Inhibitory Activities of Dimeric Ellagitannins Isolated from Cornus alba on Benign Prostatic Hypertrophy
Abstract
1. Introduction
2. Result
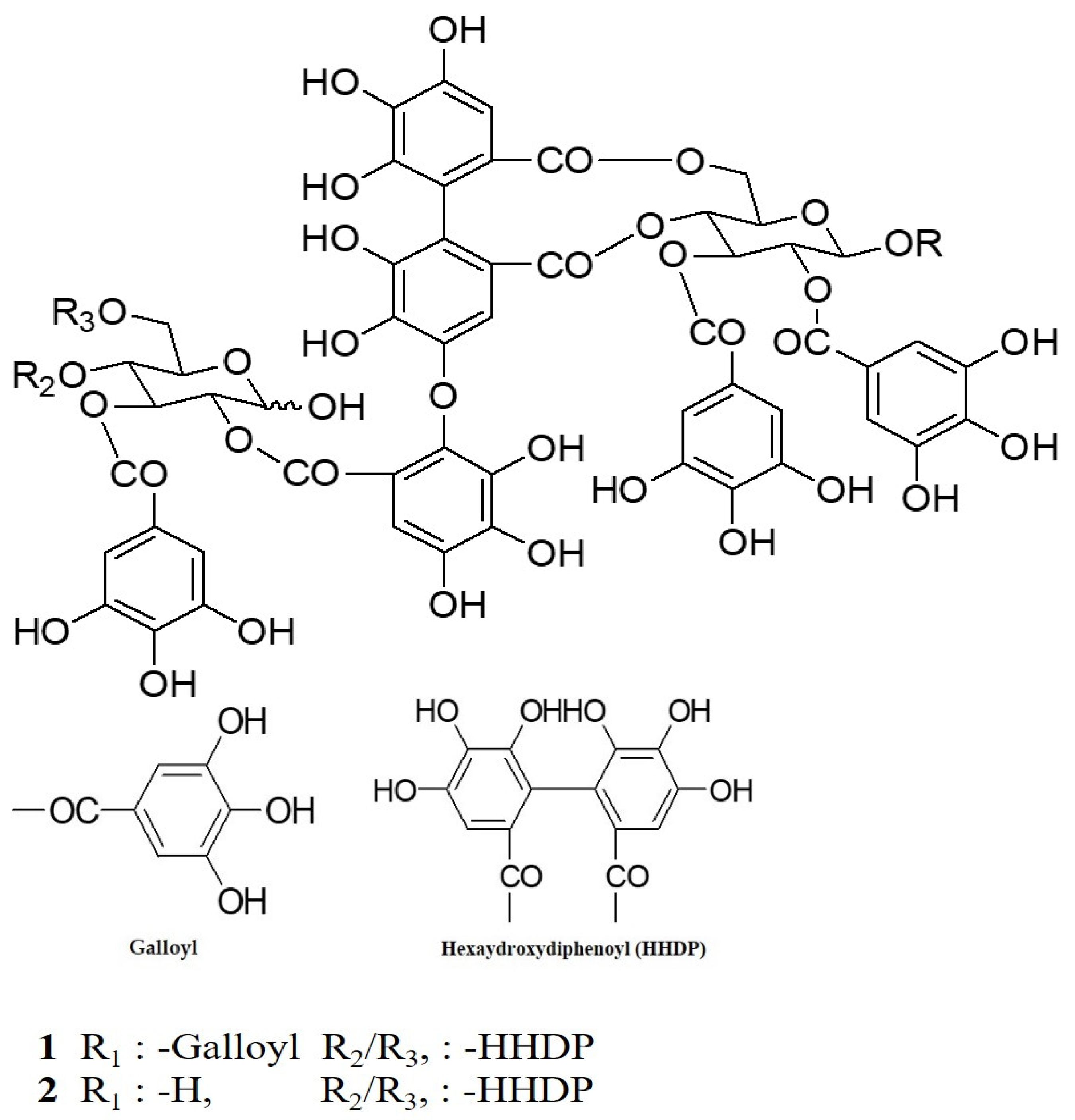
2.1. Phytochemicals from Cornus alba
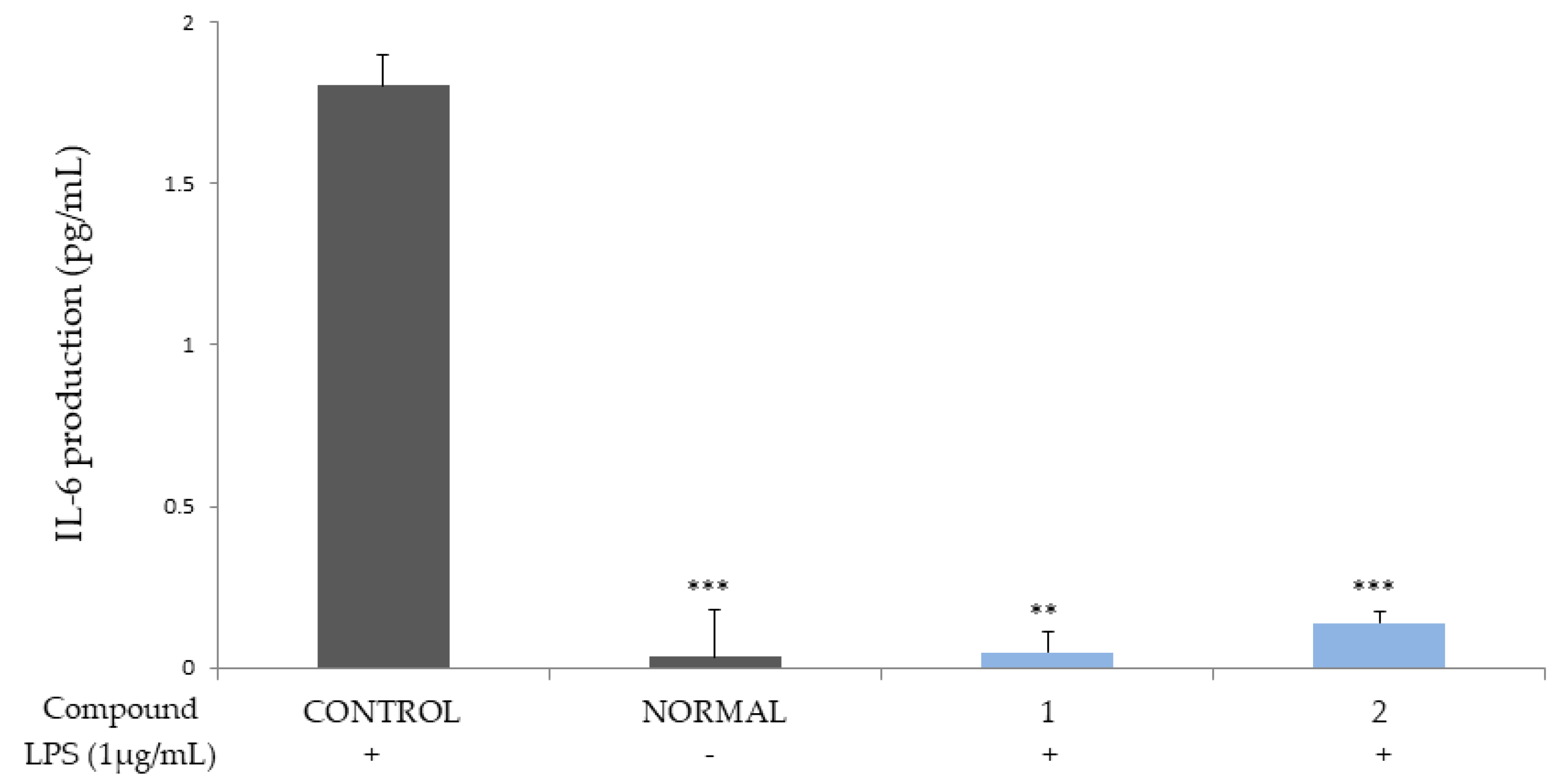
2.2. Inhibition of Cytokines Production
2.3. Anti-Proliferative Effect on BPH Cell Line
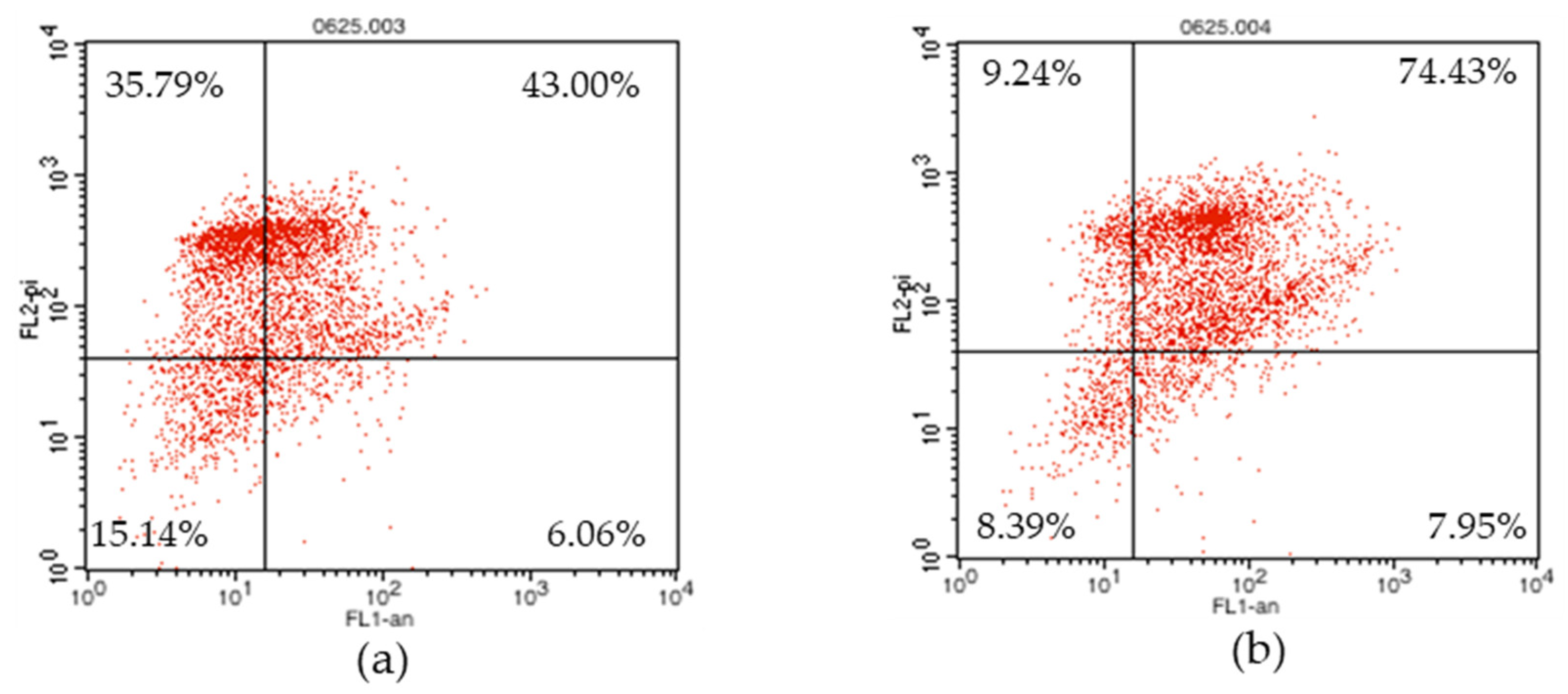
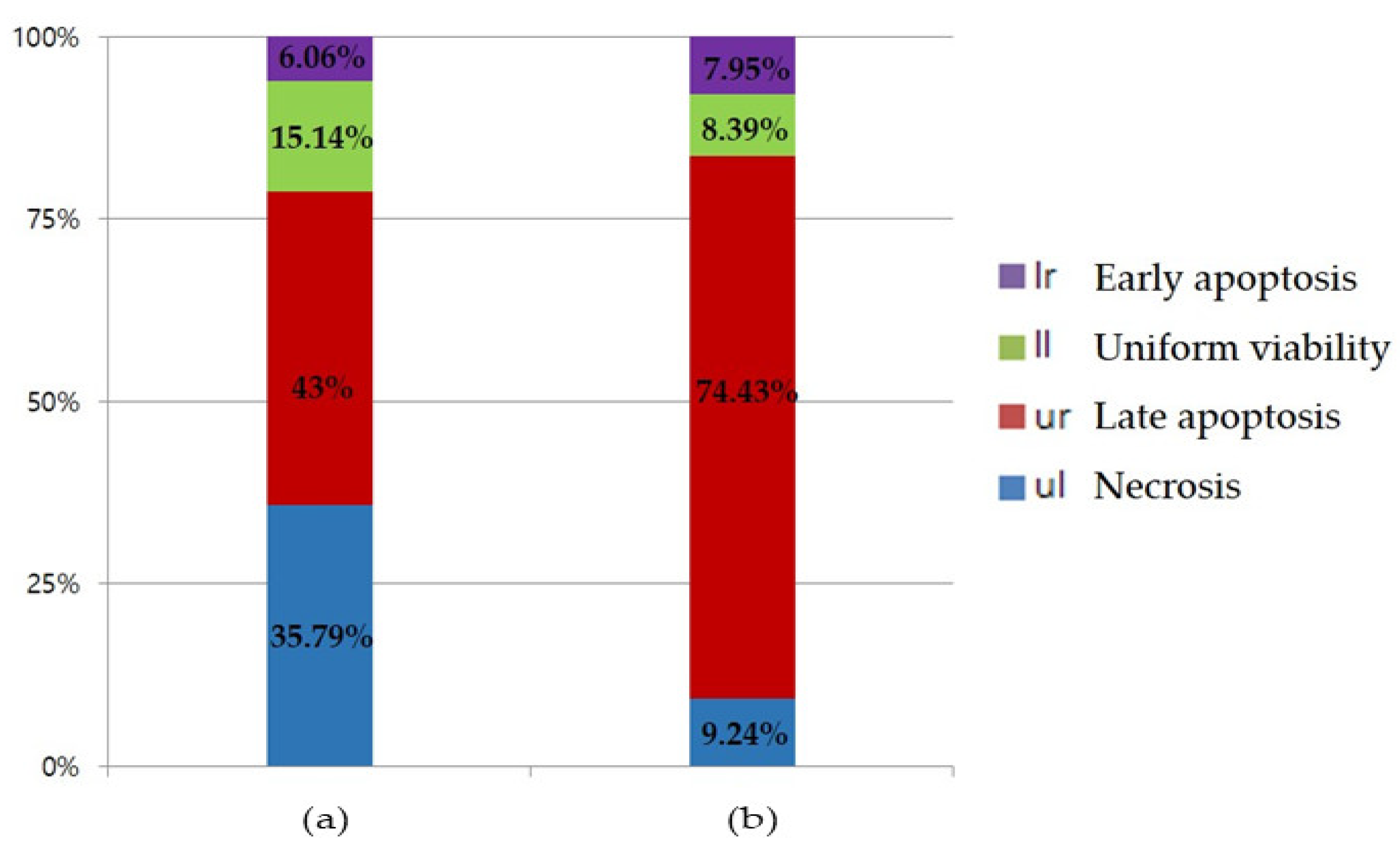
2.4. Flow Cytometry Analysis of Apoptosis
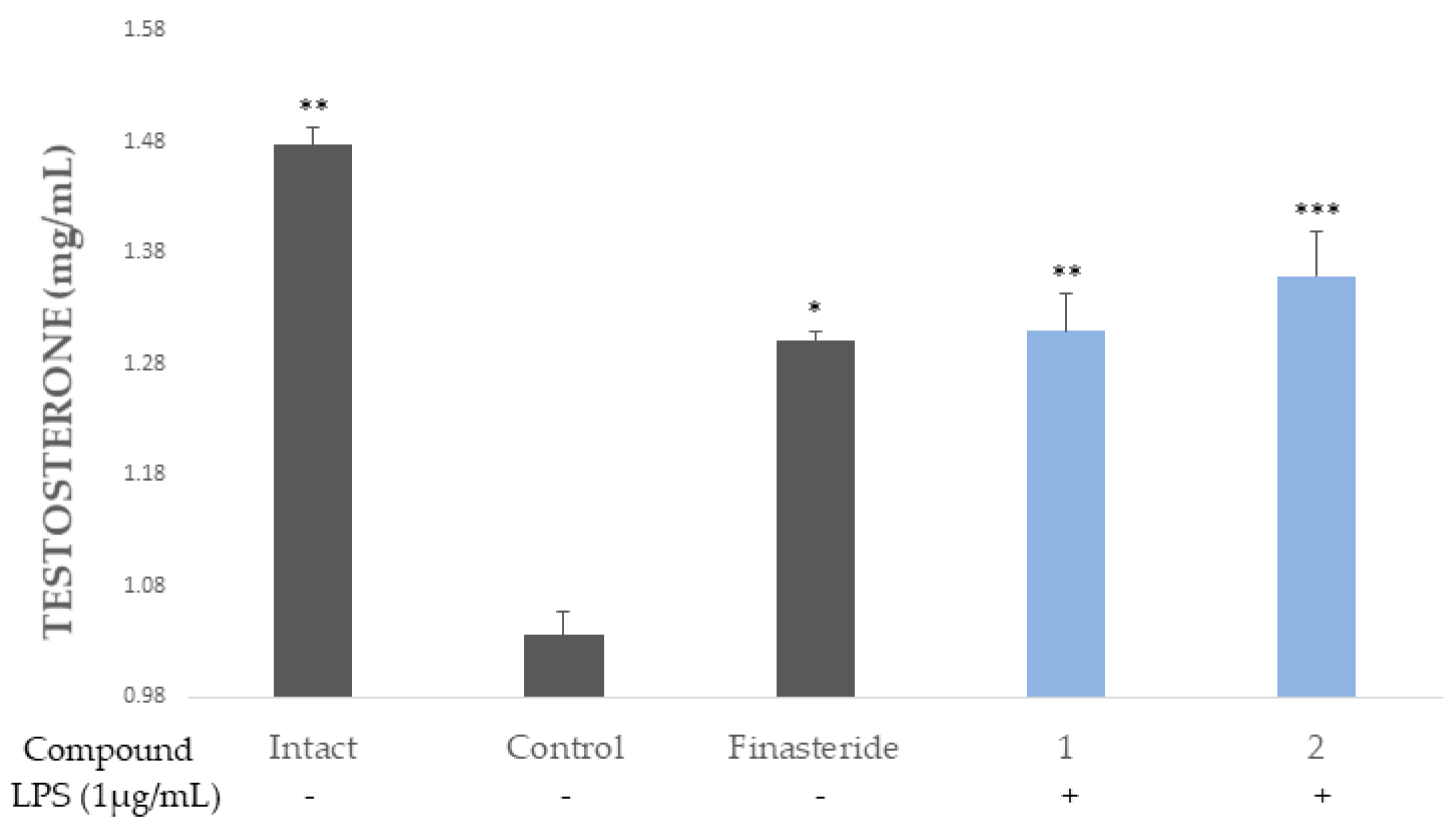
2.5. 5α-Reductase Inhibition Activity
3. Discussion
4. Materials and Methods
4.1. Compounds
4.1.1. Camptothin B (1)
4.1.2. Cornusiin A (2)
4.2. Cell Culture
4.3. Measurement of Cytokines Production
4.4. Apoptosis Activity
4.5. Measurement of Inhibitory 5α-Reductase Activity
4.6. Macrophage Differentiation and Stimulation
4.7. Preparation of Liver Microsomes
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hong, S.J. Benign prostatic hyperplasia: Multiple factors for prostate tissue change with aging. Korean J. Urol. 2005, 46, 547–554. [Google Scholar]
- Lee, E.H.; Chun, K.H.; Lee, Y.H. Benign Prostatic Hyperplasia in Community-Dwelling Elderly in Korea. J. Korean Acad. Nurs. 2005, 35, 1508–1513. [Google Scholar] [CrossRef]
- Salinas-Sánchez, A.S.; Hernández-Millán, I.; Lorenzo-Romero, J.G.; Segura-Martín, M.; Fernández-Olano, C.; Virseda-Rodriguez, J.A. Quality of life of patients on the waiting list for benign prostatic hyperplasia surgery. Qual. Life Res. 2001, 10, 543–553. [Google Scholar] [CrossRef]
- Kim, E.H.; Larson, J.A.; Andriole, G.L. Management of Benign Prostatic Hyperplasia. Annu. Rev. Med. 2016, 67, 137–151. [Google Scholar] [CrossRef]
- Guess, H.; Arrighi, H.; Metter, E.; Fozard, J. Cumulative Prevalence of Prostatism Matches the Autopsy Prevalence of Benign Prostatic Hyperplasia. Prostate 1990, 17, 241–246. [Google Scholar] [CrossRef]
- Marcelli, M.; Cunningham, G.R. Hormonal Signaling in Prostatic Hyperplasia and Neoplasia. J. Clin. Endocrinol. Metab. 1999, 84, 3463–3468. [Google Scholar] [CrossRef]
- Thompson, T.C.; Yang, G. Regulation of Apoptosis in Prostatic Disease. Prostate 2000, 45, 25–28. [Google Scholar] [CrossRef]
- Kapoor, A. Benign prostatic hyperplasia (BPH) management in the primary care setting. Can. J. Urol. 2012, 19, 10–17. [Google Scholar]
- Chaim, M.; Wendy, V.H.; Hadas, D.F. Association Between Tamsulosin and Serious Opthalmic Adverse Events in Older Men Folowing Cataract Surgery. JAMA 2009, 301, 1991–1996. [Google Scholar] [CrossRef]
- Kevin, T.M. A Review of Combination Therapy in Patients with Benign Prostatic Hyperplasis. Clin. Ther. 2007, 29, 387–398. [Google Scholar] [CrossRef]
- Popovic, B.M.; Stajner, D.; Slavko, K.; Sandra, B. Antioxidant capacity of cornelian cherry (Cornus mas L.)—Comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012, 134, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Polat, R.; Cakilcioglu, U.; Satil, F. Traditional uses of medicinal plants in Solhan (Bingöl-Turkey). J. Ethnopharmacol. 2013, 148, 951–963. [Google Scholar] [CrossRef]
- Seeram, N.P.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, Quantification, and Bioactivities of Anthocyanins in Cornus Species. J. Agric. Food Chem. 2002, 50, 2519–2523. [Google Scholar] [CrossRef]
- Vareed, S.K.; Reddy, M.K.; Schutzki, R.E.; Nair, M.G. Anthocyanins in Cornus alternifolia, Cornus controversa, Cornus kousa and Cornus florida fruits with health benefits. Life Sci. 2006, 78, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Yin, J.; Yoon, K.H.; Hwang, Y.J.; Lee, M.W. Antiproliferative effects of new Dimeric Ellagitannin from Cornus alba in prostate cancer cells including apoptosis-related S-phase arrest. Molecules 2016, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H. Anti-Proliferative Effects of Hydrolysable Tannins from Cornus alba; Chung-Ang University: Seoul, Korea; Korea Education and Research Information Service (KERIS): Daegu, Korea, 2013. [Google Scholar]
- Giri, D.; Ozen, M.; Ittmann, M. Interleukin-6 is an Autocrine Growth Factor in Human Prostate Cancer. Am. J. Pathol. 2001, 159, 2159–2165. [Google Scholar] [CrossRef]
- Hobisch, A.; Eder, I.E.; Putz, T.; Horninger, W.; Bartsch, G.; Klocker, H.; Culig, Z. Interleukin-6 Regulates Prostate-Specific Protein Expression in Prostate Carcinoma Cells by Activation of the Androgen Receptor. Cancer Res. 1998, 58, 4640–4645. [Google Scholar] [PubMed]
- Nadler, R.B.; Koch, A.E.; Calhoun, E.A.; Campbell, P.L.; Pruden, D.L.; Bennett, C.L.; Yarnold, P.R.; Schaeffer, A.J. IL-1β and TNF-α in Prostatic Secretions are Indicators in the Evaluation of Men with Chronic Prostatitis. J. Urol 2000, 164, 214–218. [Google Scholar] [CrossRef]
- De Nunzio, C.; Kramer, G.; Marberger, M.; Montironi, R.; Nelson, W.; Schröder, F.; Sciarra, A.; Tubaro, A. The Controversial Relationship between Benign Prostatic Hyperplasia and Prostate Cancer: The Role of Inflammation. Eur. Urol 2011, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.K.; Sohn, D.H.; Ryu, J. A Curcuminoid and Sesquiterpenes as Inhibitors of Macrophage TNF- α Release from Curcuma Zedoaria. Planta Med. 2001, 67, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, J.J.; Cohen, D.L.B.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar]
- Andree, H.A.; Reutelingsperger, C.P.; Hauptmann, R.; Hemker, H.C.; Hermens, W.T.; Willems, G.M. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J. Biol. Chem. 1990, 265, 4923–4928. [Google Scholar] [CrossRef]
- Kim, E.H.; Brockman, J.A.; Andriole, G.L. The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian J. Urol. 2018, 5, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, F.G.C.; Olta, A.; Gian, M.B.; Tommaso, C.; Gaetano, L.; Vittorio, M.; Gianpaolo, P.; Francesco, S.R.D.C.; Giorgio, I.R.; Kostantionos, S.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91. [Google Scholar] [CrossRef]
- Casciola-Rosen, L.; Rosen, A.; Petri, M.; Schlissel, M. Surface Blebs on Apoptotic Cells are Sites of Enhanced Procoagulant Activity: Implications for Coagulation Events and Antigenic Spread in Systemic Lupus Erythematosus. Proc. Natl. Acad. Sci. USA 1996, 93, 1624–1629. [Google Scholar] [CrossRef]
- Engeland, M.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. A Novel Assay to Measure Loss of Plasma Membrane Asymmetry during Apoptosis of Adherent Cells in Culture. Cytom. J. Int. Soc. Anal. Cytol. 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A Novel Assay for Apoptosis Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Askew, E.B.; Gampe Jr, R.T.; Stanley, T.B.; Faggart, J.L.; Wilson, E.M. Modulation of Androgen Receptor Activation Function 2 by Testosterone and Dihydrotestosterone. J. Biol. Chem. 2007, 282, 25801–25816. [Google Scholar] [CrossRef] [PubMed]
- Wilbert, D.M.; Griffin, J.E.; Wilson, J.D. Characterization of the Cytosol Androgen Receptor of the Human Prostate. J. Clin. Endocrinol. Metab. 1983, 56, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, W.J.; Griffin, J.E.; Weaver, D.D.; Carlson, B.R.; Wilson, J.D. A mutation that causes lability of the androgen receptor under conditions that normally promote transformation to the DNA-binding state. The J. Clin. Investig. 1984, 73, 1095–1104. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-H.; Park, K.-H.; Yin, J.; Kim, M.-J.; Yoon, S.-E.; Lee, S.-H.; Heo, J.-H.; Chung, H.-J.; Kim, J.-W.; Kim, K.-M.; et al. Inhibitory Activities of Dimeric Ellagitannins Isolated from Cornus alba on Benign Prostatic Hypertrophy. Molecules 2021, 26, 3446. https://doi.org/10.3390/molecules26113446
Park D-H, Park K-H, Yin J, Kim M-J, Yoon S-E, Lee S-H, Heo J-H, Chung H-J, Kim J-W, Kim K-M, et al. Inhibitory Activities of Dimeric Ellagitannins Isolated from Cornus alba on Benign Prostatic Hypertrophy. Molecules. 2021; 26(11):3446. https://doi.org/10.3390/molecules26113446
Chicago/Turabian StylePark, Dong-Hui, Kwan-Hee Park, Jun Yin, Min-Ji Kim, Seong-Eun Yoon, Sun-Ho Lee, Jun-Hyeok Heo, Hyun-Joo Chung, Jin-Wook Kim, Kyung-Mi Kim, and et al. 2021. "Inhibitory Activities of Dimeric Ellagitannins Isolated from Cornus alba on Benign Prostatic Hypertrophy" Molecules 26, no. 11: 3446. https://doi.org/10.3390/molecules26113446
APA StylePark, D.-H., Park, K.-H., Yin, J., Kim, M.-J., Yoon, S.-E., Lee, S.-H., Heo, J.-H., Chung, H.-J., Kim, J.-W., Kim, K.-M., & Lee, M.-W. (2021). Inhibitory Activities of Dimeric Ellagitannins Isolated from Cornus alba on Benign Prostatic Hypertrophy. Molecules, 26(11), 3446. https://doi.org/10.3390/molecules26113446





