Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases
Abstract
1. Introduction
2. Methods
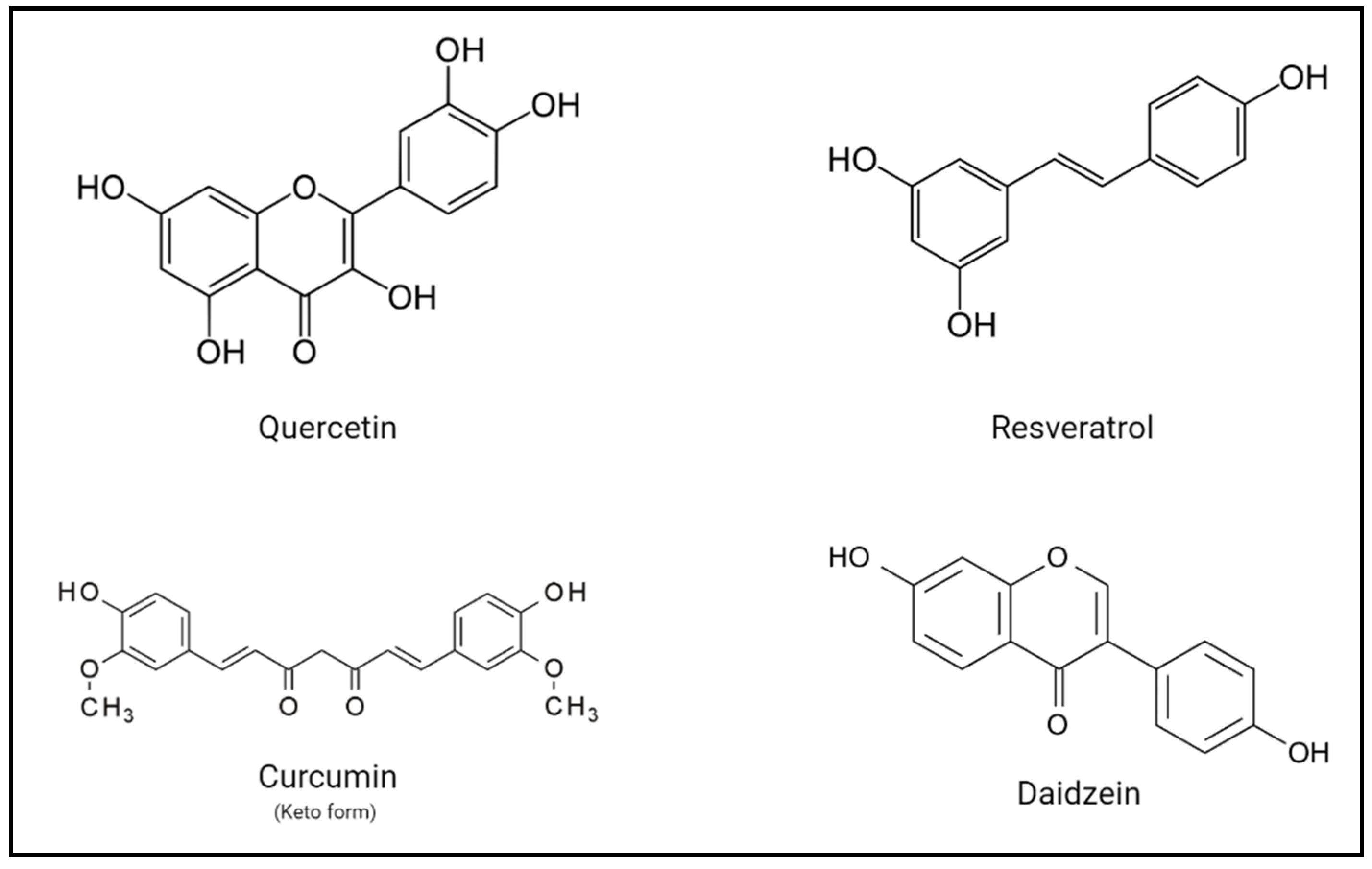
3. Polyphenols as Repurposed Drugs
4. Implications and Potential Benefits of Polyphenols on Human Health
5. Vertebrate Rho and Retinal Degeneration
5.1. Rho as a GPCR
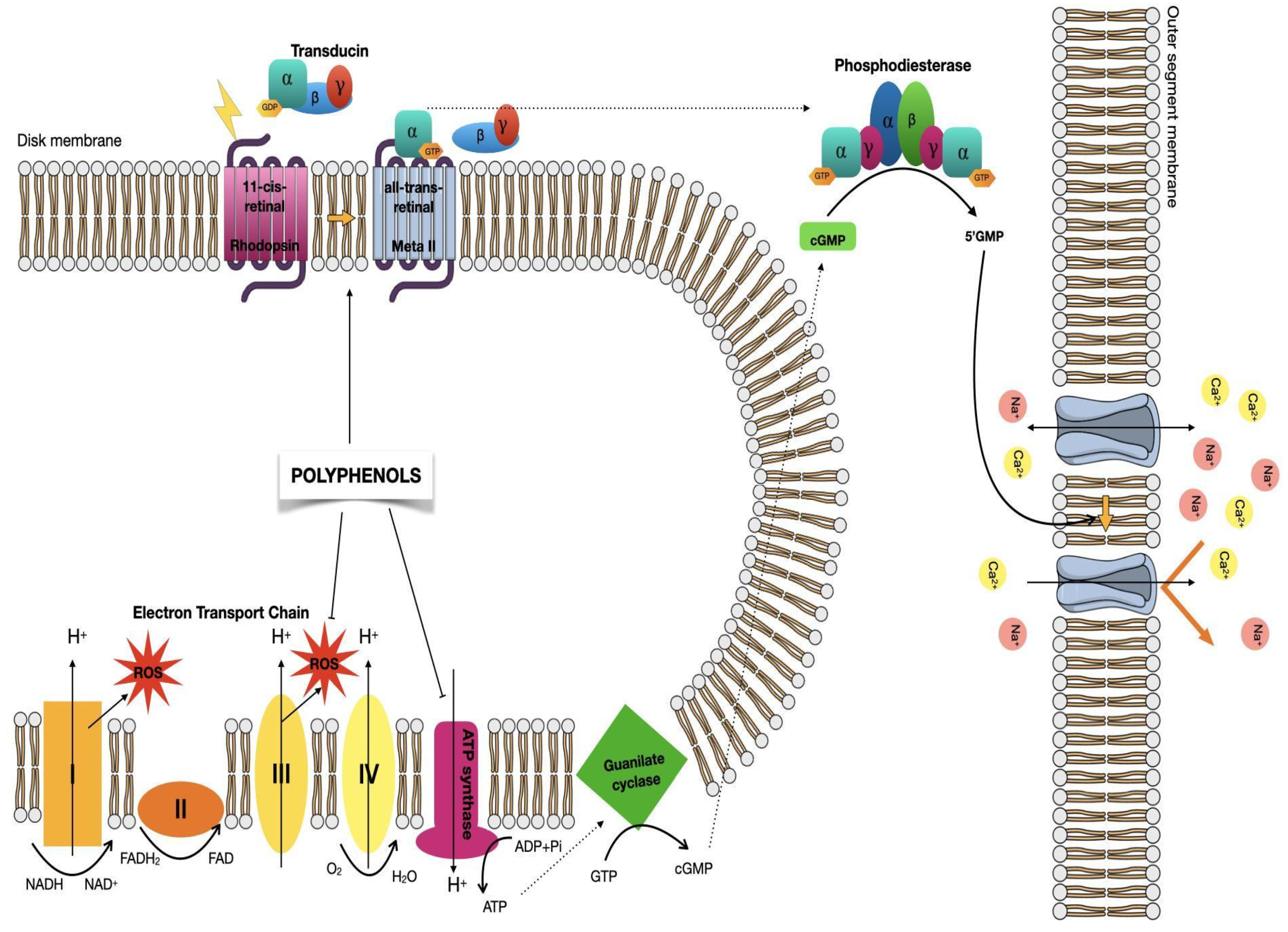
5.2. Visual Phototransduction
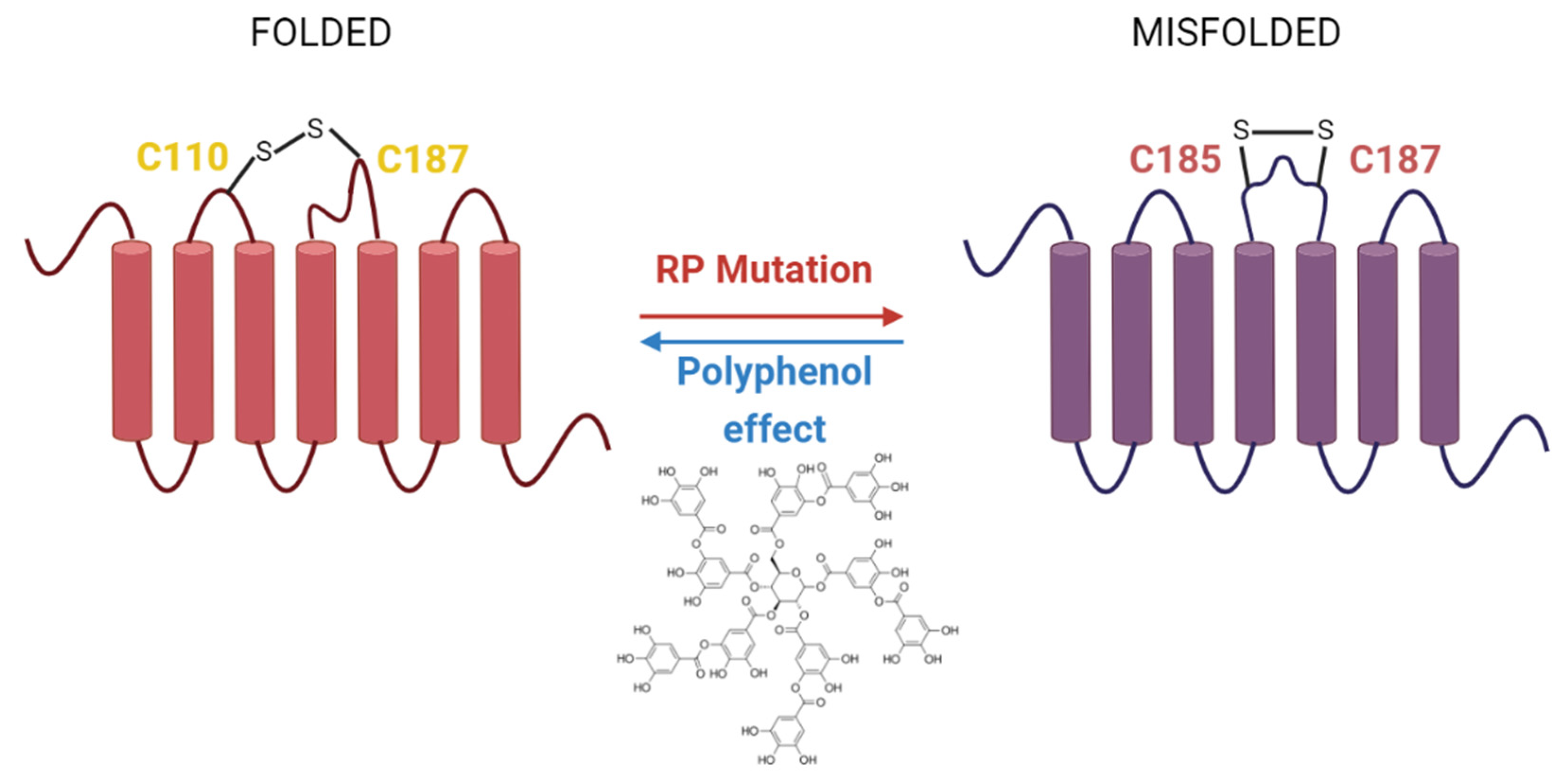
5.3. Mutations in Rho Associated with Retinal Degenerative Diseases
6. Polyphenols Effects in Retinal Degenerative Diseases
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 11CR | 11-cis-retinal |
| 9CR | 9-cis-retinal |
| AMD | Age-related macular degeneration |
| ATR | all-trans-retinal |
| cGMP | Cyclic guanosine monophosphate |
| CSNB | Congenital stationary night blindness |
| GPCR | G protein-coupled receptor |
| Gt | G-protein transducin |
| Meta II | Metarhodopsin II |
| NCDs | Non-communicable diseases |
| Rho | Rhodopsin |
| RHO | Opsin gene |
| ROS | Reactive oxygen species |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| WT | Wild type |
References
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Xiao, J.B. Stability of dietary polyphenols: It’s never too late to mend? Food Chem. Toxicol. 2018, 119, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Hollman, P.C.; Geelen, A.; Kromhout, D. Dietary flavonol intake may lower stroke risk in men and women. J. Nutr. 2010, 140, 600–604. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.R.; Atkinson, S.A.; Chang, N.; Fraga, C.G.; Levy, J.; Messina, M.; Richardson, D.P.; van Ommen, B.; Yang, Y.; Griffiths, J.C.; et al. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur. J. Nutr. 2014, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. Polyphenols and the human brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Miyata, Y.; Shida, Y.; Hakariya, T.; Sakai, H. Anti-cancer effects of green tea polyphenols against prostate cancer. Molecules 2019, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Seddon, J.M.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch. Ophthalmol. 2004, 122, 883. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Jiang, Y.; Willard, L.; Ortiz, E.; Wark, L.; Medeiros, D.; Lin, D. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp. Biol. Med. 2011, 236, 1051–1063. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, bioactivity, and synthesis of methylated flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Fraga, C.G. Plant polyphenols: How to translate their in vitro antioxidant actions to in vivo conditions. IUBMB Life 2007, 59, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as immunomodulatory compounds in the tumor microenvironment: Friends or foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Dudnik, A.; Gaspar, P.; Neves, A.R.; Forster, J. Engineering of microbial cell factories for the production of plant polyphenols with health-beneficial properties. Curr. Pharm. Des. 2018, 24, 2208–2225. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. Plant bioactives and redox signaling: (–)-Epicatechin as a paradigm. Mol. Aspects. Med. 2018, 61, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (–)-Epicatechin improves insulin sensitivity in high fat diet-fed mice. Arch. Biochem. Biophys. 2016, 599, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Prieto, M.A.; Bettaieb, A.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin prevents TNFα-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2012, 527, 113–118. [Google Scholar] [CrossRef]
- Bettaieb, A.; Cremonini, E.; Kang, H.; Kang, J.; Haj, F.G.; Oteiza, P.I. Anti-inflammatory actions of (−)-epicatechin in the adipose tissue of obese mice. Int. J. Biochem. Cell Biol. 2016, 81, 383–392. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Dearborn, P.; Robbins, M. Habitual chocolate intake and type 2 diabetes mellitus in the Maine-Syracuse Longitudinal Study: (1975–2010): Prospective observations. Appetite 2017, 108, 263–269. [Google Scholar] [CrossRef]
- Buitrago-Lopez, A.; Sanderson, J.; Johnson, L.; Warnakula, S.; Wood, A.; Di Angelantonio, E.; Franco, O.H. Chocolate consumption and cardiometabolic disorders: Systematic review and meta-analysis. BMJ 2011, 343, d4488. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodman, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: A randomized controlled clinical trial. Mol. Nutr. Food Res. 2017, 62, 1700674. [Google Scholar] [CrossRef]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of berries consumption on cardiovascular risk factors: A Meta-analysis with trial sequential analysis of randomized controlled trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef]
- Moreira, A.; Diógenes, M.J.; de Mendonça, A.; Lunet, N.; Barros, H. Chocolate consumption is associated with a lower risk of cognitive decline. J. Alzheimers Dis. 2016, 53, 85–93. [Google Scholar] [CrossRef]
- Ng, T.P.; Feng, L.; Niti, M.; Kua, E.H.; Yap, K.B. Tea consumption and cognitive impairment and decline in older Chinese adults. Am. J. Clin. Nutr. 2008, 88, 224–231. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef]
- Dong, X.; Yang, C.; Cao, S.; Gan, Y.; Sun, H.; Gong, Y.; Yang, H.; Yin, X.; Lu, Z. Tea consumption and the risk of depression: A meta-analysis of observational studies. Aust. N. Z. J. Psychiatry 2015, 49, 334–345. [Google Scholar] [CrossRef]
- Li, F.-J.; Ji, H.-F.; Shen, L. A Meta-analysis of tea drinking and risk of Parkinson’s disease. Sci. World J. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Herrera-Hernández, M.G.; Ramon, E.; Lupala, C.S.; Tena-Campos, M.; Pérez, J.J.; Garriga, P. Flavonoid allosteric modulation of mutated visual rhodopsin associated with retinitis pigmentosa. Sci. Rep. 2017, 7, 11167. [Google Scholar] [CrossRef] [PubMed]
- Bourne, H.R.; Meng, E.C. Structure. Rhodopsin sees the light. Science 2000, 289, 733–734. [Google Scholar] [CrossRef] [PubMed]
- Nickell, S.; Park, P.S.-H.; Baumeister, W.; Palczewski, K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J. Cell Biol. 2007, 177, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, B. GPCR: G protein complexes—The fundamental signaling assembly. Amino Acids 2013, 45, 1303–1314. [Google Scholar] [CrossRef]
- Katayama, K.; Gulati, S.; Ortega, J.T.; Alexander, N.S.; Sun, W.; Shenouda, M.M.; Palczewski, K.; Jastrzebska, B. Specificity of the chromophore-binding site in human cone opsins. J. Biol. Chem. 2019, 294, 6082–6093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, Q.; Wu, B. Structural studies of G protein-coupled receptors. Mol. Cells 2015, 38, 836–842. [Google Scholar] [PubMed]
- Alexander, S.P.H.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Spedding, M.; Peters, J.A.; Harmar, A.J. The concise guide to PHARMACOLOGY 2013/14: G proteincoupled receptors. Br. J. Pharmacol. 2013, 170, 1459–1581. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Lagerström, M.C.; Lundin, L.-G.; Schiöth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.C.; Cherezov, V.; Katritch, V.; Abagyan, R.; Kuhn, P.; Rosen, H.; Wüthrich, K. The GPCR network: A large-scale collaboration to determine human GPCR structure and function. Nat. Rev. Drug Discov. 2013, 12, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W.; Emmitte, K.A.; Hopkins, C.R.; Bridges, T.M.; Gregory, K.J.; Niswender, C.M.; Conn, P.J. Practical strategies and concepts in GPCR allosteric modulator discovery: Recent advances with metabotropic glutamate receptors. Chem. Rev. 2016, 116, 6707–6741. [Google Scholar] [CrossRef]
- Khoury, E.; Clément, S.; Laporte, S.A. Allosteric and biased g protein-coupled receptor signaling regulation: Potentials for new therapeutics. Front. Endocrinol. 2014, 5, 68. [Google Scholar] [CrossRef]
- Sato, J.; Makita, N.; Iiri, T. Inverse agonism: The classic concept of GPCRs revisited. Endocr. J. 2016, 63, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.R.; Abdul-Ridha, A.; Canals, M. Regulation of G protein-coupled receptors by allosteric ligands. ACS Chem. Neurosci. 2013, 4, 527–534. [Google Scholar] [CrossRef]
- Hubbard, R.; Kropf, A. The action of light on rhodopsin. Proc. Natl. Acad. Sci. USA 1958, 44, 130–139. [Google Scholar] [CrossRef]
- Nakamichi, H.; Okada, T. X-ray crystallographic analysis of 9-cis-rhodopsin, a model analogue visual pigment. J. Photochem. Photobiol. 2007, 83, 232–235. [Google Scholar] [CrossRef]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent research on polyphenolics in vision and eye health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef]
- Zhong, M.; Kawaguchi, R.; Kassai, M.; Sun, H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients 2012, 4, 2069–2096. [Google Scholar] [CrossRef]
- Park, J.H.; Scheerer, P.; Hofmann, K.P.; Choe, H.-W.; Ernst, O.P. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 2008, 454, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Garriga, P.; Manyosa, J. The eye photoreceptor protein rhodopsin. Structural implications for retinal disease. FEBS Lett. 2002, 528, 17–22. [Google Scholar] [CrossRef]
- Ridge, K.D.; Abdulaev, N.G.; Sousa, M.; Palczewski, K. Phototransduction: Crystal clear. Trends Biochem. Sci. 2003, 28, 479–487. [Google Scholar] [CrossRef]
- Travis, G.H.; Golczak, M.; Moise, A.R.; Palczewski, K. Diseases caused by defects in the visual cycle: Retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 469–512. [Google Scholar] [CrossRef]
- Calzia, D.; Barabino, S.; Bianchini, P.; Garbarino, G.; Oneto, M.; Caicci, F.; Diaspro, A.; Tacchetti, C.; Manni, L.; Candiani, S.; et al. New findings in ATP supply in rod outer segments: Insights for retinopathies. Biol. Cell. 2013, 105, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Kiser, P.D.; Golczak, M.; Palczewski, K. Chemistry of the Retinoid (Visual) Cycle. Chem. Rev. 2014, 114, 194–232. [Google Scholar] [CrossRef]
- Fan, J.; Woodruff, M.L.; Cilluffo, M.C.; Crouch, R.K.; Fain, G.L. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J. Physiol. 2005, 568, 83–95. [Google Scholar] [CrossRef]
- Toledo, D.; Ramon, E.; Aguilà, M.; Cordomí, A.; Pérez, J.J.; Mendes, H.F.; Cheetham, M.E.; Garriga, P. Molecular mechanisms of disease for mutations at Gly-90 in rhodopsin. J. Biol. Chem. 2011, 286, 39993–40001. [Google Scholar] [CrossRef]
- Palczewski, K. G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 2006, 75, 743–767. [Google Scholar] [CrossRef]
- Veleri, S.; Lazar, C.H.; Chang, B.; Sieving, P.A.; Banin, E.; Swaroop, A. Biology and therapy of inherited retinal degenerative disease: Insights from mouse models. Dis. Models Mech. 2015, 8, 109–129. [Google Scholar] [CrossRef]
- Chen, Y.; Okano, K.; Maeda, T.; Chauhan, V.; Golczak, M.; Maeda, A.; Palczewski, K. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J. Biol. Chem. 2012, 287, 5059–5069. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jang, Y.P.; Jockusch, S.; Fishkin, N.E.; Turro, N.J.; Sparrow, J.R. The all-trans- retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc. Natl. Acad. Sci. USA 2007, 104, 19273–19278. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Wu, Y.; Kim, C.Y.; Zhou, J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J. Lipid. Res. 2010, 51, 247–261. [Google Scholar] [CrossRef]
- Gao, S.; Parmar, T.; Palczewska, G.; Dong, Z.; Golczak, M.; Palczewski, K.; Jastrzebska, B. Protective effect of a locked retinal chromophore analog against light-induced retinal degeneration. Mol. Pharmacol. 2018, 94, 1132–1144. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of mitochondrial DNA damage in ROS-mediated pathogenesis of Age-related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef]
- Sawada, O.; Perusek, L.; Kohno, H.; Howell, S.J.; Maeda, A.; Matsuyama, S.; Maeda, T. All-trans-retinal induces Bax activation via DNA damage to mediate retinal cell apoptosis. Exp. Eye Res. 2014, 123, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Maeda, T.; Perusek, L.; Pearlman, E.; Maeda, A. CCL3 production by microglial cells modulates disease severity in murine models of retinal degeneration. J. Immunol. 2014, 192, 3816–3827. [Google Scholar] [CrossRef]
- Rashid, K.; Wolf, A.; Langmann, T. Microglia activation and immunomodulatory therapies for retinal degenerations. Front. Cell. Neurosci. 2018, 12, 176. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef]
- Bruschi, M.; Bartolucci, M.; Peteretto, A.; Calzia, D.; Caicci, F.; Manni, L.; Traverso, C.E.; Candiano, G.; Panfoli, I. Differential expression of the five redox complexes in the retinal mitochondria or rod outer segment disks is consistent with their different functionality. FASEB BioAdv. 2020, 2, 315–324. [Google Scholar] [CrossRef]
- Bruschi, M.; Petretto, A.; Caicci, F.; Bartolucci, M.; Calzia, D.; Santucci, L.; Manni, L.; Ramenghi, L.A.; Ghiggeri, G.; Traverso, C.E.; et al. Proteome of bovine mitochondria and rod outer segments disks: Commonalities and differences. J. Proteome Res. 2018, 17, 918–925. [Google Scholar] [CrossRef]
- Ravera, S.; Esposito, A.; Degan, P.; Caicci, F.; Calzia, D.; Perrotta, E.; Manni, L.; Bisio, A.; Iobbi, V.; Schito, A.; et al. Sclareol modulates free radical production in the retinal rod outer segment by inhibiting the ectopic f1fo-atp synthase. Free Radic. Biol. Med. 2020, 60, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.F.; Moritz, O.L.; Williams, D.S. Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res. 2016, 55, 52–81. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aquila, M.; Bellingham, J.; Li, W.; McCulley, C.; Reeves, P.J.; Cheetham, M.E. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 2018, 62, 1–23. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Hahn, L.B.; Cowley, G.S.; Olsson, J.E.; Reichel, E.; Sandberg, M.A.; Berson, E.L. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N. Engl. J. Med. 1990, 323, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Cohen, G.B.; Oprian, D.D. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature 1994, 367, 639–642. [Google Scholar] [CrossRef]
- Sieving, P.A.; Richards, J.E.; Naarendorp, F.; Bingham, E.L.; Scott, K.; Alpern, M. Dark-light: Model for night blindness from the human rhodopsin Gly-90-->Asp mutation. Proc. Natl. Acad. Sci. USA 1995, 92, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Al-Jandal, N.; Farrar, G.J.; Kiang, A.S.; Humphries, M.M.; Bannon, N.; Findlay, J.B.; Humphries, P.; Kenna, P.F. A novel mutation within the rhodopsin gene (Thr-94-Ile) causing autosomal dominant congenital stationary night blindness. Hum. Mutat. 1999, 13, 75–81. [Google Scholar] [CrossRef]
- Reiff, C.; Owczarek-Lipska, M.; Spital, G.; Roger, C.; Hinz, H.; Juschke, C.; Thiele, H.; Altmuller, J.; Nurnberg, P.; Da Costa, R.; et al. The mutation p.E113K in the Schiff base counterion of rhodopsin is associated with two distinct retinal phenotypes within the same family. Sci. Rep. 2016, 6, 36208. [Google Scholar] [CrossRef]
- Dryja, T.P.; Berson, E.L.; Rao, V.R.; Oprian, D.D. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat. Genet. 1993, 4, 280–283. [Google Scholar] [CrossRef]
- Zeitz, C.; Gross, A.K.; Leifert, D.; Kloeckener-Gruissem, B.; McAlear, S.D.; Lemke, J.; Neidhardt, J.; Berger, W. Identification and functional characterization of a novel rhodopsin mutation associated with autosomal dominant CSNB. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4105–4114. [Google Scholar] [CrossRef]
- Gross, A.K.; Rao, V.R.; Oprian, D.D. Characterization of rhodopsin congenital night blindness mutant T94I. Biochemistry 2003, 42, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Guo, Y.; Matkovic, M.; Schertler, G.; Deupi, X.; Yan, E.C.; Standfuss, J. Structural role of the T94I rhodopsin mutation in congenital stationary night blindness. EMBO Rep. 2016, 17, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shi, G.; Concepcion, F.A.; Xie, G.; Oprian, D.; Chen, J. Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J. Neurosci. 2006, 26, 11929–11937. [Google Scholar] [CrossRef]
- Tam, B.M.; Moritz, O.L. Characterization of rhodopsin P23H-induced retinal degeneration in a Xenopus laevis model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3234–3241. [Google Scholar] [CrossRef]
- Ramon, E.; del Valle, L.J.; Garriga, P. Unusual thermal and conformational properties of the rhodopsin congenital night blindness mutant Thr-94 --> Ile. J. Biol. Chem. 2003, 278, 6427–6432. [Google Scholar] [CrossRef]
- Azam, M.; Khan, M.I.; Gal, A.; Hussain, A.; Shah, S.T.; Khan, M.S.; Sadeque, A.; Bokhari, H.; Collin, R.W.J.; Orth, U.; et al. A homozygous p.Glu150Lys mutation in the opsin gene of two Pakistani families with autosomal recessive retinitis pigmentosa. Mol. Vis. 2009, 15, 2526–2534. [Google Scholar]
- Saqib, M.A.; Nikopoulos, K.; Ullah, E.; Sher Khan, F.; Iqbal, J.; Bibi, R.; Jarral, A.; Sajid, S.; Nishiguchi, K.M.; Venturini, G.; et al. Homozygosity mapping reveals novel and known mutations in Pakistani families with inherited retinal dystrophies. Sci. Rep. 2015, 5, 9965. [Google Scholar] [CrossRef] [PubMed]
- Van Schil, K.; Karlstetter, M.; Aslanidis, A.; Dannhausen, K.; Azam, M.; Qamar, R.; Leroy, B.P.; Depasse, F.; Langmann, T.; De Baere, E. Autosomal recessive retinitis pigmentosa with homozygous rhodopsin mutation E150K and non-coding cis-regulatory variants in CRX-binding regions of SAMD7. Sci. Rep. 2016, 6, 21307. [Google Scholar] [CrossRef] [PubMed]
- Collin, R.W.; van den Born, L.I.; Klevering, B.J.; de Castro-Miro, M.; Littink, K.W.; Arimadyo, K.; Azam, M.; Yazar, V.; Zonneveld, M.N.; Paun, C.C.; et al. High-resolution homozygosity mapping is a powerful tool to detect novel mutations causative of autosomal recessive RP in the Dutch population. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2227–2239. [Google Scholar] [CrossRef]
- Kartasasmita, A.; Fujiki, K.; Iskandar, E.; Sovani, I.; Fujimaki, T.; Murakami, A. A novel nonsense mutation in rhodopsin gene in two Indonesian families with autosomal recessive retinitis pigmentosa. Ophthalmic Genet. 2011, 32, 57–63. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Cowley, G.S.; McGee, T.L.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat. Genet. 1992, 1, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Lewis, R.A.; Garcia, C.A.; Ruiz, R.S.; Blanton, S.H.; Northrup, H.; et al. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: A screen of known genes in 200 families. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3052–3064. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Kemp, C.M.; Sung, C.H.; Nathans, J. Retinal function and rhodopsin levels in autosomal dominant retinitis pigmentosa with rhodopsin mutations. Am. J. Ophthalmol. 1991, 112, 256–271. [Google Scholar] [CrossRef]
- Dryja, T.P.; McEvoy, J.A.; McGee, T.L.; Berson, E.L. Novel rhodopsin mutations Gly114Val and Gln184Pro in dominant retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3124–3127. [Google Scholar]
- Shi, W.; Sports, C.D.; Raman, D.; Shirakawa, S.; Osawa, S.; Weiss, E.R. Rhodopsin arginine-135 mutants are phosphorylated by rhodopsin kinase and bind arrestin in the absence of 11-cis retinal. Biochemistry 1998, 37, 4869–4874. [Google Scholar] [CrossRef]
- Del Porto, G.; Vingolo, E.M.; David, D.; Steindl, K.; Wedemann, H.; Forte, R.; Iannccone, A.; Gal, A.; Pannarale, M.R. Clinical features of autosomal dominant retinitis pigmentosa associated with the GLY-188-ARG mutation of the rhodopsin gene. In Retinal Degeneration; Hollyfield, J.G., Anderson, R.E., LaVail, M.M., Eds.; Springer: Boston, MA, USA, 1993; pp. 91–101. [Google Scholar]
- Van Woerkom, C.; Ferrucci, S. Sector retinitis pigmentosa. Optometry 2005, 76, 309–317. [Google Scholar] [CrossRef]
- Ramon, E.; Cordomi, A.; Aguila, M.; Srinivasan, S.; Dong, X.; Moore, A.T.; Webster, A.R.; Cheetham, M.E.; Garriga, P. Differential light-induced responses in sectorial inherited retinal degeneration. J. Biol. Chem. 2014, 289, 35918–35928. [Google Scholar] [CrossRef]
- Sanchez-Reyes, O.B.; Cooke, A.L.G.; Tranter, D.B.; Rashid, D.; Eilers, M.; Reeves, P.J.; Smith, S.O. G Protein-Coupled Receptors Contain Two Conserved Packing Clusters. Biophys. J. 2017, 112, 2315–2326. [Google Scholar] [CrossRef]
- Jastrzebska, B.; Chen, Y.; Orban, T.; Jin, H.; Hofmann, L.; Palczewski, K. Disruption of rhodopsin dimerization with synthetic peptides targeting an interaction interface. J. Biol. Chem. 2015, 290, 25728–25744. [Google Scholar] [CrossRef] [PubMed]
- Kota, P.; Reeves, P.J.; Rajbhandary, U.L.; Khorana, H.G. Opsin is present as dimers in COS1 cells: Identification of amino acids at the dimeric interface. Proc. Natl. Acad. Sci. USA 2006, 103, 3054–3059. [Google Scholar] [CrossRef]
- Gunkel, M.; Schoneberg, J.; Alkhaldi, W.; Irsen, S.; Noe, F.; Kaupp, U.B.; Al-Amoudi, A. Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure 2015, 23, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ploier, B.; Caro, L.N.; Morizumi, T.; Pandey, K.; Pearring, J.N.; Goren, M.A.; Finnemann, S.C.; Graumann, J.; Arshavsky, V.Y.; Dittman, J.S.; et al. Dimerization deficiency of enigmatic retinitis pigmentosa-linked rhodopsin mutants. Nat. Commun. 2016, 7, 12832. [Google Scholar] [CrossRef]
- Davies, W.I.; Downes, S.M.; Fu, J.K.; Shanks, M.E.; Copley, R.R.; Lise, S.; Ramsden, S.C.; Black, G.C.M.; Gibson, K.; Foster, R.G.; et al. Next-generation sequencing in health-care delivery: Lessons from the functional analysis of rhodopsin. Genet. Med. 2012, 14, 891–899. [Google Scholar] [CrossRef]
- Lim, K.P.; Yip, S.P.; Cheung, S.C.; Leung, K.W.; Lam, S.T.; To, C.H. Novel PRPF31 and PRPH2 mutations and co-occurrence of PRPF31 and RHO mutations in Chinese patients with retinitis pigmentosa. Arch. Ophthalmol. 2009, 127, 784–790. [Google Scholar] [CrossRef][Green Version]
- Cideciyan, A.V.; Hood, D.C.; Huang, Y.; Banin, E.; Li, Z.Y.; Stone, E.M.; Milam, A.H.; Jacobson, S.G. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc. Natl. Acad. Sci. USA 1998, 95, 7103–7108. [Google Scholar] [CrossRef]
- Li, J.; Edwards, P.C.; Burghammer, M.; Villa, C.; Schertler, G.F. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004, 343, 1409–1438. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, A.; Man, D.; Waseem, N.; Jennings, B.J.; Ganapathiraju, M.; Gallaher, K.; Reese, E.; Bhattacharya, S.S.; Klein-Seetharaman, J. Retinitis pigmentosa associated with rhodopsin mutations: Correlation between phenotypic variability and molecular effects. Vision Res. 2006, 46, 4556–4567. [Google Scholar] [CrossRef]
- Huynh, T.P.; Mann, S.N.; Mandal, N.A. Botanical compounds: Effects on major eye diseases. Evid. Based Complement. Alternat. Med. 2013, 2013, 549174. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Utz, V.M.; Pfeifer, W.; Longmuir, S.Q.; Olson, R.J.; Wang, K.; Drack, A.V. Presentation of TRPM1-associated congenital stationary night blindness in children. JAMA Ophthalmol. 2018, 136, 389–398. [Google Scholar]
- Singh, M.S.; MacLaren, R.E. Stem cell treatment for Age-Related Macular Degeneration: The Challenges. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD78–AMD82. [Google Scholar] [CrossRef]
- Ortega, J.T.; Parmar, T.; Jastrzebska, B. Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation. J. Biol. Chem. 2019, 294, 8101–8122. [Google Scholar] [CrossRef]
- Ortega, J.T.; Parmar, T.; Golczak, M.; Jastrzebska, B. Protective effects of flavonoids in acute models of light-induced retinal degeneration. Mol. Pharmacol. 2021, 99, 60–77. [Google Scholar] [CrossRef]
- Ortega, J.T.; Jastrzebska, B. The retinoid and non-retinoid ligands of the rod visual G protein-coupled receptor. Int. J. Mol. Sci. 2019, 20, 6218. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, M.; Tuo, J.; Shen, D.; Chan, C.C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp. Eye Res. 2010, 91, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Yun, S.; Lee, H.; Yang, J. Quercetin mitigates inflammatory responses induced by vascular endothelial growth factor in mouse retinal photoreceptor cells through suppression of nuclear factor kappa B. Int. J. Mol. Sci. 2017, 18, 2497. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Mao, L.; Gong, Y.; Sun, T.; Gu, Q. Role of quercetin in protecting ARPE19 cells against H2O2 induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition. Mol. Med. Rep. 2017, 16, 3461–3468. [Google Scholar] [CrossRef]
- Kook, D.; Wolf, A.H.; Yu, A.L.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lussen, U.C. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jin, H.L.; Jang, D.S.; Jeong, K.W.; Choung, S.Y. Quercetin-3-O-α-l-arabinopyranoside protects against Oxidative Medicine and Cellular Longevity 11 retinal cell death via blue light-induced damage in human RPE cells and Balb-c mice. Food Funct. 2018, 9, 2171–2183. [Google Scholar] [CrossRef]
- Barzegar, A. Antioxidant activity of polyphenolic myricetin in vitro cell- free and cell-based systems. Mol. Biol. Res. Commun. 2016, 5, 87–95. [Google Scholar]
- Bian, M.; Zhang, Y.; Du, X.; Xu, J.; Cui, J.; Gu, J.; Zhu, W.; Zhang, T.; Chen, Y. Apigenin-7-diglucuronide protects retinas against bright light-induced photoreceptor degeneration through the inhibition of retinal oxidative stress and inflammation. Brain. Res. 2017, 1663, 141–150. [Google Scholar] [CrossRef]
- Chou, W.-W.; Wang, Y.-S.; Chen, K.-C.; Wu, J.-M.; Liang, C.-L.; Juo, S.-H.H. Tannic acid suppresses ultraviolet B-induced inflammatory signaling and complement factor B on human retinal pigment epithelial cells. Cell. Immunol. 2012, 273, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hytti, M.; Szabo, D.; Piippo, N.; Korhonen, E.; Honkakoski, P.; Kaarniranta, K.; Petrovski, G.; Kauppinen, A. Two dietary polyphenols, fisetin and luteolin, reduce inflammation but augment DNA damage-induced toxicity in human RPE cells. J. Nutr. Biochem. 2017, 42, 37–42. [Google Scholar] [CrossRef]
- Escher, P.; Schorderet, D.F.; Cottet, S. Altered expression of the transcription factor Mef2c during retinal degeneration in Rpe65–/– mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5933–5940. [Google Scholar] [CrossRef]
- Wolf, A.; Aslanidis, A.; Langmann, T. Retinal expression and localization of Mef2c support its important role in photoreceptor gene expression. Biochem. Biophys. Res. Commun. 2017, 483, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Calzia, D.; Degan, P.; Caicci, F.; Bruschi, M.; Manni, L.; Ramenghi, L.A.; Candiano, G.; Traverso, C.E.; Panfoli, I. Modulation of the rod outer segment aerobic metabolism diminishes the production of radicals due to light absorption. Free Radic. Biol. Med. 2018, 17, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roque, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Veltmann, M.; Hollborn, M.; Reichenbach, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Osmotic induction of angiogenic growth factor expression in human retinal pigment epithelial cells. PLoS ONE 2016, 11, e0147312. [Google Scholar] [CrossRef][Green Version]
- Yoon, S.M.; Lee, B.L.; Guo, Y.R.; Choung, S.Y. Preventive effect of Vaccinium uliginosum L. extract and its fractions on age-related macular degeneration and its action mechanisms. Arch. Pharm. Res. 2016, 39, 21–32. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Arch. Pharm. Res. 2017, 160, 45–55. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, T.; Jiang, Y.; Wu, L.; Cai, X.; Sun, X.; Sun, X. Photooxidative damage in retinal pigment epithelial cells via GRP78 and the protective role of grape skin polyphenols. Food Chem. Toxicol. 2014, 74, 216–224. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M., Jr.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed Res. Int. 2013, 2013, 915436. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: Phytochemical analysis and antioxidant effect. Cell. Mol. Biol. 2012, 58, 174–181. [Google Scholar] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Petrs-Silva, H.; Linden, R. Advances in gene therapy technologies to treat retinitis pigmentosa. Clin. Ophthalmol. 2014, 8, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Novelli, E.; Piano, I.; Gargini, C.; Strettoi, E. Pharmacological approaches to retinitis pigmentosa: A laboratory perspective. Prog. Retin. Eye Res. 2015, 48, 62–81. [Google Scholar] [CrossRef]
- Bernier, V.; Bichet, D.G.; Bouvier, M. Pharmacological chaperone action on G-protein-coupled receptors. Curr. Opin. Pharmacol. 2004, 4, 528–533. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A favonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Dong, Z.; Jin, H.; Sawada, O.; Gao, S.; Utkhede, D.; Monk, W.; Palczewska, G.; Palczewski, K. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Investig. Ophthalmol. Vis. Sci. 2013, 54, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Maeda, A.; Casadesus, G.; Palczewski, K.; Margaron, P. Evaluation of 9-cis-retinyl acetate therapy in Rpe65–/– mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4368–4378. [Google Scholar] [CrossRef] [PubMed]
- Van Hooser, J.P.; Liang, Y.; Maeda, T.; Kuksa, V.; Jang, G.-F.; He, Y.-G.; Rieke, F.; Fong, H.K.W.; Detwiler, P.B.; Palczewski, K. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J. Biol. Chem. 2002, 277, 19173–19182. [Google Scholar] [CrossRef]
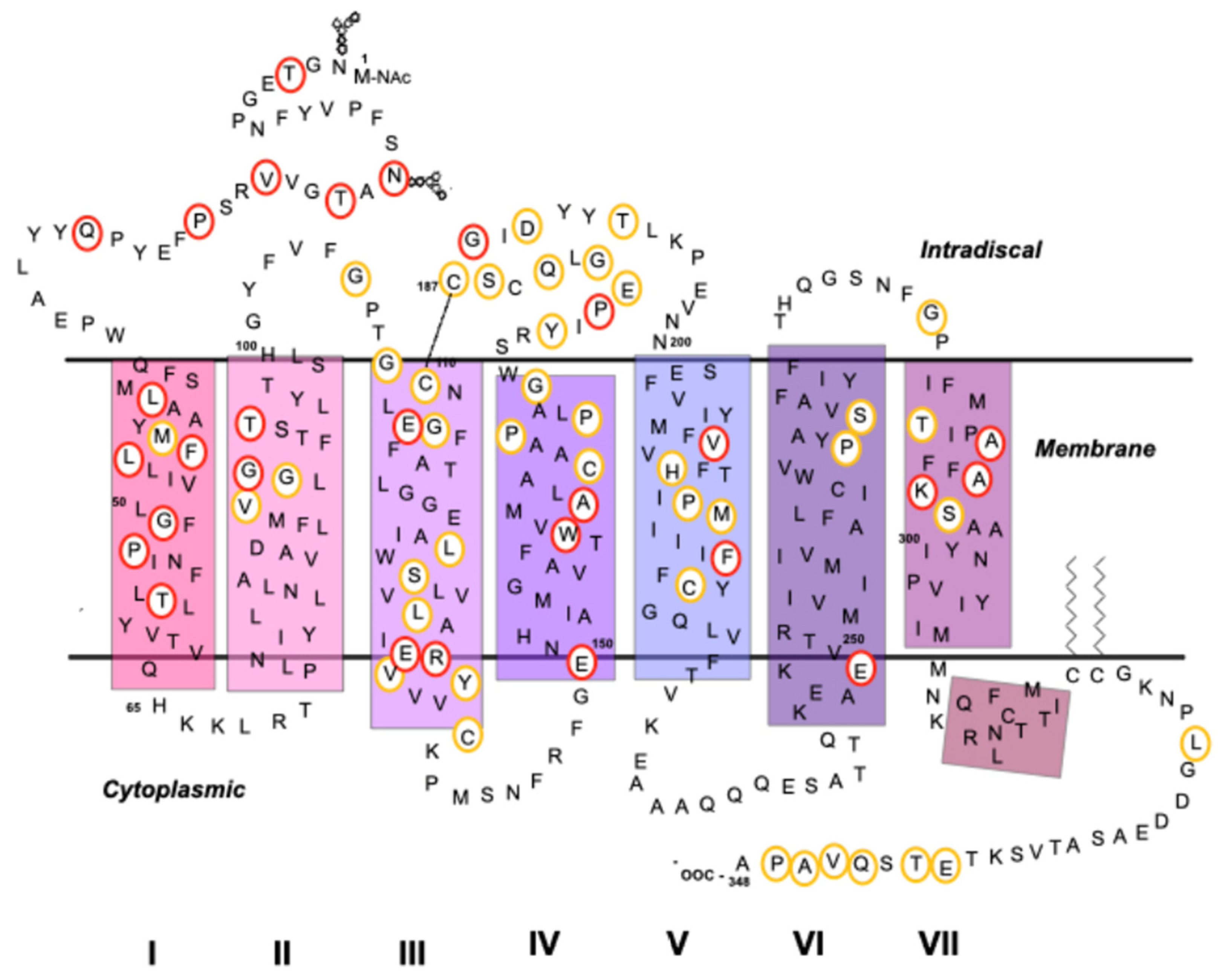
| Mutation | Behavior/Effect | Class/Misfolds | References |
|---|---|---|---|
| G90X | Causes thermal instability and/or abnormal photoproduct formation in inducing a RP phenotype. | VI/No | [45] |
| T94I | Induces constitutive activation of the opsin in the absence of chromophore and in the dark. | VI/No | [94] |
| E113K | Associated with the two distinct phenotypes of RP and CSNB in independent members of the same family. | Unclassified | [91] |
| A292E | Anomalously activates transducin when the chromophore is missing. | Unclassified | [92] |
| P23H | Destabilizes outer rod segments via the formation of aggregates due to retention in the ER. | II/Yes | [87] |
| E150 | No observed biochemical or cellular defects or not studied in detail. | Unclassified | [101] |
| W161X | No observed biochemical or cellular defects or not studied in detail. | Unclassified | [103] |
| G114V | No observed biochemical or cellular defects or not studied in detail. | Unclassified | [107] |
| Q184P | No observed biochemical or cellular defects or not studied in detail. | Unclassified | [107] |
| R135X | Affects endocytosis | III/No | [108] |
| G188R | Forms aggregates due to retention in the ER and cannot be easily constituted with 11CR. | II/Yes | [109] |
| Compound | Condition/Cell Lines | Effect | References |
|---|---|---|---|
| Quercetin | Oxidative stress conditions. Assay in vitro in human hepatoma HepG2 cells. | Activates the Nrf2-ARE signaling pathway and exhibits anti-oxidative stress activity alone and together with kaempferol and pterostilbene. | [123] |
| Oxidative stress conditions. Assay in vitro in human RPE cells and in Ccl2/Cx3cr1 double knock-out mice. | Protects RPE cells from oxidative stress via inhibiting pro-inflammatory molecules and the intrinsic apoptosis pathway. | [129] | |
| VEGF-treated mouse photoreceptor-derived 661W cells. | Inhibits the production of inflammatory proteins in VEGF-stimulated 661W cells. | [130] | |
| Oxidative stress conditions. ARPE-19 human retinal pigment epithelial cells. | Protects ARPE-19 cells from H2O2-induced cytotoxicity by activating the Nrf2 pathway, inhibiting ER stress and targeting anti-apoptotic proteins. | [131] | |
| Oxidative stress conditions. Assay in vitro in human RPE cells. | Protects RPE cells from oxidative damage and cellular senescence in a dose-dependent manner. | [132] | |
| Oxidative stress conditions. Assay in vitro and in vivo in human RPE cells. | Protects against blue light-induced retinal damage. | [133] | |
| Myricetin | Human MCF-7 breast cancer cells. | Reduces and scavenges intracellular ROS. | [134] |
| Apigenin | Bright light-exposed BALB/c mice. | Confers retinal protection by inhibiting retinal oxidative stress and retinal inflammatory responses. | [135] |
| Tannic acid | Assay in vitro in human RPE cells (ARPE-19). | Protects RPE against ultraviolet B radiation via the inhibition of the inflammatory response. | [136] |
| Fisetin/Luteolin | Assay in vitro in human RPE cells (ARPE-19). | Anti-inflammatory and cytoprotective effects when used as dietary supplements. | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Gonzalez, P.; Mas-Sanchez, A.; Garriga, P. Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases. Molecules 2021, 26, 3407. https://doi.org/10.3390/molecules26113407
Fernandez-Gonzalez P, Mas-Sanchez A, Garriga P. Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases. Molecules. 2021; 26(11):3407. https://doi.org/10.3390/molecules26113407
Chicago/Turabian StyleFernandez-Gonzalez, Pol, Aina Mas-Sanchez, and Pere Garriga. 2021. "Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases" Molecules 26, no. 11: 3407. https://doi.org/10.3390/molecules26113407
APA StyleFernandez-Gonzalez, P., Mas-Sanchez, A., & Garriga, P. (2021). Polyphenols and Visual Health: Potential Effects on Degenerative Retinal Diseases. Molecules, 26(11), 3407. https://doi.org/10.3390/molecules26113407






