Highly Sensitive and Selective Fluorescence “Turn-On” Detection of Pb (II) Based on Fe3O4@Au–FITC Nanocomposite
Abstract
1. Introduction
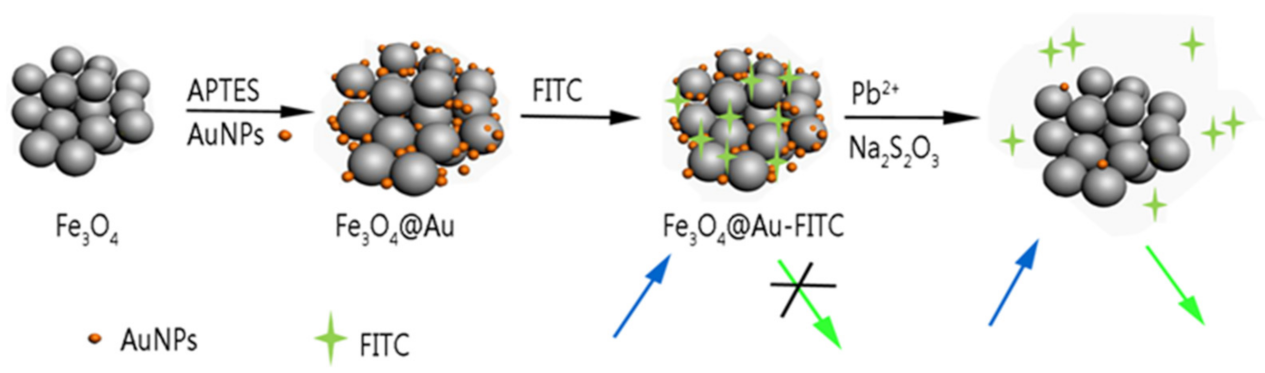
2. Results
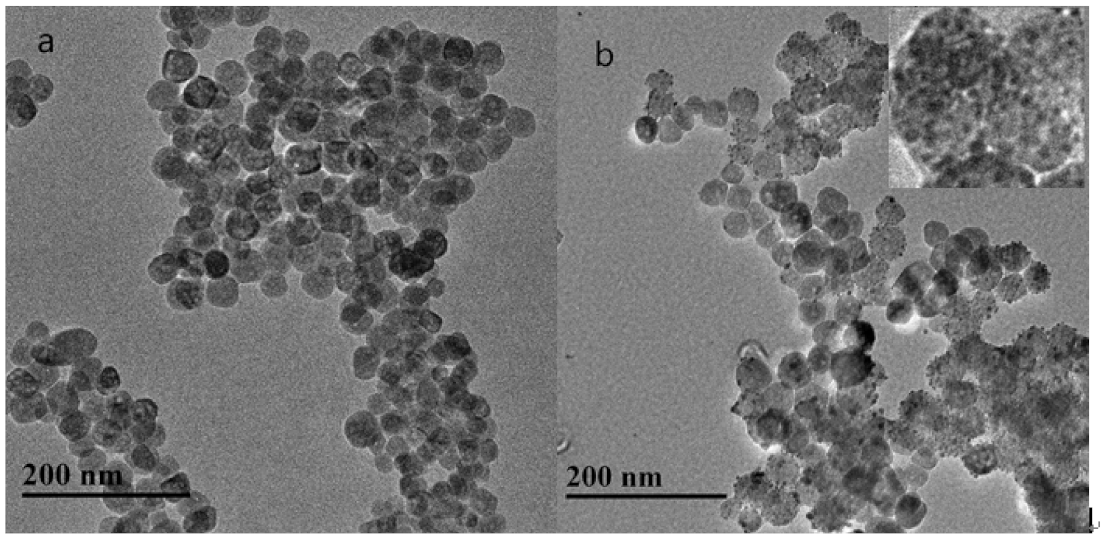
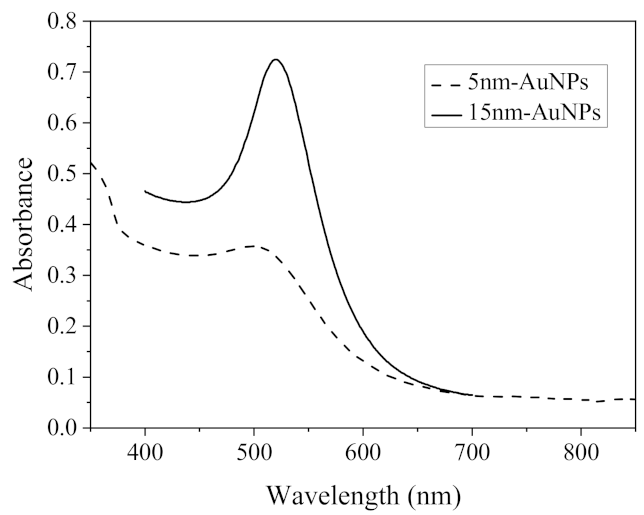
2.1. Characterization of NPs
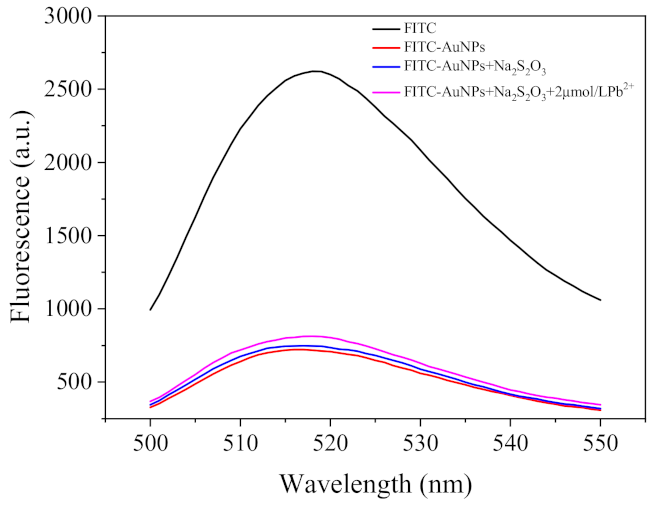
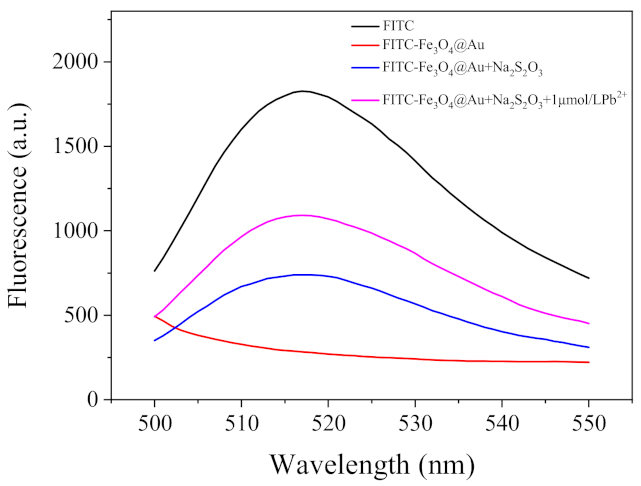
2.2. Etching of Fe3O4@AuNPs–FITC by Lead and Thiosulfate Ions
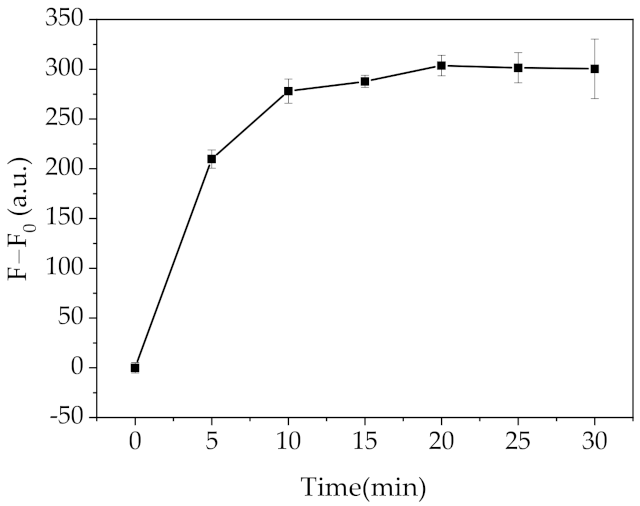
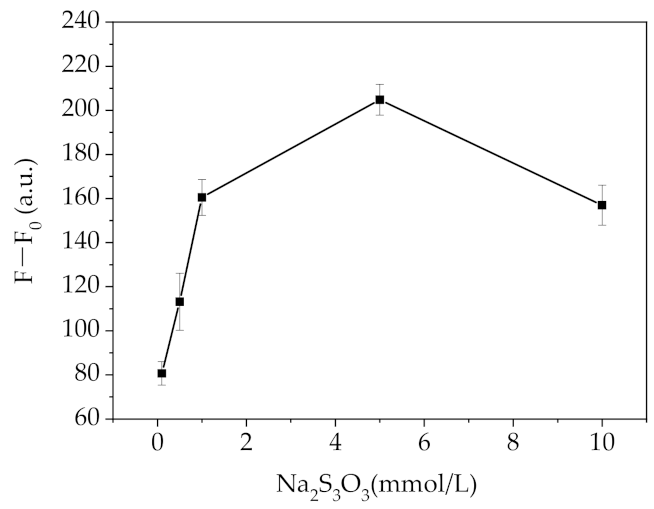
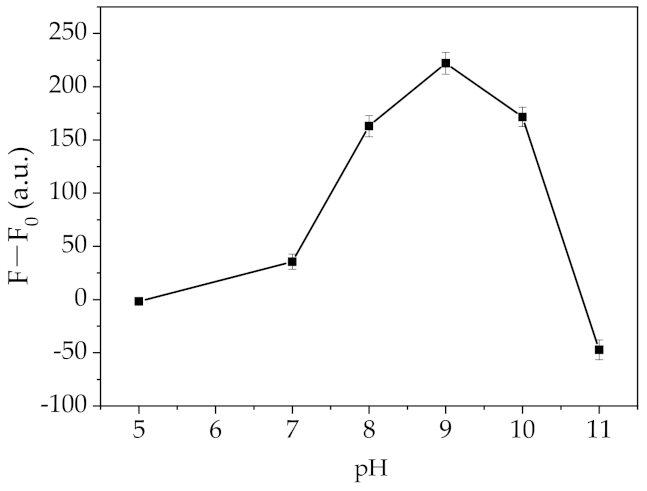
2.3. Optimization of the Fluorescent Assay
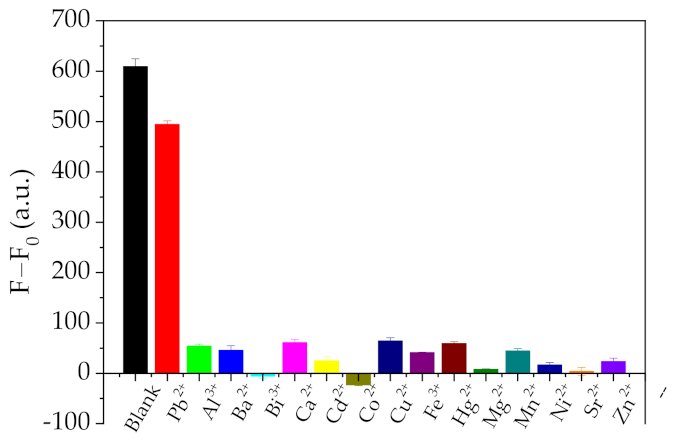
2.4. Selectivity of Pb2+ Sensing
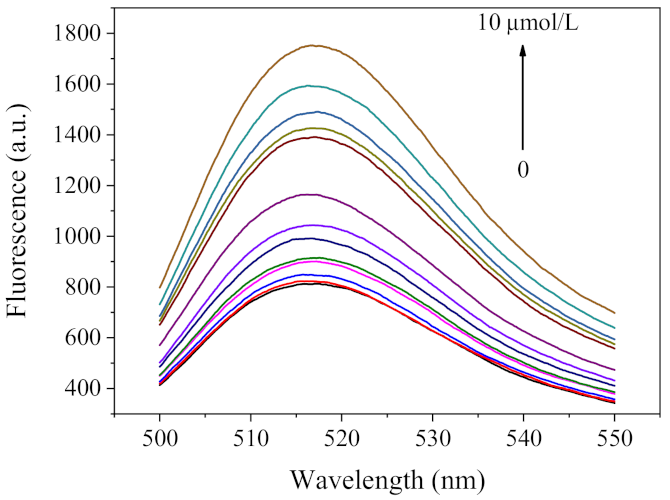
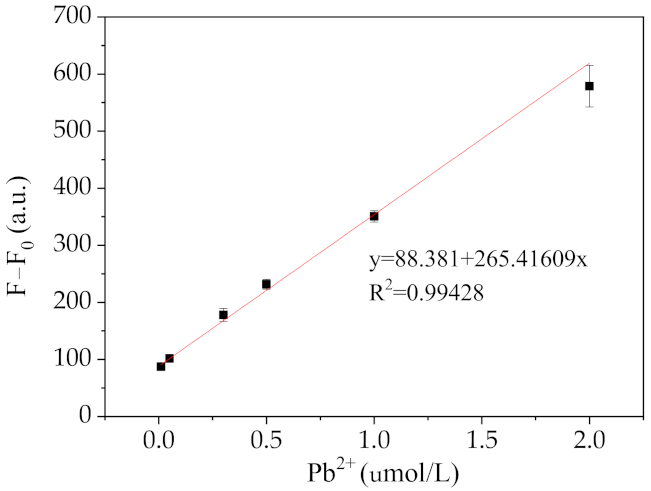
2.5. Analytical Performance of Pb2+ Sensing
2.6. Application of Pb2+ Sensing
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Synthesis of Au Nanoparticles (AuNPs)
3.4. Amine-Functionalization of Fe3O4 Nanoparticles Using APTES
3.5. Synthesis of Fe3O4@AuNCs-FITC
3.6. Fluorescent Detection of Pb2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knecht, M.R.; Sethi, M. Bio-inspired colorimetric detection of Hg2+ and Pb2+ heavy metal ions using Au nanoparticles. Anal. Bioanal. Chem. 2009, 394, 33–46. [Google Scholar] [CrossRef]
- Lan, Y.-J.; Lin, Y.-W. A non-aggregation colorimetric method for trace lead(II) ions based on the leaching of gold nanorods. Anal. Methods UK 2014, 6, 7234–7242. [Google Scholar] [CrossRef]
- Li, Y.F.; Chen, C.Y.; Li, B.; Sun, J.; Wang, J.X.; Gao, Y.X.; Zhao, Y.L.; Chai, Z.F. Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS. J. Anal. Atom. Spectrom. 2006, 21, 94–96. [Google Scholar] [CrossRef]
- Mamatha, P.; Venkateswarlu, G.; Thangavel, S.; Sahayam, A.C.; Swamy, A.V.N. Methodology for the Speciation of Cr(III) and Cr(VI) in Leaf Powders Using the Sulfate Form of Dowex-1 After Microwave Extraction and ICP-OES Detection. Atom. Spectrosc. 2019, 40, 31–35. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhang, Y.Y.; Cheng, M.P.; Mergny, J.L.; Lin, Q.M.; Zhou, J.; Ju, H.X. Highly active G-quadruplex/hemin DNAzyme for sensitive colorimetric determination of lead(II). Microchim. Acta 2019, 186, 786. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Y.; Duan, N.; Wang, Z.P. A Colorimetric Aptamer Sensor Based on the Enhanced Peroxidase Activity of Functionalized Graphene/Fe3O4-AuNPs for Detection of Lead (II) Ions. Catalysts 2020, 10, 600. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, Y.X.; Meng, F.D.; Wang, B.X.; Cheng, Y.X.; Zhu, C.J. N-doped carbon dots synthesized by rapid microwave irradiation as highly fluorescent probes for Pb2+ detection. New J. Chem. 2015, 39, 3357–3360. [Google Scholar] [CrossRef]
- Chen, M.; Hassan, M.; Li, H.H.; Chen, Q.S. Fluorometric determination of lead(II) by using aptamer-functionalized upconversion nanoparticles and magnetite-modified gold nanoparticles. Microchim. Acta 2020, 187, 85. [Google Scholar] [CrossRef]
- Chen, S.H.; Li, Y.X.; Li, P.H.; Xiao, X.Y.; Jiang, M.; Li, S.S.; Zhou, W.Y.; Yang, M.; Huang, X.J.; Liu, W.Q. Electrochemical spectral methods for trace detection of heavy metals: A review. Trac Trend. Anal. Chem. 2018, 106, 139–150. [Google Scholar] [CrossRef]
- Li, P.H.; Song, Z.Y.; Yang, M.; Chen, S.H.; Xiao, X.Y.; Duan, W.C.; Li, L.N.; Huang, X.J. Electrons in Oxygen Vacancies and Oxygen Atoms Activated by Ce3+/Ce4+ Promote High-Sensitive Electrochemical Detection of Pb(II) over Ce-Doped alpha-MoO3 Catalysts. Anal. Chem. 2020, 92, 16089–16096. [Google Scholar] [CrossRef]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. Nanomaterial-based fluorescent sensors for the detection of lead ions. J. Hazard. Mater. 2021, 407, 124379. [Google Scholar] [CrossRef]
- Zamojc, K.; Kamrowski, D.; Zdrowowicz, M.; Wyrzykowski, D.; Wiczk, W.; Chmurzynski, L.; Makowska, J. A Pentapeptide with Tyrosine Moiety as Fluorescent Chemosensor for Selective Nanomolar-Level Detection of Copper(II) Ions. Int. J. Mol. Sci. 2020, 21, 743. [Google Scholar] [CrossRef]
- Lee, J.F.; Chen, H.L.; Lee, G.S.; Tseng, S.C.; Lin, M.H.; Liau, W.B. Photosensized Controlling Benzyl Methacrylate-Based Matrix Enhanced Eu3+ Narrow-Band Emission for Fluorescence Applications. Int. J. Mol. Sci. 2012, 13, 3718–3737. [Google Scholar] [CrossRef]
- Qi, Z.L.; Cheng, Y.H.; Xu, Z.; Chen, M.L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. Int. J. Mol. Sci. 2020, 21, 5839. [Google Scholar] [CrossRef]
- Kurshanov, D.A.; Khavlyuk, P.D.; Baranov, M.A.; Dubavik, A.; Rybin, A.V.; Fedorov, A.V.; Baranov, A.V. Magneto-Fluorescent Hybrid Sensor CaCO3-Fe3O4-AgInS2/ZnS for the Detection of Heavy Metal Ions in Aqueous Media. Materials 2020, 13, 4373. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhuri, A.R.; Laha, D.; Mahto, T.K.; Karmakar, P.; Sahu, S.K. Green synthesis of carbon dots from Ocimum sanctum for effective fluorescent sensing of Pb2+ ions and live cell imaging. Sens. Actuat. B Chem. 2017, 242, 679–686. [Google Scholar] [CrossRef]
- Niu, X.F.; Zhong, Y.B.; Chen, R.; Wang, F.; Liu, Y.J.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuator B Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Shu, T.; Su, L.; Wang, J.; Li, C.; Zhang, X. Chemical etching of bovine serum albumin-protected Au25 nanoclusters for label-free and separation-free detection of cysteamine. Biosens. Bioelectron. 2015, 66, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Chen, Z.P.; Wang, S.S.; Qu, C.L.; Chen, L.X.; Wang, Z. Colorimetric sensing of copper(II) based on catalytic etching of gold nanoparticles. Talanta 2013, 112, 37–42. [Google Scholar] [CrossRef]
- Liu, W.Q.; Hou, S.; Yan, J.; Zhang, H.; Ji, Y.L.; Wu, X.C. Quantification of proteins using enhanced etching of Ag coated Au nanorods by the Cu2+/bicinchoninic acid pair with improved sensitivity. Nanoscale 2016, 8, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.H.; Gu, W.; Peng, W.D.; Li, B.Y.; Chen, N.N.; Zhao, K.; Xian, Y.Z. Sensitive Pb2+ Probe Based on the Fluorescence Quenching by Graphene Oxide and Enhancement of the Leaching of Gold Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.U.; Ma, Y.H.; Hu, J.; Xu, Y.Y.; Wang, W.F.; Chen, X.G. Preparation and characterization of magnetic gold shells using different sizes of gold nanoseeds and their corresponding effects on catalysis. RSC Adv. 2014, 4, 5012–5020. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Peng, C.F.; Pan, N.; Xie, Z.J.; Wu, L.L. Highly sensitive and selective colorimetric detection of Hg2+ based on the separation of Hg2+ and formation of catalytic DNA-gold nanoparticles. Anal. Methods UK 2016, 8, 1021–1025. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Leng, Y.M.; Miao, L.J.; Xin, J.W.; Wu, A.G. The colorimetric detection of Pb2+ by using sodium thiosulfate and hexadecyl trimethyl ammonium bromide modified gold nanoparticles. Dalton Trans. 2013, 42, 5485–5490. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chang, H.T.; Shiang, Y.C.; Hung, Y.L.; Chiang, C.K.; Huang, C.C. Colorimetric Assay for Lead Ions Based on the Leaching of Gold Nanoparticles. Anal. Chem. 2009, 81, 9433–9439. [Google Scholar] [CrossRef]
- Shi, X.H.; Gu, W.; Zhang, C.L.; Zhao, L.Y.; Peng, W.D.; Xian, Y.Z. A label-free colorimetric sensor for Pb2+ detection based on the acceleration of gold leaching by graphene oxide. Dalton Trans. 2015, 44, 4623–4629. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Edit. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
- Xia, Q.D.; Fu, S.S.; Ren, G.J.; Chai, F.; Jiang, J.J.; Qu, F.Y. Fabrication of magnetic bimetallic Fe3O4@Au-Pd hybrid nanoparticles with recyclable and efficient catalytic properties. RSC Adv. 2016, 6, 55248–55256. [Google Scholar] [CrossRef]
- Wang, S.S.; Wang, X.K.; Zhang, Z.Y.; Chen, L.X. Highly sensitive fluorescence detection of copper ion based on its catalytic oxidation to cysteine indicated by fluorescein isothiocyanate functionalized gold nanoparticles. Colloid Surf. A 2015, 468, 333–338. [Google Scholar] [CrossRef]
- Wu, S.; Li, D.; Wang, J.; Zhao, Y.; Dong, S.; Wang, X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sens. Actuator B Chem. 2017, 238, 427–433. [Google Scholar] [CrossRef]
- Zhong, Y.P.; Wang, Q.P.; He, Y.; Ge, Y.L.; Song, G.W. A novel fluorescence and naked eye sensor for iodide in urine based on the iodide induced oxidative etching and aggregation of Cu nanoclusters. Sens. Actuator B Chem. 2015, 209, 147–153. [Google Scholar] [CrossRef]

| Nanoprobes | Linear Range | LOD | Time | Ref. |
|---|---|---|---|---|
| CTAB modified AuNPs | 1.0~6.0 µmol/L | 75 nmol/L | 30 min | [25] |
| AuNPs and graphene oxide | 0.1~20 µmol/L | 50 nmol/L | 20 min | [27] |
| AuNPs | 0.0025~10 µmol/L | 0.5 nmol/L | 2 h | [26] |
| Fe3O4@Au–FITC | 0.02~2.0 µmol/L | 5.2 nmol/L | 12 min | This work |
| Concentration (nmol/L) | Result (nmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|
| 50 | 53.2 | 106.4 | 8.3 |
| 100 | 98.3 | 98.3 | 2.5 |
| 150 | 159.0 | 106.0 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Ren, B.; Peng, C.; Zhang, C.; Wei, X. Highly Sensitive and Selective Fluorescence “Turn-On” Detection of Pb (II) Based on Fe3O4@Au–FITC Nanocomposite. Molecules 2021, 26, 3180. https://doi.org/10.3390/molecules26113180
Cai Y, Ren B, Peng C, Zhang C, Wei X. Highly Sensitive and Selective Fluorescence “Turn-On” Detection of Pb (II) Based on Fe3O4@Au–FITC Nanocomposite. Molecules. 2021; 26(11):3180. https://doi.org/10.3390/molecules26113180
Chicago/Turabian StyleCai, Yina, Binxue Ren, Chifang Peng, Cunzheng Zhang, and Xinlin Wei. 2021. "Highly Sensitive and Selective Fluorescence “Turn-On” Detection of Pb (II) Based on Fe3O4@Au–FITC Nanocomposite" Molecules 26, no. 11: 3180. https://doi.org/10.3390/molecules26113180
APA StyleCai, Y., Ren, B., Peng, C., Zhang, C., & Wei, X. (2021). Highly Sensitive and Selective Fluorescence “Turn-On” Detection of Pb (II) Based on Fe3O4@Au–FITC Nanocomposite. Molecules, 26(11), 3180. https://doi.org/10.3390/molecules26113180






