Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity
Abstract
1. Introduction
2. Results
2.1. Essential Oil Extraction
2.2. Essential Oil Composition
2.3. Antioxidant Capacity
3. Discussion
3.1. Essential Oil Extraction
3.2. Essential Oil Composition
3.3. Antioxidant Capacity
4. Materials and Methods
4.1. Plant Material Collection and Preparation
4.2. Essential Oil Extraction
4.3. Essential Oil Analysis
4.4. Antioxidant Activity
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- LUCAS. Land Use and Cover Area frame Survey. EUROSTAT. 2018. Available online: https://ec.europa.eu/eurostat/web/lucas/data/primary-data (accessed on 25 March 2021).
- Bongers, F.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.; Villar, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Rundel, P.; Arroyo, M.; Cowling, R.; Keeley, J.; Lamont, B.; Pausas, J.; Vargas, P. Fire and Plant Diversification in Mediterranean-Climate Regions. Front. Plant Sci. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Francos, M.; Úbeda, X.; Pereira, P.; Alcañiz, M. Long-term impact of wildfire on soils exposed to different fire severities. A case study in Cadiretes Massif (NE Iberian Peninsula). Sci. Total Environ. 2018, 615, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C. Human health impacts of forest fires in the Southern United States: A literature review. J. Ecol. Antropol. 2003, 7, 39–59. [Google Scholar] [CrossRef]
- Liu, Z.; Wimberly, M. Direct and indirect effects of climate change on projected future fire regimes in the western United States. Sci. Total Environ. 2016, 542, 65–75. [Google Scholar] [CrossRef]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Verkerk, P.; de Arano, I.; Palahi, M. The bio-economy as an opportunity to tackle wildfires in Mediterranean forest ecosystems. For. Pol. Econ. 2018, 86, 1–3. [Google Scholar] [CrossRef]
- Nazzaro, F.; De Martino, L.; Fratianni, F.; De Feo, V. Essential oils from Mediterranean aromatic plants. In The Mediterranean Diet. An Evidence-Based Approach, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier: London, UK, 2020; Volume 49, pp. 555–564. [Google Scholar]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Sant′Ana, A.S.; Dominguez, R.; Rodriguez-Lazaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- GBIF. GBIF Secretariat (2019). GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://www.gbif.org/es/species/6 (accessed on 26 October 2020).
- MFE25. Mapa Forestal de España. MAPAMA. Dirección General de Desarrollo Rural. 2018. Available online: https://www.miteco.gob.es/es/cartografia-y-sig/ide/descargas/biodiversidad/mfe.aspx (accessed on 25 March 2021).
- MFE200. Mapa Forestal de España. Dirección General de Medio Natural y Política Forestal. Ministerio de Medio Ambiente, y Medio Rural y Marino. 1997. Available online: https://www.miteco.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/mfe200_descargas.aspx/ (accessed on 25 March 2021).
- Hernández, M.; Sotomayor, J.; Jordán, M. Rosemary (Rosmarinus officinalis L.) oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V., Ed.; Elsevier: London, UK, 2016. [Google Scholar]
- Jeronimo, E.; Cachucho, L.; Soldado, D.; Guerreiro, O.; Bessa, R.; Alves, S. Fatty Acid Content and Composition of the Morphological Fractions of Cistus Ladanifer L. and Its Seasonal Variation. Molecules 2020, 25, 1550. [Google Scholar] [CrossRef] [PubMed]
- Orav, A.; Koel, M.; Kailas, T.; Muurisepp, M.; Kaljurand, M. Comparative analysis of the composition of essential oils and supercritical carbon dioxide extracts from the berries and needles of Estonian juniper (Juniperus communis L.). Procedia Chem. 2010, 2, 161–167. [Google Scholar] [CrossRef]
- Said, Z.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Rigou, P.; Remini, H.; Adjaoud, A.; Khoudja, N.; Madani, K. Essential oils composition, antibacterial and antioxidant activities of hydrodistillated extract of Eucalyptus globulus fruits. Ind. Crops. Prod. 2016, 89, 167–175. [Google Scholar] [CrossRef]
- Emami, A.; Javadi, B.; Hassanzadeh, M. Antioxidant activity of the essential oils of different parts of Juniperus communis subsp hemisphaerica and Juniperus oblonga. Pharm Biol. 2007, 45, 769–776. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef]
- Arnal-Schnebelen, B.; Hadji-Minaglow, F.; Peroteau, J.; Ribeyre, F.; de Billerbeck, V. Essential oils in infectious gynaecological disease: A statistical study of 658 cases. Int. J. Aromather. 2004, 14, 192–197. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.; Boira, H. Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Nat. Prod. Res. 2012, 26, 1602–1609. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing. Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops. Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Ind. Crops. Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Daghbouche, S.; Ammar, I.; Rekik, D.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of phenological stages on essential oil composition of Cytisus triflorus L’Her. J. King Saud Univ. Sci. 2020, 32, 2383–2387. [Google Scholar] [CrossRef]
- Alipour, M.; Saharkhiz, M. Phytotoxic activity and variation in essential oil content and composition of Rosemary (Rosmarinus officinalis L.) during different phenological growth stages. Biocatal. Agric. Biotechnol. 2016, 7, 271–278. [Google Scholar] [CrossRef]
- Vaiciulyte, V.; Butkiene, R.; Loziene, K. Effects of meteorological conditions and plant growth stage on the accumulation of carvacrol and its precursors in Thymus pulegioides. Phytochemistry 2016, 128, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Usano-Alemany, J.; Pala-Paul, J.; Herraiz-Penalver, D. Essential oil yields and qualities of different clonal lines of Salvia lavandulifolia monitored in Spain over four years of cultivation. Ind. Crops. Prod. 2016, 80, 251–261. [Google Scholar] [CrossRef]
- Raal, A.; Kanut, M.; Orav, A. Annual Variation of Yield and Composition of the Essential Oil of Common Juniper (Juniperus communis L.) Branches from Estonia. Balt. For. 2010, 16, 50–56. [Google Scholar]
- Mendes, B.S.; Gonçalves, M.M.B.P. Composition and functional properties of essential oils extracted from forest waste biomass. Studia Suplemento temático 2013, 1–4. [Google Scholar]
- Gomes, P.; Mata, V.; Rodrigues, A. Characterization of the Portuguese-grown Cistus ladanifer essential oil. J. Essent. Oil Res. 2005, 17, 160–165. [Google Scholar] [CrossRef]
- Robles, C.; Bousquet-Melou, A.; Garzino, S.; Bonin, G. Comparison of essential oil composition of two varieties of Cistus ladanifer. Biochem. Syst. Ecol. 2003, 31, 339–343. [Google Scholar] [CrossRef]
- Teixeira, S.; Mendes, A.; Alves, A.; Santos, L. Simultaneous distillation-extraction of high-value volatile compounds from Cistus ladanifer L. Anal. Chim. Acta 2007, 584, 439–446. [Google Scholar] [CrossRef]
- Guimaraes, R.; Sousa, M.; Ferreira, I. Contribution of essential oils and phenolics to the antioxidant properties of aromatic plants. Ind. Crops Prod. 2010, 32, 152–156. [Google Scholar] [CrossRef]
- Tavares, C.; Martins, A.; Faleiro, M.; Miguel, M.; Duarte, L.; Gameiro, J.; Roseiro, L.; Figueiredo, A. Bioproducts from forest biomass: Essential oils and hydrolates from wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crops Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Boukhatem, M.; Amine, F.; Kameli, A.; Saidi, F.; Walid, K.; Mohamed, S. Quality assessment of the essential oil from Eucalyptus globulus Labill of Blida (Algeria) origin. ILCPA 2014, 36, 303–315. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Loran, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagan, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar Cuéllar, A.; Hussein Yunus, R. Evaluation of the yield and the antimicrobial activity of the essential oils from: Eucalyptus globulus, Cymbopogon citratus and Rosmarinus officinalis in Mbarara district (Uganda). Rev. Colomb. Cienc. Anim. 2009, 1, 240–249. [Google Scholar] [CrossRef][Green Version]
- Dagne, E.; Bisrat, D.; Alemayehu, M.; Worku, T. Essential oils of twelve Eucalyptus species from Ethiopia. J. Essent. Oil Res. 2000, 12, 467–470. [Google Scholar] [CrossRef]
- Li, H.; Madden, J. Analysis of leaf oils from a Eucalyptus species trial. Biochem. Syst. Ecol. 1995, 23, 167–177. [Google Scholar] [CrossRef]
- Zrira, S.; Benjilali, B. Seasonal changes in the volatile oil and cineole contents of five Eucalyptus species growing in Morocco. J. Essent. Oil Res. 1996, 8, 19–24. [Google Scholar] [CrossRef]
- Li, H.; Madden, J.; Davies, N. Variation in leaf oils of Eucalyptus nitens and E. Denticulata. Biochem. Syst. Ecol. 1994, 22, 631–640. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Russo, M.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Koundal, R.; Kumar, A.; Thakur, S.; Agnihotri, V.; Chand, G.; Singh, R. Seasonal variation in phytochemicals of essential oil from Juniperus communis needles in western Himalaya. J. Essent. Oil Res. 2015, 27, 406–411. [Google Scholar] [CrossRef]
- Rostaefar, A.; Hassani, A.; Sefidkon, F. Seasonal variations of essential oil content and composition in male and female plants of Juniperus communis L. ssp hemisphaerica growing wild in Iran. J. Essent. Oil Res. 2017, 29, 357–360. [Google Scholar] [CrossRef]
- Radoukova, T.; Zheljazkov, V.; Semerdjieva, I.; Dincheva, I.; Stoyanova, A.; Kacaniova, M.; Markovic, T.; Radanovic, D.; Astatkie, T.; Salamon, I. Differences in essential oil yield, composition, and bioactivity of three juniper species from Eastern Europe. Ind. Crops Prod. 2018, 124, 643–652. [Google Scholar] [CrossRef]
- Macchioni, F.; Cioni, P.; Flamini, G.; Morelli, I.; Maccioni, S.; Ansaldi, M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P-halepensis, P-pinaster and P. nigra from central Italy. Flavour. Fragr. J. 2003, 18, 139–143. [Google Scholar] [CrossRef]
- Rezzoug, S. Optimization of Steam Extraction of Oil from Maritime Pine Needles. J. Wood Chem. Technol. 2009, 29, 87–100. [Google Scholar] [CrossRef]
- Amri, I.; Hanana, M.; Gargouri, S.; Jamoussi, B.; Hamrouni, L. Comparative study of two coniferous species (Pinus pinaster Aiton and Cupressus sempervirens L. var. dupreziana [A. Camus] Silba) essential oils: Chemical composition and biological activity. Chil. J. Agric. Res. 2013, 73, 259–266. [Google Scholar]
- Mimoune, N.; Mimoune, D.; Yataghene, A. Chemical composition and antimicrobial activity of the essential oils of Pinus pinaster. J. Coast. Life Med. 2013, 1, 55–59. [Google Scholar]
- Tumen, I.; Hafizoglu, H.; Kilic, A.; Donmez, I.; Sivrikaya, H.; Reunanen, M. Yields and Constituents of Essential Oil from Cones of Pinaceae spp. Natively Grown in Turkey. Molecules 2010, 15, 5797–5806. [Google Scholar] [CrossRef]
- Judzentiene, A.; Kupcinskiene, E. Chemical composition on essential oils from needles of Pinus sylvestris L. grown in northern Lithuania. J. Essent. Oil Res. 2008, 20, 26–29. [Google Scholar] [CrossRef]
- Koukos, P.; Papadopoulou, K.; Papagiannopoulos, A.; Patiaka, D. Essential oils of the twigs of some conifers grown in Greece. Holz Als Roh-Und Werkstoff 2001, 58, 437–438. [Google Scholar] [CrossRef]
- Ustun, O.; Sezik, E.; Kurkcuoglu, M.; Baser, K. Study of the essential oil composition of Pinus sylvestris from Turkey. Chem. Nat. Compd. 2006, 42, 26–31. [Google Scholar] [CrossRef]
- Venskutonis, P.; Vyskupaityte, K.; Plausinaitis, R. Composition of essential oils of Pinus sylvestris L. from different locations of Lithuania. J. Essent. Oil Res. 2000, 12, 559–565. [Google Scholar] [CrossRef]
- Labokas, J.; Loziene, K.; Jureviciute, R. Preconditions for industrial use of foliage as felling by-product of Scots pine for essential oil production. Ind. Crops Prod. 2017, 109, 542–547. [Google Scholar] [CrossRef]
- Varela, F.; Navarrete, P.; Cristobal, R.; Fanlo, M.; Melero, R.; Sotomayor, J.; Jordan, M.; Cabot, P.; de Ron, D.; Calvo, R.; et al. Variability in the chemical composition of wild Rosmarinus officinalis L. Int. Med. Aromat. Plants Conf. Culin. Herbs 2009, 826, 167–174. [Google Scholar] [CrossRef]
- Guillén, M.; Cabo, N.; Burillo, J. Characterisation of the essential oils of some cultivated aromatic plants of industrial interest. J. Sci. Food Agric. 1996, 70, 359–363. [Google Scholar] [CrossRef]
- Boutekedjiret, C.; Belabbes, R.; Bentahar, F.; Bressiere, J. Study of Rosmarinus officinalis L. essential oil yield and composition as a function of the plant life cycle. J. Essent. Oil Res. 1999, 11, 238–240. [Google Scholar] [CrossRef]
- Serralutzu, F.; Stangoni, A.; Amadou, B.; Tijan, D.; Re, G.; Marceddu, S.; Dore, A.; Bullitta, S. Essential oil composition and yield of a Rosmarinus officinalis L. natural population with an extended flowering season in a coastal Mediterranean environment and perspectives for exploitations. Genet. Resour. Crop Evol. 2020, 67, 1777–1793. [Google Scholar] [CrossRef]
- Hussain, A.; Anwar, F.; Chatha, S.; Jabbar, A.; Mahboob, S.; Nigam, P. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef]
- Atti-Santos, A.; Rossato, M.; Pauletti, G.; Rota, L.; Rech, J.; Pansera, M.; Agostini, F.; Serafini, L.; Moyne, P. Physico-chemical evaluation of Rosmarinus officinalis L. essential oils. Braz. Archz. Biol. Technol. 2005, 48, 1035–1039. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Tomás-Menor, L.; Morales-Soto, A.; Martí Bruñá, N.; Saura López, D.; Segura-Carretero, A.; Micol, V. Rockroses (Cistus sp.) oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 649–658. [Google Scholar]
- Frazao, D.; Raimundo, J.; Domingues, J.; Quintela-Sabaris, C.; Goncalves, J.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.; Fernandez-Lopez, J.; Amensour, M.; Abrini, J. Identification of flavonoid content and chemical composition of the essential oils of Moroccan herbs: Myrtle (Myrtus communis L.), rockrose (Cistus ladanifer L.) and Montpellier cistus (Cistus monspeliensis L.). J. Essent. Oil Res. 2011, 23, 1–9. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.; Hammani, K. Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladaniferus subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Barbosa, L.; Filomeno, C.; Teixeira, R. Chemical Variability and Biological Activities of Eucalyptus spp. Essential Oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [PubMed]
- Ložienė, K.; Venskutonis, P. Juniper (Juniperus communis L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 495–500. [Google Scholar]
- Adams, R.; Beauchamp, P.; Dev, V.; Bathala, R. The Leaf Essential Oils of Juniperus communis L. Varieties in North America and the NMR and MS Data for Isoabienol. J. Essent. Oil Res. 2010, 22, 23–28. [Google Scholar] [CrossRef]
- Hadaruga, N.; Branic, A.; Hadaruga, D.; Gruia, A.; Plesa, C.; Costescu, C.; Ardelean, A.; Lupea, A. Comparative study of Juniperus communis and Juniperus virginiana essential oils: TLC and GC analysis. JPC-J. Planar. Chromat. 2011, 24, 130–135. [Google Scholar] [CrossRef]
- Dob, T.; Berramdane, T.; Chelghoum, C. Analysis of essential oil from the needles of Pinus pinaster growing in Algeria. Chem. Nat. Compd. 2005, 41, 545–548. [Google Scholar] [CrossRef]
- Petrakis, P.; Tsitsimpikou, C.; Tzakou, O.; Couladis, M.; Vagias, C.; Roussis, V. Needle volatiles from five Pinus species growing in Greece. Flavour. Fragr. J. 2001, 16, 249–252. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Gardeli, C.; Mallouchos, A.; Papaioannou, M.; Komaitis, M. Variation of the chemical profile and antioxidant behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller grown in Greece. J. Agric. Food Chem. 2008, 56, 7254–7264. [Google Scholar] [CrossRef]
- Salido, S.; Altarejos, J.; Nogueras, M.; Sanchez, A.; Luque, P. Chemical composition and seasonal variations of rosemary oil from southern Spain. J. Essent. Oil Res. 2003, 15, 10–14. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Upadhyay, N.; Singh, V.; Dwivedy, A.; Das, S.; Chaudhari, A.; Dubey, N. Cistus ladanifer L. essential oil as a plant based preservative against molds infesting oil seeds, aflatoxin B 1 secretion, oxidative deterioration and methylglyoxal biosynthesis. LWT 2018, 92, 395–403. [Google Scholar] [CrossRef]
- Miguel, M.G.; Gago, C.; Antunes, M.D.; Lagoas, S.; Faleiro, M.L.; Megías, C.; Cortés-Giraldo, I.; Vioque, J.; Figueiredo, A.C. Antibacterial, antioxidant, and antiproliferative activities of Corymbia citriodora and the essential oils of eight Eucalyptus species. Medicines 2018, 5, 61. [Google Scholar] [CrossRef]
- Bentayeb, K.; Vera, P.; Rubio, C.; Nerín, C. The additive properties of Oxygen Radical Absorbance Capacity (ORAC) assay: The case of essential oils. Food Chem. 2014, 148, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Shibamoto, T. Antioxidant activities of volatile components isolated from Eucalyptus species. J. Sci. Food Agric. 2001, 81, 1573–1579. [Google Scholar] [CrossRef]
- Ben Marzoug, H.N.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Couderc, F.; Abderraba, M.; Romdhane, M. Eucalyptus (gracilis, oleosa, salubris, and salmonophloia) essential oils: Their chemical composition and antioxidant and antimicrobial activities. J. Med. Food 2010, 13, 1005–1012. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Gruľová, D.; Zheljazkov, V.Z.; De Feo, V.; Camele, I. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic activities. J. Appl. Microbiol. 2020, 129, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Kacaniova, M.; Dincheva, I.; Radoukova, T.; Semerdjieva, I.B.; Astatkie, T.; Schlegel, V. Essential oil composition, antioxidant and antimicrobial activity of the galbuli of six juniper species. Ind. Crops Prod. 2018, 124, 449–458. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovič, N.; Horská, E.; Salamon, I.; Bobková, A.; Hleba, L.; Fiskelová, M.; Vatľák, A.; Petrová, J.; Bobko, M. Antibacterial activity against Clostridium genus and antiradical activity of the essential oils from different origin. J. Environ. Sci. Health B 2014, 49, 505–512. [Google Scholar] [CrossRef]
- Tümen, İ.; Akkol, E.K.; Taştan, H.; Süntar, I.; Kurtca, M. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine (Pinus pinaster Ait). J. Ethnopharmacol. 2018, 211, 235–246. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour. Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Mezza, G.N.; Borgarello, A.V.; Grosso, N.R.; Fernandez, H.; Pramparo, M.C.; Gayol, M.F. Antioxidant activity of rosemary essential oil fractions obtained by molecular distillation and their effect on oxidative stability of sunflower oil. Food Chem. 2018, 242, 9–15. [Google Scholar] [CrossRef] [PubMed]
- ISO 18134–2:2017. Solid Biofuels–Determination of Moisture Content–Oven Dried Method–Part 2: Total Moisture–Simplified Method; European Commission: Brussels, Belgium.
- ISO 7609:1985. Analysis by Gas Chromatography on Capillary Columns–General Method; European Commission: Brussels, Belgium.
- Adams, R. Identification of Essential Oil Components by Gras Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IN, USA, 2007; p. 804. [Google Scholar]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
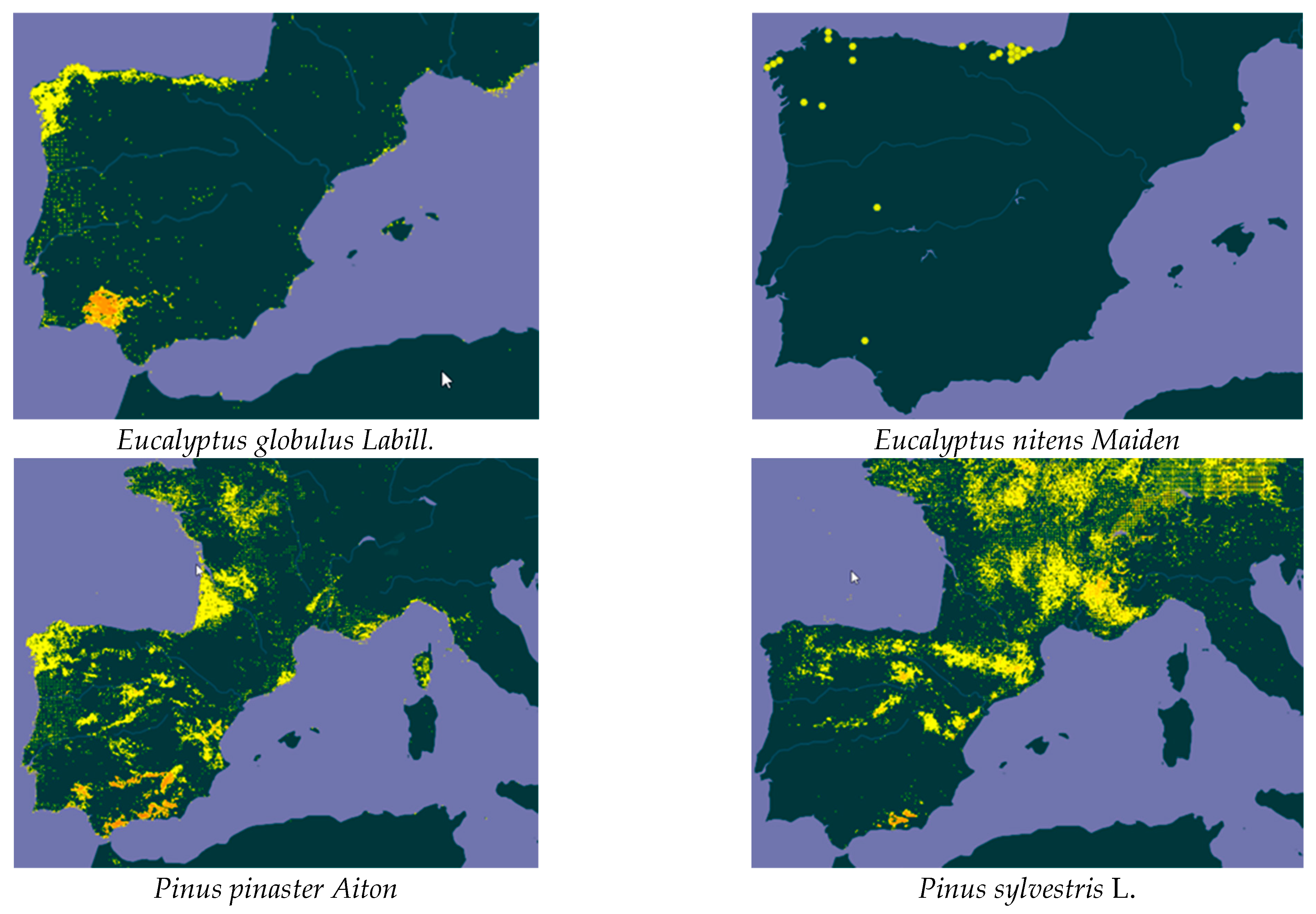
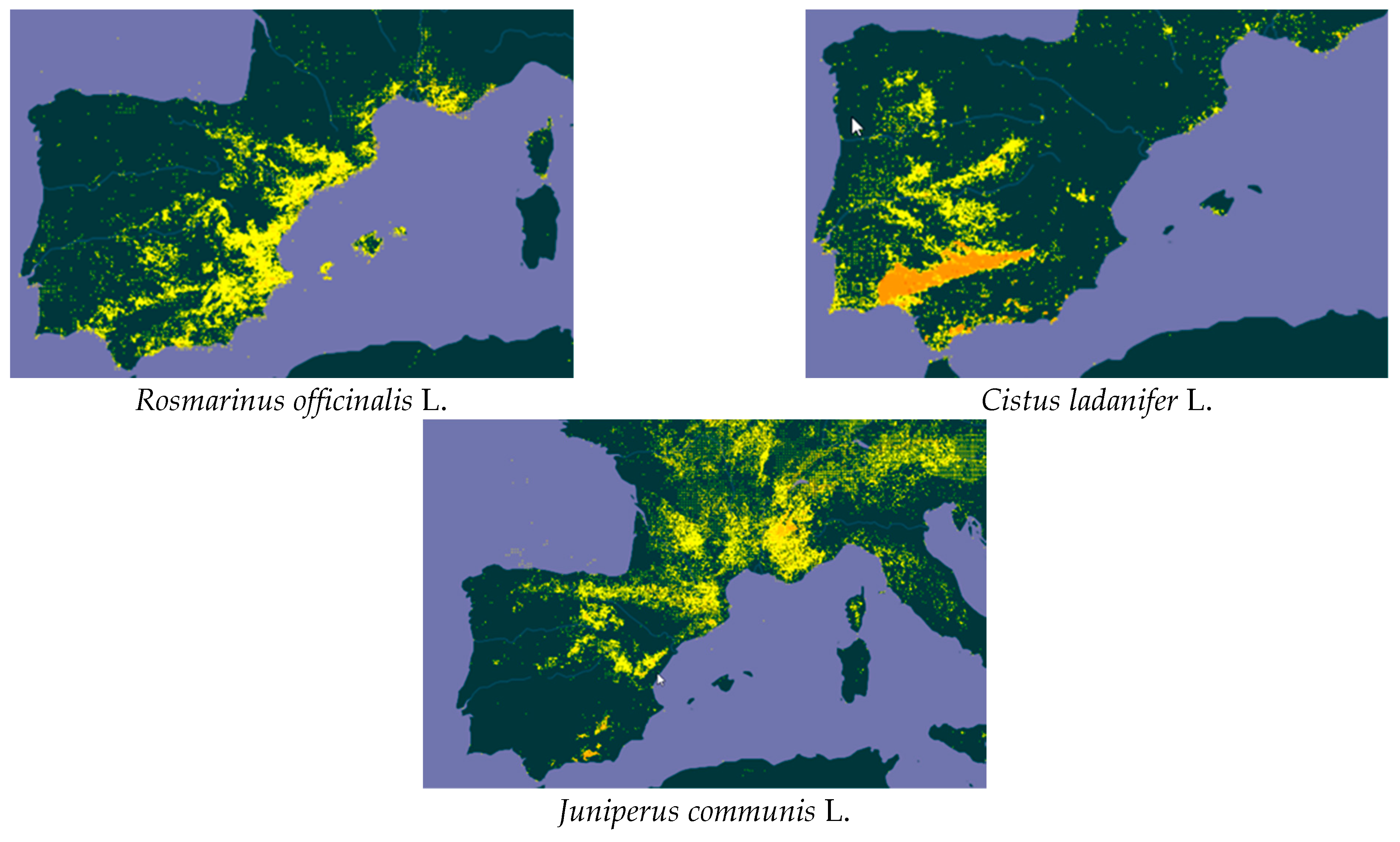
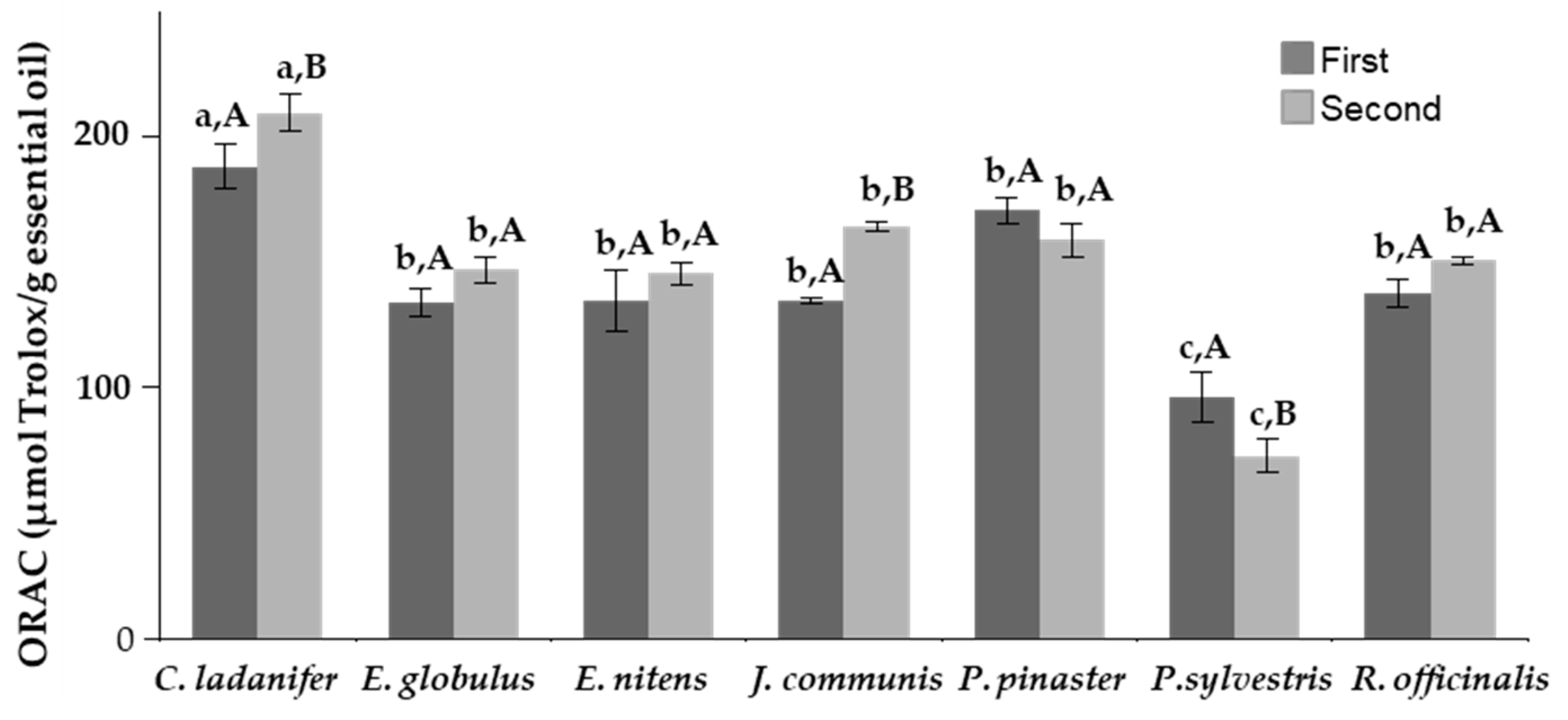
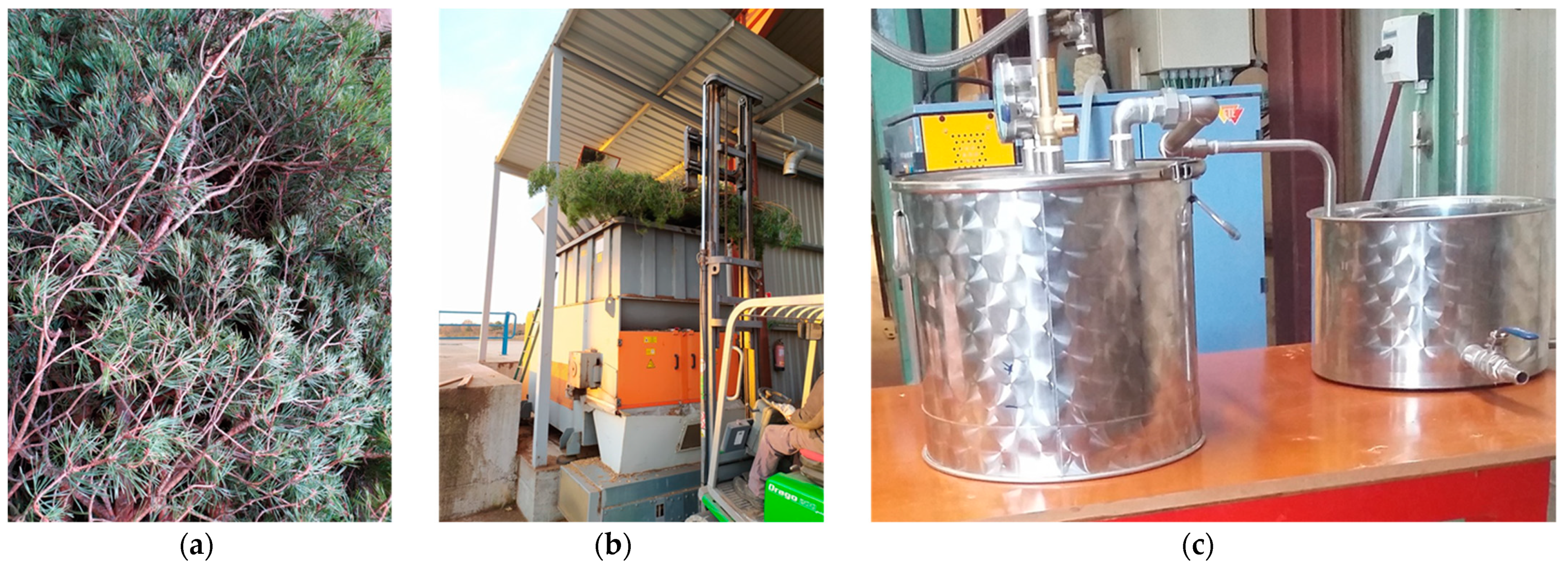
| Species | Collection Period | Moisture Content (%, w.m.) | Aver. Yield (%, d.b.) | Std. Dev. | p-Value of the F-Test |
|---|---|---|---|---|---|
| C. ladanifer | First | 20.2 | 0.036 | 0.0023 | 0.8652 |
| C. ladanifer | Second | 19.2 | 0.037 | 0.0093 | |
| E. globulus | First | 57.7 | 1.59 | 0.021 | 0.0039 |
| E. globulus | Second | 77.5 | 1.93 | 0.095 | |
| E. nitens | First | 45.5 | 0.45 | 0.0025 | 0.0001 |
| E. nitens | Second | 32.8 | 0.57 | 0.012 | |
| J. communis | First | 35.7 | 0.37 | 0.011 | 0.0115 |
| J. communis | Second | 39.2 | 0.31 | 0.020 | |
| P. pinaster | First | 42.4 | 0.22 | 0.0035 | 0.0001 |
| P. pinaster | Second | 36.4 | 0.17 | 0.0045 | |
| P. sylvestris | First | 44.3 | 0.15 | 0.0046 | 0.1079 |
| P. sylvestris | Second | 51.0 | 0.18 | 0.026 | |
| R. officinalis | First | 35.3 | 0.50 | 0.010 | 0.0028 |
| R. officinalis | Second | 32.6 | 0.44 | 0.015 |
| Component Groups | Relative Area (%) | ||||||
|---|---|---|---|---|---|---|---|
| C. lad. | E. glob. | E. nit. | J. com. | P. pin. | P. syl. | R. off. | |
| Monoterpene hydrocarbons (1) | 62.47 | 27.06 | 16.19 | 59.19 | 67.90 | 74.24 | 46.01 |
| Monoterpene hydrocarbons (2) | 49.46 | 25.18 | 14.90 | 79.58 | 60.57 | 84.84 | 37.75 |
| Oxygenated monoterpenes (1) | 14.22 | 52.19 | 79.58 | 3.39 | 0.75 | 0.80 | 43.41 |
| Oxygenated monoterpenes (2) | 14.35 | 68.85 | 80.46 | 8.46 | 1.27 | 0.48 | 53.95 |
| Sesquiterpene hydrocarbons (1) | 5.38 | 13.25 | 1.29 | 34.20 | 19.27 | 19.54 | 4.75 |
| Sesquiterpene hydrocarbons (2) | 6.99 | 1.75 | 1.17 | 8.67 | 33.53 | 12.67 | 4.12 |
| Oxygenated sesquiterpenes (1) | 9.47 | 3.55 | 0.40 | 1.65 | 0.97 | 0.91 | 0.03 |
| Oxygenated sesquiterpenes (2) | 15.35 | 2.65 | 0.54 | 0.95 | 0.91 | 0.48 | 0.33 |
| Others (1) | 2.77 | 0.42 | 1.30 | 0.52 | 9.88 | 0.12 | 4.17 |
| Others (2) | 3.35 | 0.25 | 1.61 | 0.71 | 2.73 | 0.72 | 2.98 |
| Total identified (1) | 94.31 | 96.47 | 98.76 | 98.95 | 98.77 | 95.61 | 98.37 |
| Total identified (2) | 89.50 | 98.68 | 98.68 | 98.37 | 99.01 | 99.19 | 99.13 |
| Species | Ref. | Collection Months | Origin | Plant Part | Distil. Method | Distil. Time (h) | Sample Weight | Yield (%w/w d.b.) | Yield (%v/w d.b.) |
|---|---|---|---|---|---|---|---|---|---|
| C. lad. | [34] | n.a. | Portugal | twigs | SD | n.a. | 50 g | 0.33 | - |
| [35] | July–August | Portugal | twigs | HD Clev | 2 | 20 g | 0.25 | - | |
| [36] | May | France | twigs | SD | 2 | 100 g | 0.10 | - | |
| [37] | July | Portugal | twigs | SDE | 1 | 15 g | 0.15 | - | |
| [38] | July | Portugal | leaves | HD Clev | 3 | 100 g | 0.63 | - | |
| [39] | March | Portugal | twigs | SD | 1.5 | 100 kg | - | 0.01 | |
| [39] | August | Portugal | twigs | SD | 1.5 | 100 kg | - | 0.04 | |
| [39] | August | Portugal | twigs | HD | 3 | 270 g | - | 0.15 | |
| E. glob. | [40] | n.a. | Algeria | twigs | SD | n.a. | 600 kg | 0.5 | - |
| [41] | n.a. | Morocco | leaves | HD Clev | 2 | n.a. | 2.7 | - | |
| [42] | March | Uganda | leaves | HD Clev | n.a. | n.a. | - | 0.2 | |
| [43] | n.a. | Ethiopia | leaves | HD Clev | 3 | n.a. | 1.1 | - | |
| [44] | March | Australia | leaves | Glass dist | 6 | 100 g | 3.9 | - | |
| [45] | n.a. | Morocco | leaves | SD | n.a. | n.a. | 2.3 | - | |
| E. nit. | [43] | n.a. | Ethiopia | leaves | HD Clev | 3 | n.a. | 1.4 | - |
| [44] | March | Australia | leaves | Glass dist | 6 | 100 g | 0.75 | - | |
| [46] | November | Australia | leaves | Glass dist | 6 | 150 g | 0.7–1.5 | - | |
| J. com. | [17] | February | Estonia | leaves | SDE | 2 | 10 g | 0.70 | - |
| [47] | October–December | Sardinia | leaves | SD Clev | n.a. | 100 g | - | 0.19 | |
| [33] | May–April | Estonia | branches | HD Clev | 1.5 | 20 g | 0.05–0.70 | - | |
| [48] | April–December | India | leaves | SD Clev | 3 | 1000 g | 0.70 | - | |
| [49] | May–November | Iran | twigs | HD Clev | 3.5 | 30 g | - | 2.43 | |
| [50] | Summer | Bulgaria | leaves | HD Clev | 2 | 50 g | - | 0.30–0.60 | |
| [50] | Summer | Serbia | leaves | HD Clev | 2 | 50 g | - | 0.53–0.86 | |
| P. pin. | [51] | February | Italy | twigs | HD Clev | 2 | n.a. | 0.18 | - |
| [52] | n.a. | France | leaves | HD Clev | 2 | 50 g | 0.82 | - | |
| [53] | August | Tunisia | needles | HD Clev | 3 | 100 g | 0.4 | - | |
| [54] | March | Algeria | needles | HD Clev | 4 | 100 g | 0.61 | - | |
| P. syl. | [55] | September | Turkey | cones | HD Clev | 3 | 100 g | 0.13 | |
| [56] | July | Lithuania | leaves | HD Clev | 2 | 50 g | 0.25 | - | |
| [57] | April | Greece | twigs | HD | 3 | 80 g | 0.52 | - | |
| [58] | n.a. | Turkey | leaves | HD Clev | 3 | n.a. | 0.22–0.82 | - | |
| [59] | July | Lithuania | leaves | HD Clev | 2 | 20 g | - | 1 | |
| [60] | January | Lithuania | leaves | HD Clev | 2 | 70 g | 0.43–0.64 | - | |
| R. off. | [42] | March | Uganda | leaves | HD Clev | n.a. | n.a. | - | 1 |
| [61] | n.a. | Spain | twigs | HD Clev | 2 | 100 g | 1.88 | - | |
| [62] | n.a. | Spain | twigs | SD | n.a. | n.a. | 1.1 | - | |
| [41] | n.a. | Morocco | leaves | HD Clev | 2 | n.a. | 1.8 | - | |
| [63] | October–May | Algeria | twigs | HD | n.a. | n.a. | 0.64–1.07 | - | |
| [64] | January–December | Italy | twigs | SD | n.a. | 900 g | 0.055–0.77 | - | |
| [65] | November–December | Pakistan | leaves | HD Clev | 3 | n.a. | 0.93 | - | |
| [66] | n.a. | Brazil | twigs | SD | 1.5 | 60–310 kg | - | 0.37–0.49 |
| Species | Coordinates (WGS84 Google) | 1st Collection Date | 2nd Collection Date |
|---|---|---|---|
| Cistus ladanifer | X: −2.979643 Y: 41.09917 | September 2018 | October 2019 |
| Eucalyptus globulus | X: −5.31016776 Y: 43.5112467 | July 2018 | June 2020 |
| Eucalyptus nitens | X: −7.3083651 Y: 41.9822278 | July 2018 | April 2020 |
| Juniperus communis | X: −2.463912 Y: 42.004695 | May 2018 | June 2020 |
| Pinus pinaster | X: −2.490868 Y: 41.601647 | July 2018 | April 2020 |
| Pinus sylvestris | X: −2.478987 Y: 42.033522 | July 2018 | April 2020 |
| Rosmarinus officinalis | X: −1.988602 Y: 41.562386 | July 2018 | April 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity. Molecules 2021, 26, 3257. https://doi.org/10.3390/molecules26113257
Mediavilla I, Guillamón E, Ruiz A, Esteban LS. Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity. Molecules. 2021; 26(11):3257. https://doi.org/10.3390/molecules26113257
Chicago/Turabian StyleMediavilla, Irene, Eva Guillamón, Alex Ruiz, and Luis Saúl Esteban. 2021. "Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity" Molecules 26, no. 11: 3257. https://doi.org/10.3390/molecules26113257
APA StyleMediavilla, I., Guillamón, E., Ruiz, A., & Esteban, L. S. (2021). Essential Oils from Residual Foliage of Forest Tree and Shrub Species: Yield and Antioxidant Capacity. Molecules, 26(11), 3257. https://doi.org/10.3390/molecules26113257






