https://doi.org/10.3390/molecules26133802
Kurzydłowski D, Chumak T, Rogoża J, Listkowski A. Hydrogen-Bonded Cyclic Dimers at Large Compression: The Case of 1H-pyrrolo[3,2-h]quinoline and 2-(2′-pyridyl)pyrrole. Molecules. 2021; 26(13):3802. https://doi.org/10.3390/molecules26133802
Chicago/Turabian StyleKurzydłowski, Dominik, Taisiia Chumak, Jakub Rogoża, and Arkadiusz Listkowski. 2021. "Hydrogen-Bonded Cyclic Dimers at Large Compression: The Case of 1H-pyrrolo[3,2-h]quinoline and 2-(2′-pyridyl)pyrrole" Molecules 26, no. 13: 3802. https://doi.org/10.3390/molecules26133802
APA StyleKurzydłowski, D., Chumak, T., Rogoża, J., & Listkowski, A. (2021). Hydrogen-Bonded Cyclic Dimers at Large Compression: The Case of 1H-pyrrolo[3,2-h]quinoline and 2-(2′-pyridyl)pyrrole. Molecules, 26(13), 3802. https://doi.org/10.3390/molecules26133802






