177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MSn, and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng
Abstract
1. Introduction
2. Results
2.1. Identification of Saponins in Wild Ginseng, Ginseng under Forest and Cultivated Ginseng with HPLC-IT-TOF-MSn
2.2. Potential New Compounds Found in Wild Ginseng, Ginseng under Forest and Cultivated Ginseng via HPLC-IT-TOF-MSn
| RT (min) | Molecular Formula | Measured (m/z) | Predicted (m/z) | Error (ppm) | Ion | n-ESI-MSn Data | Identification | Source | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.938 | C52H88O23 | 1079.5628 | 1079.5644 | −1.48 | [M − H]− | MS2[1079.5627]: 962.5320(100), 961.5308(76.47), 781.4758(11.26) MS3[1079.5627→961.5361]: 799.4771(4.42), 781.4756(100), 635.4042(23.49), 617.4012(5.15) | (B3-b)-glc-glc-rha-C4H6O4 | W-GS (no. 19) |
| 2 | 4.147 | C51H80O21 | 1027.5105 | 1027.5119 | −1.36 | [M − H]− | MS2[1027.5104]: 847.4492(100) MS3[1027.5104→847.4492]: 701.3909(100), 521.3270(57.60), 491.2823(5.26) | Unknown(C33H50O7)-glc-glc-rha | C-GS (no. 25) |
| 3 | 6.350 | C48H80O20 | 1021.5112 | 1021.5225 | −11.06 | [M + HCOO]− | MS2[1021.5113]: 975.5109(100), 815.4821(11.98) MS3[1021.5113→975.5104]: 815.4704(47.05), 669.4099(100) MS4[1021.5113→975.5104→669.4099]: 507.3565(100) | (C3-b)/(C1-b)/(B10-b)-glc-C6H8O5-rha | W-GS (no. 19 and 20) |
| 4 | 6.970 | C45H68O16 | 863.4456 | 863.4435 | 2.43 | [M + HCOO]− | MS2[863.4454]: 717.3812(100), 537.3186(31.79), 391.2789(14.69) | Unknown(C33H48O7)-glc-rha | C-GS (no. 25) |
| 5 | 9.168 | C45H70O17 | 881.4549 | 881.4540 | 1.02 | [M + HCOO]− | MS2[881.4550]: 823.4218(17.73), 735.4004(35.88), 677.3571(97.45), 555.3343(15.43), 515.3014(8.70), 497.2947(100) | Unknown(C30H44O7)-glc-rha-C3H6O | C-GS (no. 25) |
| 6 | 9.447 | C35H40O7 | 617.2671 | 617.2756 | −13.77 | [M + HCOO]− | MS2[617.2656]: 571.2617(100) MS3[617.2656→571.2617]: 439.1954(100), 277.1775(100) | Unknown(C30H32O3)-xyl | W-GS (no. 19) and F-GS (no. 18) |
| 7 | 11.687 | C54H84O26 | 573.2556 | 573.2553 | 0.52 | [M − 2H]2− | MS2[573.2557(2)]: 985.4663(100) MS3[573.2557(2)→985.4663]: 643.3430(100), 463.2941(23.54) | Unknown(C30H44O6)-glc-glc-glc-glc | C-GS (no. 25) |
| 8 | 15.098 | C45H70O16 | 865.4600 | 865.4591 | 1.04 | [M − H]− | MS2[865.4600]: 719.3996(93.02), 539.3384(100), 509.2889(5.33) | Unknown(C33H50O7)-glc-rha | C-GS (no. 25) |
| 9 | 19.317 | C48H80O18 | 989.5305 | 989.5327 | −2.22 | [M + HCOO]− | MS2[989.5299]: 943.5149(100) MS3[989.5299→943.5148]: 763.4574(100), 617.3911(15.50) | PPD-gluA-glc-rha | W-GS (no. 19) |
| 10 | 20.045 | C36H64O10 | 701.4468 | 701.4482 | −2.00 | [M + HCOO]− | MS2[701.4473]: 655.4324(100), 493.3847(58.09) MS3[701.4473→654.4171]: 493.3859(100), 392.2902(25.12) | (B1-b)-glc | W-GS (no. 19, 20) and C-GS (no. 25) |
| 11 | 20.270 | C42H74O14 | 843.4735 | 843.4748 | −1.54 | [M + HCOO]− | MS2[843.4733]: 799.4767(100), 617.4006(18.51) MS3[843.4733→799.4764]: 653.4222(44.40), 635.4042(44.40), 491.3685(88.79), 391.2842(100) | Unknown(C31H52O7)-glc-rha | W-GS (no. 19 and 20) |
| 12 | 26.555 | C38H66O13 | 775.4551 | 775.4485 | 8.51 | [M + HCOO]− | MS2[775.4549]: 729.4399(100) MS3[775.4549→730.4454]: 583.3904(33.33), 421.3375(100) | Unknown(C26H46O4)-glc-rha | W-GS (no. 19) |
| 13 | 40.932 | C41H68O13 | 813.4639 | 813.4642 | −0.37 | [M + HCOO]− | MS2[813.4639]: 767.4578(100), 635.4058(34.42), 489.3553(4.17) | (B8-b)-rha-xyl | W-GS (no. 19 and 20) |
| 14 | 41.082 | C54H92O24 | 561.2898 | 561.2917 | −3.39 | [M − 2H]2− | MS2[561.2900(2)]: 961.5367(89.98), 799.4831(88.49), 781.4794(100), 637.4221(54.76), 475.3830(47.19), 375.2964(7.86) | (B4-a)-6-glc-20-glc(-glc)-glc | W-GS (no. 20) |
| 15 | 44.095 | C39H68O14 | 805.4600 | 805.4591 | 1.12 | [M + HCOO]− | MS2[805.4597]: 759.4501(100) MS3[805.4597→759.4501]: 597.4013(31.25), 435.3427(100), 375.2780(7.99) MS4[805.4597→759.4501→597.4014]: 435.3394(100) | Unknown(C27H48O4)-glc-glc | W-GS (no. 19 and 20) |
| 16 | 45.002 | C38H64O14 | 743.4226 | 743.4223 | 0.40 | [M − H]− | MS2[743.4225]: 597.3617(70.84), 435.3087(100), 417.2935(7.98) | Unknown(C26H44O5)-glc-rha | W-GS (no. 19) |
2.3. Quantitative Analysis of Seven Ginsenosides in Ginseng with “Quantitative Analysis of Multi-Component with Single Marker” (QAMS) Method
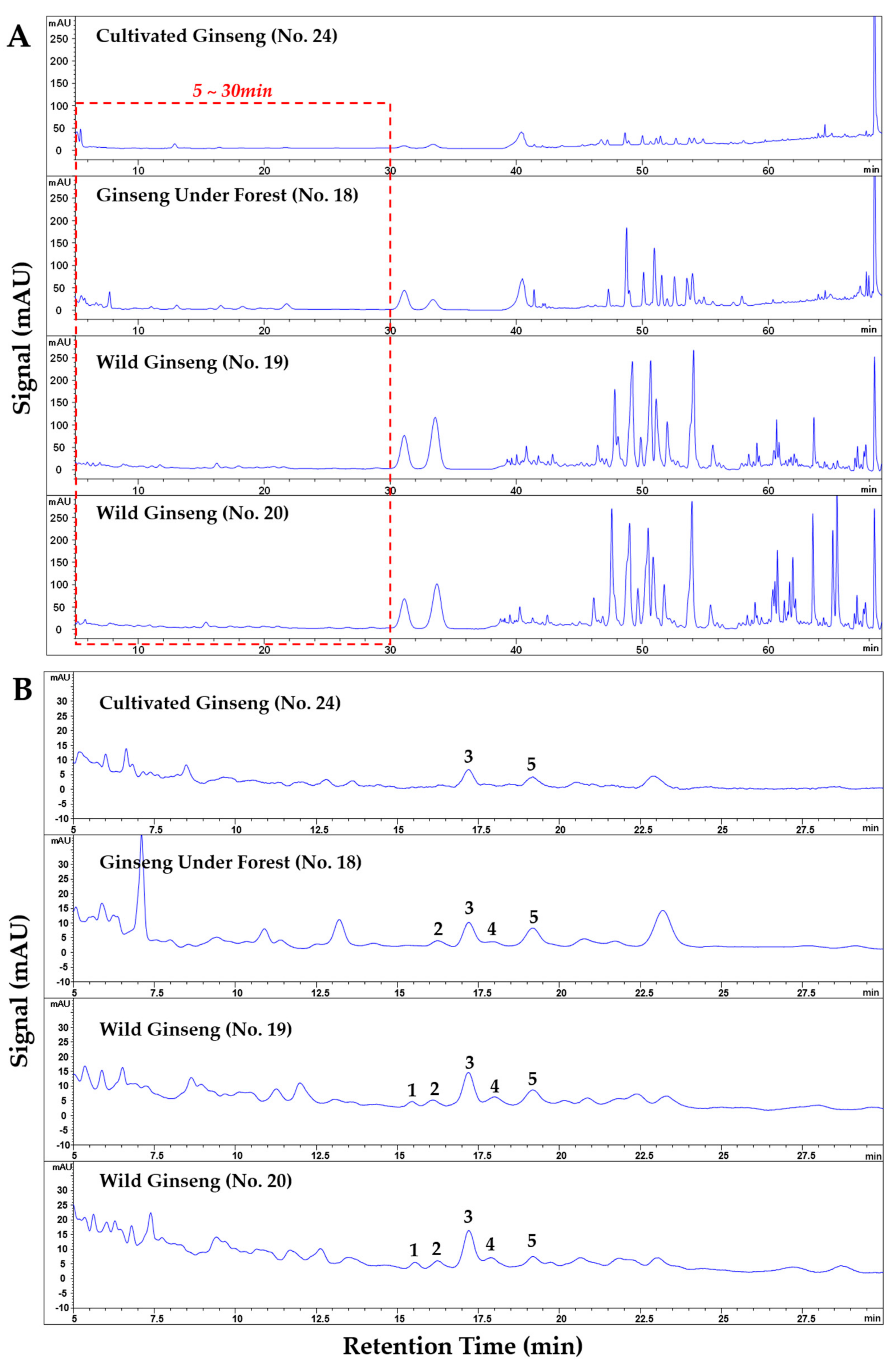
2.4. Fingerprints Comparison of Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng
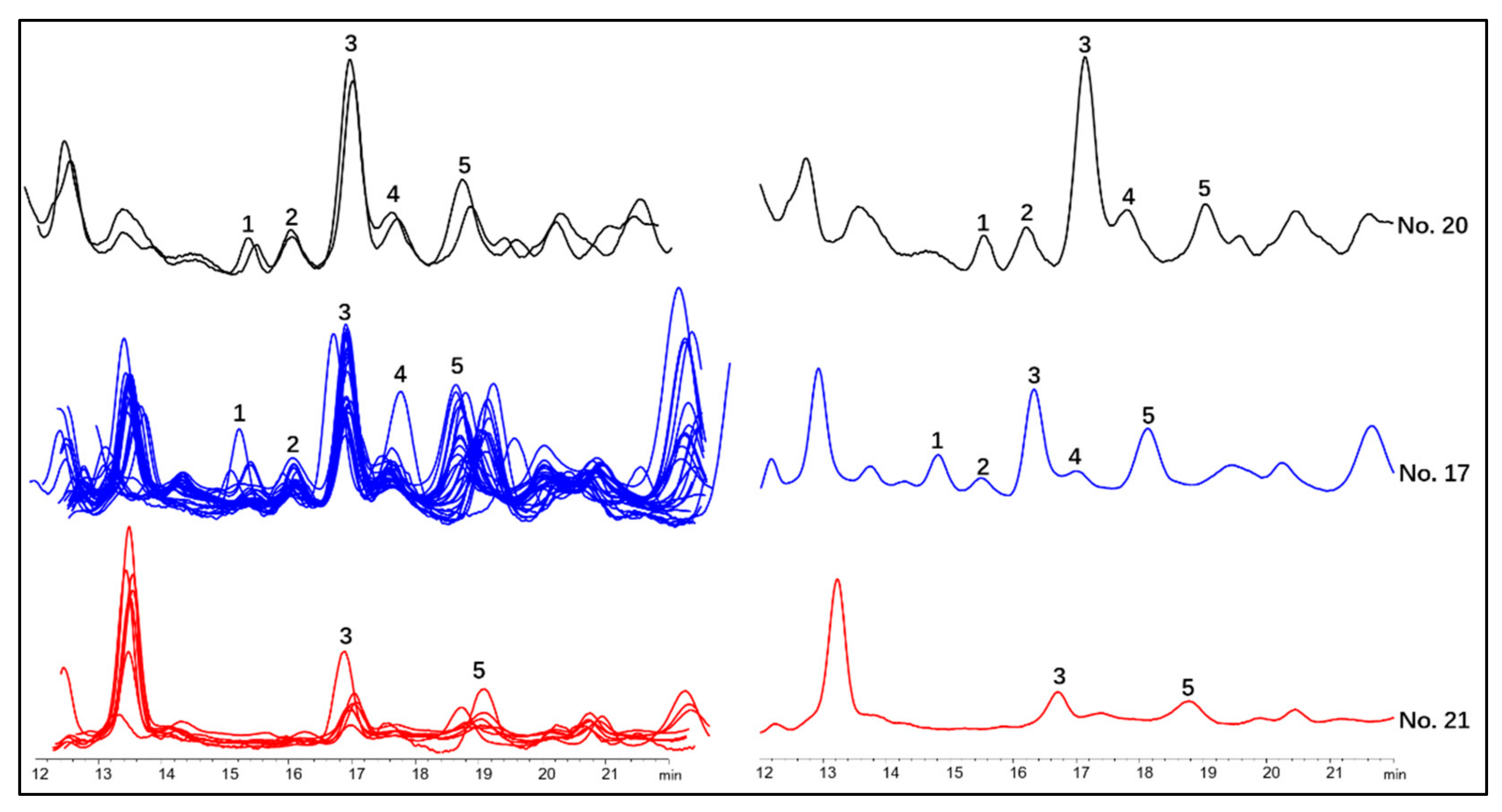
2.5. Differentiation of Wild Ginseng, Ginseng under Forest and Cultivated Ginseng with Characteristic Peak Pattern of Chromatogram
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials
| No. | Type | Age/Year | Production Area | No. | Type | Age/Year | Production Area |
|---|---|---|---|---|---|---|---|
| 1 | F-GS 1 | 12 | Xintunzi village | 21 | C-GS 5 | 6 | Fusong village |
| 2 | F-GS | 12 | Xintunzi village | 22 | C-GS | 6 | Fusong village |
| 3 | F-GS | 12 | Xintunzi village | 23 | C-GS | 6 | Fusong village |
| 4 | F-GS | 12 | Xintunzi village | 24 | C-GS | 6 | Fusong village |
| 5 | F-GS | 12 | Xintunzi village | 25 | C-GS | 6 | Fusong village |
| 6 | F-GS | 22 | Yanjiang village | 26 | C-GS | 6 | N |
| 7 | F-GS | 17 | Yanjiang village | 27 | F-GS | 9 | N |
| 8 | F-GS | 12 | Yanjiang village | 28 | F-GS | 9 | N |
| 9 | F-GS | 16 | Yanjiang village | 29 | F-GS | 9 | N |
| 10 | F-GS | 15 | Donggang village | 30 | F-GS | 13 | N |
| 11 | F-GS | 8 | Donggang village | 31 | F-GS | 11 | N |
| 12 | F-GS | 9 | Ningkuandian county | 32 | F-GS | 11 | N |
| 13 | F-GS | 6 | Ningkuandian county | 33 | F-GS | 8 | N |
| 14 | F-GS | 13 | Xintunzi village | 34 | F-GS | 15 | N |
| 15 | F-GS | 17 | Xintunzi village | 35 | F-GS | 15 | N |
| 16 | F-GS | 17 | Xintunzi village | 36 | C-GS | N | Jilin province |
| 17 | F-GS | 17 | Yanjiang village | 37 | C-GS | N | Jilin province |
| 18 | F-GS | 25 | Yanjiang village | 38 | C-GS | N | Ji’an city |
| 19 | W-GS 2 | ~50 3 | N 4 | 39 | C-GS | N | N |
| 20 | W-GS | ~100 3 | N | 40 | C-GS | 6 | Fusong village |
4.3. Semi-Micro Sample Preparation Method for Ginseng Sample Preparation
4.4. Chromatographic Conditions
4.5. HPLC-IT-TOF-MSn and “5-Point Screening” Method
4.6. “Quantitative Analysis of Multi-Components with Single Marker” (QAMS) Method
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, J.J.; Qi, F.H.; Wang, Z.X.; Zhang, Z.K.; Pan, N.; Huai, L.; Qu, S.Y.; Zhao, L. A review of traditional Chinese medicine for treatment of glioblastoma. Biosci. Trends 2019, 13, 476–487. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, Y. Current evaluation of the millennium phytomedicine- ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr. Med. Chem. 2009, 16, 2924–2942. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Ye, M.; Qiao, X.; Liu, C.F.; Miao, W.J.; Bo, T.; Tao, H.Y.; Guo, D.A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal. Chim. Acta 2012, 739, 56–66. [Google Scholar]
- Lai, C.J.S.; Tan, T.; Zeng, S.L.; Qi, L.W.; Liu, X.G.; Dong, X.; Li, P.; Liu, E.H. An integrated high resolution mass spectrometric data acquisition method for rapid screening of saponins in Panax notoginseng (Sanqi). J. Pharm. Biomed. Anal. 2015, 109, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C.; Hu, X.; Cai, G.Q.; Zhang, Y.H.; Deng, J.Y. Determination of seven ginsenosides in wild ginseng by RP-HPLC. Chin. Tradit. Patent Med. 2012, 34, 1954–1957. [Google Scholar]
- Xiao, X.Y.; Yin, J.F.; Zhang, N.P.; Lin, R.C. Study on the relation between duration of cultivation of plant and contents of the eight kinds of ginsenoside in the Panax ginseng by RP-HPLC. Chin. J. Pharm. Anal. 2004, 24, 238–244. [Google Scholar]
- Yamaguchi, H.; Matsuura, H.; Kasai, R.; Tanaka, O.; Satake, M.; Kohda, H.; Izumi, H.; Nuno, M.; Katsuki, S.; Isoda, S.; et al. Analysis of saponins of wild Panax ginseng. Chem. Pharm. Bull. 1988, 10, 4177–4181. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Li, K.X.; Shi, D.W.; Sun, Q.S. Study on characteristics and microscopic characteristics of wild ginseng, wild ginseng under forest, unimplanted ginseng, and cultivated ginseng. Chin. Tradit. Herb. Drugs 2013, 43, 2304–2307. [Google Scholar]
- Liu, D.; Li, Y.G.; Xu, H.; Sun, S.Q.; Wang, Z.T. Differentiation of the root of cultivated ginseng, mountain cultivated ginseng and mountain wild ginseng using FT-IR and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 2008, 883–884, 228–235. [Google Scholar] [CrossRef]
- Xu, X.F.; Cheng, X.L.; Lin, Q.H.; Li, S.S.; Jia, Z.; Han, T.; Lin, R.C.; Wang, D.; Wei, F.; Li, X.R. Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy. J. Ginseng. Res. 2016, 40, 344–350. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, H.; Tan, J.; Wang, C.; Wang, H.; Wu, F.; Dong, Q.; Liu, Y.; Li, P.; Liu, J. UPLC-QTOF/MS-based nontargeted metabolomic analysis of mountain- and garden-cultivated ginseng of different ages in northeast China. Molecules 2019, 24, 33. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, X.; Cai, S.Q.; Komatsu, K.; Namba, T. Analysis of the constituents in the Chinese drug notoginseng by liquid chromatography-electrospray mass spectrometry. J. Chin. Pharm. Sci. 2004, 13, 225–237. [Google Scholar]
- Wang, J.; Sha, Y.; Li, W.; Tezuka, Y.; Kadota, S.; Li, X. Quinquenoside L9 from leaves and stems of Panax quinquefolium L. J. Asian Nat. Prod. Res. 2001, 3, 293–297. [Google Scholar] [CrossRef]
- Tung, N.H.; Song, G.Y.; Minh, C.V.; Kiem, P.V.; Jin, L.G.; Boo, H.J.; Kang, H.K.; Kim, Y.H. Steamed ginseng-leaf components enhance cytotoxic effects on human leukemia HL-60 cells. Chem. Pharm. Bull. 2010, 58, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Tuoheti, T.; Rasheed, H.A.; Meng, L.; Dong, M.S. High hydrostatic pressure enhances the anti-proliferative properties of lotus bee pollen on the human prostate cancer PC-3 cells via increased metabolites. J. Ethnopharmacol. 2020, 261, 113057. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lv, J.J.; Xu, M.; Wang, D.; Zhu, H.T.; Yang, C.R.; Zhang, Y.J. Dammarane-type saponins from steamed leaves of Panax notoginseng. Nat. Prod. Bioprospect. 2011, 1, 124–128. [Google Scholar] [CrossRef]
- Cui, X.M.; Jiang, Z.Y.; Zeng, J.; Zhou, J.M.; Chen, J.J.; Zhang, X.M.; Xu, L.S.; Wang, Q. Two new dammarane triterpene glycosides from the rhizomes of Panax notoginseng. J. Asian Nat. Prod. Res. 2008, 10, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Lee, Y.H.; Kim, S.I. Ginsenoside Rf2, a new dammarane glycoside from Korean red ginseng (Panax ginseng). Arch. Pharm. Res. 1998, 21, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.J.; Hu, J.; Jia, L.; Zhang, C.X.; Yang, W.Z.; Zhang, P.; Guo, D.A. Profiling and identification of chemical components of Shenshao Tablet and its absorbed components in rats by comprehensive HPLC/DAD/ESI-MSn analysis. Chin. J. Nat. Med. 2018, 16, 791–800. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yang, Y.; Du, M.; Mao, S.; Chen, C.; Liu, Y.; Li, S. Rapid analysis of ginsenosides in dried fresh ginseng by ultra-performance liquid chromatography with quadrupole time-of-flight mass spectrometry. China Med. Her. 2015, 12, 130–136. [Google Scholar]
- Hong, C.; Yang, P.; Li, S.; Guo, Y.; Wang, D.; Wang, J. In vitro/in vivo metabolism of ginsenoside Rg5 in rat using ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. Molecules 2018, 23, 2113. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Jia, X.H.; Zhu, S.; Komatsu, K.; Wang, X.; Cai, S.Q. A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject. Talanta 2015, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.H.; Wang, C.Q.; Liu, J.H.; Li, X.W.; Wang, X.; Shang, M.Y.; Cai, S.Q.; Zhu, S.; Komatsu, K. Comparative studies of saponins in 1-3-year-old main roots, fibrous roots, and rhizomes of Panax notoginseng, and identification of different parts and growth-year samples. J. Nat. Med. 2013, 67, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, C.Q.; Xu, F.; Jia, X.H.; Liu, G.X.; Yang, S.C.; Long, G.Q.; Chen, Z.J.; Wei, F.G.; Yang, S.Z.; et al. A semimicroquality evaluation method on Panax notoginseng and its application in analysis of continuous cropping obstacles research samples. China J. Chin. Mater. Med. 2016, 41, 3773–3781. [Google Scholar]






| Number of Saponins | W-GS (nos. 20 and 19) | F-GS (no. 18) | C-GS (no. 25) | Total 1 |
|---|---|---|---|---|
| Total identified in each ginseng type | 177 (161 in no. 20; 112 in no. 19) | 56 | 60 | 199 |
| First time found in each ginseng type | 162 | 5 | 9 | 184 |
| First time found in ginseng 2 | 54 (45 in no. 20; 41 in no.19) | 5 | 9 | 62 |
| Potential new compounds | 11 (7 in no. 20; 10 in no. 19) | 1 | 6 | 16 |
| Rhamnosides identified | 41 (39 in no. 20; 29 in no. 19) | 8 | 11 | 48 |
| Xylosides identified | 41 (39 in no. 20; 27 in no. 19) | 17 | 16 | 42 |
| Sample no. | Rg1 | Re | Rf | Rb1 | Rg2 | Rh1 | Rd | Total |
|---|---|---|---|---|---|---|---|---|
| Wild Ginseng (W-GS, n = 2) | ||||||||
| 19 | 7.43 | 13.91 | 1.03 | 6.12 | 1.64 | 7.46 | 12.94 | 50.52 |
| 20 | 6.58 | 12.36 | 1.61 | 12.34 | 1.99 | 7.65 | 12.98 | 55.51 |
| Ginseng Under Forest (F-GS, n = 27) | ||||||||
| 1 | 1.00 | 1.78 | 0.29 | 1.88 | 0.32 | 0.84 | 0.52 | 6.63 |
| 2 | 1.49 | 1.85 | 0.39 | 1.95 | 1.36 | 0.73 | 0.66 | 8.44 |
| 3 | 1.58 | 1.22 | 0.39 | 2.11 | 0.10 | 0.93 | 0.96 | 7.29 |
| 4 | 5.20 | 2.41 | 1.03 | 5.24 | 2.67 | 1.10 | 0.64 | 18.30 |
| 5 | 4.20 | 2.91 | 0.90 | 5.30 | 2.71 | 0.82 | 1.04 | 17.88 |
| 6 | 2.46 | 2.30 | 0.53 | 3.29 | 0.27 | 1.43 | 0.75 | 11.04 |
| 7 | 4.24 | 2.45 | 0.78 | 4.41 | 2.26 | 0.83 | 0.80 | 15.77 |
| 8 | 2.57 | 2.43 | 0.83 | 6.08 | 2.95 | 1.45 | 0.84 | 17.16 |
| 9 | 3.50 | 2.57 | 0.75 | 4.39 | 2.37 | 1.49 | 0.90 | 15.97 |
| 10 | 3.08 | 3.42 | 0.87 | 4.36 | 2.46 | 1.26 | 0.77 | 16.22 |
| 11 | 2.14 | 2.42 | 0.55 | 3.71 | 0.29 | 1.16 | 0.76 | 11.02 |
| 12 | 1.26 | 1.62 | 0.34 | 1.72 | 0.88 | 0.28 | 0.19 | 6.31 |
| 13 | 2.09 | 2.43 | 0.51 | 3.37 | 1.22 | 0.64 | 0.31 | 10.57 |
| 14 | 1.01 | 1.49 | 0.37 | 1.24 | 0.65 | 0.25 | 0.68 | 5.69 |
| 15 | 1.84 | 2.71 | 0.49 | 2.52 | 1.31 | 0.83 | 0.67 | 10.36 |
| 16 | 2.09 | 2.69 | 0.49 | 3.57 | 1.89 | 0.55 | 0.52 | 11.80 |
| 17 | 2.99 | 1.90 | 0.57 | 2.53 | 1.85 | 0.51 | 0.56 | 10.89 |
| 18 | 4.48 | 2.92 | 0.79 | 5.39 | 3.36 | 1.35 | 0.45 | 18.74 |
| 27 | 2.16 | 2.22 | 0.57 | 4.64 | 1.22 | 0.20 | 1.00 | 12.01 |
| 28 | 2.56 | 3.26 | 0.66 | 3.80 | 1.30 | 0.26 | 0.92 | 12.76 |
| 29 | 1.61 | 2.78 | 0.44 | 3.33 | 0.29 | 1.19 | 0.67 | 10.31 |
| 30 | 3.01 | 2.28 | 0.76 | 6.99 | 0.28 | 1.22 | 0.90 | 15.44 |
| 31 | 2.91 | 2.26 | 0.77 | 3.25 | 0.95 | 0.28 | 0.91 | 11.32 |
| 32 | 4.59 | 2.54 | 0.79 | 7.57 | 1.23 | 0.38 | 0.77 | 17.86 |
| 33 | 6.22 | 2.22 | 1.19 | 4.51 | 0.62 | 0.37 | 0.70 | 15.83 |
| 34 | 2.49 | 2.18 | 0.81 | 2.03 | 0.95 | 0.15 | 0.71 | 9.31 |
| 35 | 4.11 | 3.50 | 0.74 | 5.39 | 2.02 | 0.51 | 1.49 | 17.76 |
| Cultivated Ginseng (C-GS, n = 11) | ||||||||
| 21 | 0.47 | 0.96 | 0.22 | 0.57 | 0.11 | 0.25 | 0.41 | 2.99 |
| 22 | 0.56 | 0.43 | 0.16 | 0.45 | 2.32 | 0.19 | 0.36 | 4.46 |
| 23 | 0.84 | 0.67 | 0.23 | 0.76 | 0.09 | 0.16 | 0.40 | 3.14 |
| 24 | 0.55 | 1.13 | 0.25 | 0.78 | 0.13 | 0.26 | 0.56 | 3.66 |
| 25 | 4.23 | 3.45 | 0.95 | 6.13 | 0.51 | 3.42 | 0.86 | 19.55 |
| 26 | 2.68 | 2.91 | 0.51 | 3.38 | 2.02 | 1.73 | 1.68 | 14.92 |
| 36 | 2.82 | 1.89 | 0.63 | 4.36 | 3.96 | 0.83 | 1.12 | 15.62 |
| 37 | 2.90 | 1.98 | 0.54 | 5.11 | 4.21 | 0.89 | 1.16 | 16.79 |
| 38 | 0.33 | 0.30 | 0.14 | 0.89 | 0.14 | 1.23 | 0.48 | 3.50 |
| 39 | 2.68 | 1.47 | 0.69 | 2.58 | 0.78 | 2.08 | 0.52 | 10.80 |
| 40 | 1.41 | 0.96 | 0.28 | 1.23 | 0.11 | 0.05 | 0.17 | 4.22 |
| Summary of Content | Rg1 | Re | Rf | Rb1 | Rg2 | Rh1 | Rd | Total |
|---|---|---|---|---|---|---|---|---|
| Wild Ginseng (W-GS, n = 2) | ||||||||
| Range | 6.58–7.43 | 12.36–13.91 | 1.03–1.61 | 6.12–12.34 | 1.64–1.99 | 7.46–7.65 | 12.94–12.98 | 50.52–55.51 |
| Ginseng Under Forest (F-GS, n = 27) | ||||||||
| Range | 1.00–6.22 | 1.22–3.50 | 0.29–1.19 | 1.24–7.57 | 0.10–3.36 | 0.15–1.49 | 0.19–1.49 | 5.69–18.74 |
| Mean ± SD | 2.85 ± 1.33 | 2.40 ± 0.55 | 0.65 ± 0.22 | 3.87 ± 1.63 | 1.40 ± 0.94 | 0.78 ± 0.43 | 0.74 ± 0.25 | 14.21 ± 10.85 |
| Median | 2.56 | 2.42 | 0.66 | 3.71 | 1.23 | 0.82 | 0.75 | 11.80 |
| Cultivated Ginseng (C-GS, n = 11) | ||||||||
| Range | 0.33–4.23 | 0.30–3.45 | 0.14–0.95 | 0.45–6.13 | 0.09–4.21 | 0.05–3.42 | 0.17–1.68 | 2.99–19.55 |
| Mean ± SD | 1.77 ± 1.34 | 1.47 ± 1.01 | 0.42 ± 0.26 | 2.39 ± 2.06 | 1.31 ± 1.58 | 1.01 ± 1.05 | 0.70 ± 0.45 | 9.06 ± 6.53 |
| Median | 1.41 | 1.13 | 0.28 | 1.23 | 0.51 | 0.83 | 0.52 | 4.46 |
| Summary of Content | Rg1 | Re | Rf | Rb1 | Rg2 | Rh1 | Rd | Total |
|---|---|---|---|---|---|---|---|---|
| Ginseng Under Forest ≥ 15 years old (n = 10) | ||||||||
| Range | 1.84–4.48 | 1.90–3.50 | 0.49–0.87 | 2.03–5.39 | 0.27–3.36 | 0.15–1.49 | 0.45–1.49 | 9.31–18.74 |
| Mean ± SD | 3.13 ± 0.93 | 2.66 ± 0.51 | 0.68 ± 0.14 | 3.79 ± 1.19 | 1.87 ± 0.86 | 0.89 ± 0.47 | 0.76 ± 0.29 | 13.79 ± 3.44 |
| Median | 3.04 | 2.63 | 0.74 | 3.96 | 1.96 | 0.83 | 0.73 | 13.78 |
| Ginseng Under Forest and Cultivated Ginseng < 15 years old (n = 24) | ||||||||
| Range | 0.47–6.22 | 0.43–3.45 | 0.16–1.19 | 0.45–7.57 | 0.09–2.95 | 0.05–3.42 | 0.17–1.68 | 2.99–19.55 |
| Mean ± SD | 2.34 ± 1.56 | 2.03 ± 0.82 | 0.56 ± 0.29 | 3.33 ± 2.12 | 1.01 ± 0.91 | 0.76 ± 0.74 | 0.70 ± 0.33 | 10.74 ± 5.43 |
| Median | 2.12 | 2.24 | 0.51 | 3.35 | 0.76 | 0.51 | 0.69 | 10.80 |
| Ginseng Under Forest and Cultivated Ginseng between 10 to 15 years old (n = 13) | ||||||||
| Range | 1.00–5.10 | 1.22–3.50 | 0.29–1.03 | 1.24–7.57 | 0.10–2.95 | 0.15–1.45 | 0.52–1.49 | 5.69–18.30 |
| Mean ± SD | 2.86 ± 1.37 | 2.33 ± 0.67 | 0.69 ± 0.24 | 4.10 ± 2.15 | 1.43 ± 1.01 | 0.76 ± 0.42 | 0.83 ± 0.24 | 13.02 ± 4.96 |
| Median | 2.91 | 2.28 | 0.77 | 4.36 | 1.23 | 0.82 | 0.77 | 15.44 |
| Ginseng Under Forest ≥ 10 years old (n = 20) | ||||||||
| Range | 1.00–5.20 | 1.22–3.50 | 0.29–1.03 | 1.24–7.57 | 0.10–3.36 | 0.15–1.49 | 0.45–1.49 | 5.69–18.74 |
| Mean ± SD | 2.94 ± 1.24 | 2.39 ± 0.57 | 0.67 ± 0.21 | 3.97 ± 1.82 | 1.60 ± 1.00 | 0.84 ± 0.43 | 0.78 ± 0.23 | 13.19 ± 4.36 |
| Median | 2.95 | 2.42 | 0.76 | 3.96 | 1.60 | 0.83 | 0.76 | 13.62 |
| Ginseng Under Forest and Cultivated Ginseng < 10 years old (n = 14) | ||||||||
| Range | 0.47–6.22 | 0.43–3.45 | 0.16–1.19 | 0.45–6.13 | 0.09–2.32 | 0.05–3.42 | 0.17–1.68 | 2.99–19.55 |
| Mean ± SD | 2.06 ± 1.58 | 1.96 ± 0.99 | 0.49 ± 0.29 | 2.74 ± 1.81 | 0.79 ± 0.73 | 0.72 ± 0.92 | 0.64 ± 0.40 | 9.41 ± 5.34 |
| Median | 1.85 | 2.22 | 0.48 | 3.35 | 0.56 | 0.27 | 0.62 | 10.44 |
| Contents—Ages | Rg1 | Re | Rf | Rb1 | Rg2 | Rh1 | Rd | Total |
|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | 0.33 | 0.37 | 0.30 | 0.29 | 0.45 | 0.15 | 0.09 | 0.38 |
| p-Value | 0.0550 | 0.0297 | 0.0795 | 0.0986 | 0.0082 | 0.4108 | 0.6130 | 0.0247 |
| cRT (min) 1 | Correlation Coefficient (p-Value) | Identification | ||
|---|---|---|---|---|
| Wild Ginseng no. 19 | Wild Ginseng no. 20 | |||
| 1 | 15.682 | 0.4572 (0.0030) | 0.4510 (0.0046) | (B4-b)-glc-xyl 2 |
| 2 | 16.576 | 0.4096 (0.0092) | 0.4389 (0.00461) | (B3-b)-glc-rha 2 |
| 3 | 17.491 | 0.3398 (0.0319) | NC 4 | Ginsenoside Re4 or its isomer 2 |
| 4 | 32.061 | 0.6414 (<0.0001) | 06259 (0.00002) | Ginsenoside Rg1 3 |
| 5 | 34.474 | 0.6805 (<0.0001) | 0.5963 (0.00005) | Ginsenoside Re 3 |
| 6 | 47.367 | 0.6188 (<0.0001) | 0.6438 (<0.0001) | Ginsenoside Rf 3 |
| 7 | 47.630 | 0.5426 (0.0003) | 0.4938 (0.0012) | Notoginsenoside Fc 2 |
| 8 | 48.778 | 0.6295 (<0.0001) | 0.7030 (<0.0001) | Ginsenoside Rb1 3 |
| 9 | 50.122 | 0.6753 (<0.0001) | 0.6209 (<0.0001) | Ginsenoside F3 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Q.; Yi, L.-W.; Zhao, L.; Zhou, Y.-Z.; Guo, F.; Huo, Y.-S.; Zhao, D.-Q.; Xu, F.; Wang, X.; Cai, S.-Q. 177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MSn, and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng. Molecules 2021, 26, 3371. https://doi.org/10.3390/molecules26113371
Wang C-Q, Yi L-W, Zhao L, Zhou Y-Z, Guo F, Huo Y-S, Zhao D-Q, Xu F, Wang X, Cai S-Q. 177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MSn, and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng. Molecules. 2021; 26(11):3371. https://doi.org/10.3390/molecules26113371
Chicago/Turabian StyleWang, Chao-Qun, Li-Wei Yi, Lin Zhao, Yu-Zhen Zhou, Fang Guo, Yu-Shu Huo, Da-Qing Zhao, Feng Xu, Xuan Wang, and Shao-Qing Cai. 2021. "177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MSn, and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng" Molecules 26, no. 11: 3371. https://doi.org/10.3390/molecules26113371
APA StyleWang, C.-Q., Yi, L.-W., Zhao, L., Zhou, Y.-Z., Guo, F., Huo, Y.-S., Zhao, D.-Q., Xu, F., Wang, X., & Cai, S.-Q. (2021). 177 Saponins, Including 11 New Compounds in Wild Ginseng Tentatively Identified via HPLC-IT-TOF-MSn, and Differences among Wild Ginseng, Ginseng under Forest, and Cultivated Ginseng. Molecules, 26(11), 3371. https://doi.org/10.3390/molecules26113371





