The Role of Serotonin in Breast Cancer Stem Cells
Abstract
1. Introduction
1.1. Breast Cancer Clinical and Molecular Subtypes
1.2. Breast Cancer Stem Cells
1.3. Epithelial to Mesenchymal Transition and BTIC, Therapeutic Implications
2. Discovery of BTIC Targeting Small Molecules
2.1. Identification of an Abundant Source of BTIC
2.2. Phenotypic Screen in Mouse BTIC-Enriched Tumor Cells Identifies Neurotransmitter Antagonists
2.3. 5-HT and Proteins Required for Its Synthesis, Transport, and Activity via 5-HTRs Are Expressed in Mouse Mammary Tumor Cells
2.4. 5-HT Is Synthesized by Mouse Mammary Tumor Cells, Which Express TPH1 and SERT
2.5. Serotonergic Antagonists Target BTIC from a Mouse Model of HER2-Overexpressing Breast Cancer
2.6. Serotonergic Antagonist Function by an Irreversible Mechanism to Reduce BTIC Frequency
2.7. Serotonergic Antagonists Synergize with Chemotherapy to Shrink Mouse Mammary Tumors
2.8. Sertraline in Combination with Docetaxel Reduces Tumor Cell Proliferation and Induces Programmed Cell Death in Mammary Tumor Allografts
2.9. Summary
3. 5-HT and Human Cancers
3.1. Human Breast Tumors, Patient-Derived Xenografts and Breast Tumor Cell Lines Synthesize 5-HT and Express Proteins Required for Its Biosynthesis and Activity
3.2. Selective Antagonists of Multiple 5-HT System Pathway Components Reduce BTIC Frequency in Human Breast Tumor Cell Lines and Synergize with Chemotherapy to Shrink Breast Tumor Xenografts
3.3. Functional Evidence for 5-HTRs in Human Breast Cancer and Other Cancers
4. Monoamine Oxidase A (MAO-A)
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| 5-HT | 5-Hydroxytryptamine |
| WB | Western blot |
| 5-HTRs | 5-HT receptors |
| TNBC | Triple-negative breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| BTICs | Breast tumor-initiating cell |
| EMT | Epithelial to mesenchymal transition |
| GPCRs | G-protein coupled receptors |
| SERT | Serotonin reuptake transporter |
| 5-HT | 5-hydroxytryptamine |
| SSRI | Selective serotonin reuptake inhibitors |
| TPH1 | Tryptophan hydroxylase 1 |
| MAO | Monoamine oxidase |
| MESC | Mammary epithelial stem cells |
| TIC | Tumor-initiating cells |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| IHC | Immunohistochemistry |
| IF | Immunofluorescence |
| RTK | Receptor tyrosine kinase |
| 5-CT | 5-carboxamidotryptamine |
| ERKs | Extracellular regulated kinases |
| cAMP | Cyclic adenosine monophosphate |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Wagner, U.; Henninghausen, L. Mammary Cancer in Humans and Mice: A Tutorial for Comparative Pathology. Veter. Pathol. 2001, 38, 357–358. [Google Scholar] [CrossRef]
- Rosner, A.; Miyoshi, K.; Landesman-Bollag, E.; Xu, X.; Seldin, D.C.; Moser, A.R.; MacLeod, C.L.; Shyamala, G.; Gillgrass, A.E.; Cardiff, R.D. Pathway Pathology: Histological Differences Between ErbB/Ras and Wnt Pathway Transgenic Mammary Tumors. Am. J. Pathol. 2002, 161, 1087–1097. [Google Scholar] [CrossRef]
- Lin, E.Y.; Jones, J.G.; Li, P.; Zhu, L.; Whitney, K.D.; Muller, W.J.; Pollard, J.W. Progression to Malignancy in the Polyoma Middle T Oncoprotein Mouse Breast Cancer Model Provides a Reliable Model for Human Diseases. Am. J. Pathol. 2003, 163, 2113–2126. [Google Scholar] [CrossRef]
- Herschkowitz, J.I.; Simin, K.; Weigman, V.J.; Mikaelian, I.; Usary, J.; Hu, Z.; Rasmussen, K.E.; Jones, L.P.; Assefnia, S.; Chandrasekharan, S.; et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007, 8, R76. [Google Scholar] [CrossRef]
- Dick, J.E. Stem cell concepts renew cancer research. Blood 2008, 112, 4793–4807. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Scheel, C.; Weinberg, R.A. Cancer stem cells and epithelial–mesenchymal transition: Concepts and molecular links. Semin. Cancer Biol. 2012, 22, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Brueckmann, I.; Scheel, C.; Kaestli, A.J.; Wiggins, P.A.; Rodrigues, L.O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA 2011, 108, 7950–7955. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F. Clinical and Therapeutic Implications of Cancer Stem Cells. N. Engl. J. Med. 2019, 380, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lewis, M.T.; Huang, J.; Gutierrez, C.; Osborne, C.K.; Wu, M.-F.; Hilsenbeck, S.G.; Pavlick, A.; Zhang, X.; Chamness, G.C.; et al. Intrinsic Resistance of Tumorigenic Breast Cancer Cells to Chemotherapy. J. Natl. Cancer Inst. 2008, 100, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I.; et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar] [CrossRef] [PubMed]
- Kurpios, N.A.; Girgis-Gabardo, A.; Hallett, R.M.; Rogers, S.; Gludish, D.W.; Kockeritz, L.; Woodgett, J.; Cardiff, R.; Hassell, J.A. Single Unpurified Breast Tumor-Initiating Cells from Multiple Mouse Models Efficiently Elicit Tumors in Immune-Competent Hosts. PLoS ONE 2013, 8, e58151. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.S.; Sen, A.; Kallos, M.S.; Behie, L.A.; Girgis-Gabardo, A.; Kurpios, N.; Barcelon, M.; Hassell, J.A. Large-Scale Expansion of Mammary Epithelial Stem Cell Aggregates in Suspension Bioreactors. Biotechnol. Prog. 2008, 21, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Sci. 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Guy, C.T.; Webster, M.A.; Schaller, M.; Parsons, T.J.; Cardiff, R.D.; Muller, W.J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10578–10582. [Google Scholar] [CrossRef] [PubMed]
- Nociari, M.M.; Shalev, A.; Benias, P.; Russo, C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Methods 1998, 213, 157–167. [Google Scholar] [CrossRef]
- Hallett, R.M.; Girgis-Gabardo, A.; Gwynne, W.D.; Giacomelli, A.O.; Bisson, J.N.P.; Jensen, J.E.; Dvorkin-Gheva, A.; Hassell, J.A. Serotonin transporter antagonists target tumor-initiating cells in a transgenic mouse model of breast cancer. Oncotarget 2016, 7, 53137–53152. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Imaoka, T.; Vomachka, A.J.; Gudelsky, G.A.; Hou, Z.; Mistry, M.; Bailey, J.P.; Nieport, K.M.; Walther, D.J.; Bader, M.; et al. Serotonin Regulates Mammary Gland Development via an Autocrine-Paracrine Loop. Dev. Cell 2004, 6, 193–203. [Google Scholar] [CrossRef]
- Collier, R.; Hernandez, L.; Horseman, N. Serotonin as a homeostatic regulator of lactation. Domest. Anim. Endocrinol. 2012, 43, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Horseman, N.D. Multiple Cellular Responses to Serotonin Contribute to Epithelial Homeostasis. PLoS ONE 2011, 6, e17028. [Google Scholar] [CrossRef] [PubMed]
- Horseman, N.D.; Collier, R.J. Serotonin: A Local Regulator in the Mammary Gland Epithelium. Annu. Rev. Anim. Biosci. 2014, 2, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, A.; Bonizzi, G.; Pasi, C.E.; Faretta, M.; Ronzoni, S.; Giulini, B.; Brisken, C.; Minucci, S.; Di Fiore, P.P.; Pelicci, P.G. The Tumor Suppressor p53 Regulates Polarity of Self-Renewing Divisions in Mammary Stem Cells. Cell 2009, 138, 1083–1095. [Google Scholar] [CrossRef]
- Liao, M.-J.; Zhang, C.C.; Zhou, B.; Zimonjic, D.B.; Mani, S.A.; Kaba, M.; Gifford, A.; Reinhardt, F.; Popescu, N.C.; Guo, W.; et al. Enrichment of a Population of Mammary Gland Cells that Form Mammospheres and Have In vivo Repopulating Activity. Cancer Res. 2007, 67, 8131–8138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Deng, T.; Lehal, R.S.; Kim, J.; Zacksenhaus, E. Identification of Tumorsphere- and Tumor-Initiating Cells in HER2/Neu-Induced Mammary Tumors. Cancer Res. 2007, 67, 8671–8681. [Google Scholar] [CrossRef]
- Fillmore, C.M.; Kuperwasser, C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hallett, R.M.; Kondratyev, M.K.; Giacomelli, A.O.; Nixon, A.M.L.; Girgis-Gabardo, A.; Ilieva, D.; Hassell, J.A. Small Molecule Antagonists of the Wnt/Beta-Catenin Signaling Pathway Target Breast Tumor-Initiating Cells in a Her2/Neu Mouse Model of Breast Cancer. PLoS ONE 2012, 7, e33976. [Google Scholar] [CrossRef] [PubMed]
- Kondratyev, M.; Kreso, A.; Hallett, R.M.; Girgis-Gabardo, A.; Barcelon, M.E.; Ilieva, D.; Ware, C.; Majumder, P.K.; Hassell, J.A. Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene 2011, 31, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nat. Cell Biol. 2008, 456, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Waitches, G.; Birchmeier, C.; Fasano, O.; Wigler, M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 1986, 45, 711–719. [Google Scholar] [CrossRef]
- Julius, D.; Livelli, T.; Jessell, T.; Axel, R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science 1989, 244, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Varrault, A.; Bockaert, J.; Waeber, C. Activation of 5-HT1A receptors expressed in NIH-3T3 cells induces focus formation and potentiates EGF effect on DNA synthesis. Mol. Biol. Cell 1992, 3, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Julius, D.; Huang, K.N.; Livelli, T.J.; Axel, R.; Jessell, T.M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc. Natl. Acad. Sci. USA 1990, 87, 928–932. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, M.; Degese, M.S.; Gutkind, J.S. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr. Opin. Cell Biol. 2014, 27, 126–135. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef]
- Wu, V.; Yeerna, H.; Nohata, N.; Chiou, J.; Harismendy, O.; Raimondi, F.; Inoue, A.; Russell, R.B.; Tamayo, P.; Gutkind, J.S. Illuminating the Onco-GPCRome: Novel G protein–coupled receptor-driven oncocrine networks and targets for cancer immunotherapy. J. Biol. Chem. 2019, 294, 11062–11086. [Google Scholar] [CrossRef] [PubMed]
- Arang, N.; Gutkind, J.S. G Protein-Coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett. 2020, 594, 4201–4232. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, W.D.; Hallett, R.M.; Girgis-Gabardo, A.; Bojovic, B.; Dvorkin-Gheva, A.; Aarts, C.; Dias, K.; Bane, A.; Hassell, J.A. Serotonergic system antagonists target breast tumor initiating cells and synergize with chemotherapy to shrink human breast tumor xenografts. Oncotarget 2017, 8, 32101–32116. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Maggiolini, M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 2010, 10, 47–60. [Google Scholar] [CrossRef]
- Gutierrez, A.N.; McDonald, P.H. GPCRs: Emerging anti-cancer drug targets. Cell Signal. 2018, 41, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.H.; Johannessen, C.M.; Piccioni, F.; Tamayo, P.; Kim, J.W.; Van Allen, E.M.; Corsello, S.M.; Capelletti, M.; Calles, A.; Butaney, M.; et al. A Functional Landscape of Resistance to ALK Inhibition in Lung Cancer. Cancer Cell 2015, 27, 397–408. [Google Scholar] [CrossRef]
- Johannessen, C.M.; Johnson, L.A.; Piccioni, F.; Townes, A.; Frederick, D.T.; Donahue, M.K.; Narayan, R.; Flaherty, K.T.; Wargo, J.A.; Root, D.E.; et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nat. Cell Biol. 2013, 504, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Konermann, S.; Brigham, M.; Trevino, A.E.; Joung, J.; Abudayyeh, O.O.; Barcena, C.; Hsu, P.D.; Habib, N.; Gootenberg, J.; Nishimasu, H.; et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nat. Cell Biol. 2015, 517, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Malta, T.M.; Sokolov, A.; Gentles, A.J.; Burzykowski, T.; Poisson, L.; Weinstein, J.N.; Kamińska, B.; Huelsken, J.; Omberg, L.; Gevaert, O.; et al. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell 2018, 173, 338–354.e15. [Google Scholar] [CrossRef]
- Fröbe, A.; Čičin-Šain, L.; Jones, G.; Soldič, Ž.; Lukač, J.; Bolanča, A.; Kusič, Z. Plasma free serotonin as a marker for early detection of breast cancer recurrence. Anticancer. Res. 2014, 34, 1167–1169. [Google Scholar]
- Leoncikas, V.; Wu, H.; Ward, L.T.; Kierzek, A.; Plant, N.J. Generation of 2,000 breast cancer metabolic landscapes reveals a poor prognosis group with active serotonin production. Sci. Rep. 2016, 6, 19771. [Google Scholar] [CrossRef] [PubMed]
- Sonier, B.; Arseneault, M.; Lavigne, C.; Ouellette, R.J.; Vaillancourt, C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem. Biophys. Res. Commun. 2006, 343, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Horseman, N.D. Biphasic Regulation of Mammary Epithelial Resistance by Serotonin through Activation of Multiple Pathways. J. Biol. Chem. 2008, 283, 30901–30910. [Google Scholar] [CrossRef] [PubMed]
- Kopparapu, P.K.; Tinzl, M.; Anagnostaki, L.; Persson, J.L.; Dizeyi, N. Expression and localization of serotonin receptors in human breast cancer. Anticancer. Res. 2013, 33, 363–370. [Google Scholar]
- Gautam, J.; Banskota, S.; Regmi, S.C.; Ahn, S.; Jeon, Y.H.; Jeong, H.; Kim, S.J.; Nam, T.-G.; Jeong, B.-S.; Kim, J.-A. Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol. Cancer 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Marshall, A.M.; Hernandez, L.L.; Buckley, A.R.; Horseman, N.D. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009, 11, R81. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, W.D.; Shakeel, M.S.; Wu, J.; Hallett, R.M.; Girgis-Gabardo, A.; Dvorkin-Gheva, A.; Hassell, J.A. Monoamine oxidase-A activity is required for clonal tumorsphere formation by human breast tumor cells. Cell. Mol. Biol. Lett. 2019, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, J.; Escrich, E.; Ribalta, T.; Muntané, J.; Unzeta, M. Amine oxidase activities in rat breast cancer induced experimentally with 7,12-dimethylbenz(α)anthracene. Biochem. Pharmacol. 1991, 42, 263–269. [Google Scholar] [CrossRef]
- Lizcano, J.M.; Escrich, E.; Tipton, K.F.; Unzeta, M. Amine oxidase activities in chemically-induced mammary cancer in the rat. Amine Oxidases Impact Neurobiol. 1990, 32, 323–326. [Google Scholar] [CrossRef]
- Pai, V.P.; Hernandez, L.L.; Stull, M.A.; Horseman, N.D. The Type 7 Serotonin Receptor, 5-HT7, Is Essential in the Mammary Gland for Regulation of Mammary Epithelial Structure and Function. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L. The Serotonin Receptors: From Molecular Pharmacology to Human Thereapeutics; Springer Science & Business Media: Berlin, Germany, 2006; pp. 537–565. [Google Scholar]
- Sutkeviciute, I.; Vilardaga, J.-P. Structural insights into emergent signaling modes of G protein–coupled receptors. J. Biol. Chem. 2020, 295, 11626–11642. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.-M.; Gainetdinov, R.; Caron, M.G. Akt/GSK3 Signaling in the Action of Psychotropic Drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, W.D.; Shakeel, M.S.; Girgis-Gabardo, A.; Kim, K.H.; Ford, E.; Dvorkin-Gheva, A.; Aarts, C.; Isaac, M.; Al-Awar, R.; Hassell, J.A. Antagonists of the serotonin receptor 5A target human breast tumor initiating cells. BMC Cancer 2020, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Banús-Mulet, A.; Etxabe, A.; Cornet-Masana, J.M.; Torrente, M.Á.; Lara-Castillo, M.C.; Palomo, L.; Nomdedeu, M.; Díaz-Beyá, M.; Solé, F.; Nomdedeu, B.; et al. Serotonin receptor type 1B constitutes a therapeutic target for MDS and CMML. Sci. Rep. 2018, 8, 13883. [Google Scholar] [CrossRef] [PubMed]
- Etxabe, A.; Lara-Castillo, M.C.; Cornet-Masana, J.M.; Banús-Mulet, A.; Nomdedeu, M.; Torrente, M.A.; Pratcorona, M.; Díaz-Beyá, M.; Esteve, J.; Risueño, R.M. Inhibition of serotonin receptor type 1 in acute myeloid leukemia impairs leukemia stem cell functionality: A promising novel therapeutic target. Leukemia 2017, 31, 2288–2302. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, T.; Wang, Z.; Wu, X.; Gu, Y.; Huang, Q.; Wang, J.; Xie, J. 5-HT7 Receptor Contributes to Proliferation, Migration and Invasion in NSCLC Cells. OncoTargets Ther. 2020, 13, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, N.; Ashour, A.A.; Alpay, S.N.; Ozpolat, B. Down-Regulation of 5-HT1B and 5-HT1D Receptors Inhibits Proliferation, Clonogenicity and Invasion of Human Pancreatic Cancer Cells. PLoS ONE 2014, 9, e105245. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.E.; Angus, J.A. 5-carboxamidotryptamine elicits 5-HT2 and 5-HT3 receptor-mediated cardiovascular responses in the conscious rabbit: Evidence for 5-HT release from platelets. J. Cardiovasc. Pharmacol. 1989, 13, 557–564. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Breakefield, X.O. Human monoamine oxidase A gene determines levels of enzyme activity. Am. J. Hum. Genet. 1991, 49, 383–392. [Google Scholar] [PubMed]
- Ohi, Y.; Umekita, Y.; Yoshioka, T.; Souda, M.; Rai, Y.; Sagara, Y.; Sagara, Y.; Sagara, Y.; Tanimoto, A. Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology 2011, 59, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Song, Y.; Wang, S.; Huang, X.; Xuan, Q.; Kang, X.; Zhang, Q. CD44+/CD24- phenotype predicts a poor prognosis in triple-negative breast cancer. Oncol. Lett. 2017, 14, 5890–5898. [Google Scholar] [CrossRef] [PubMed]
- Rybaczyk, L.A.; Bashaw, M.J.; Pathak, D.R.; Huang, K. An indicator of cancer: Downregulation of Monoamine Oxidase-A in multiple organs and species. BMC Genom. 2008, 9, 134. [Google Scholar] [CrossRef]
- Liao, C.-P.; Lin, T.-P.; Li, P.-C.; Geary, L.A.; Chen, K.; Vaikari, V.P.; Wu, J.B.; Lin, C.-H.; Gross, M.E.; Shih, J.C. Loss of MAOA in epithelia inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate. Oncogene 2018, 37, 5175–5190. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, L.; Ma, Y.; Huang, B.; Xiu, Z.; Zhang, P.; Zhou, K.; Tang, X. Increased expression of monoamine oxidase A is associated with epithelial to mesenchymal transition and clinicopathological features in non-small cell lung cancer. Oncol. Lett. 2017, 15, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, A.; Spiekerkoetter, E.; Martinez, E.C.; Ambartsumian, N.; Sheward, W.J.; MacLean, M.R.; Harmar, A.J.; Schmidt, A.-M.; Lukanidin, E.; Rabinovitch, M. Interdependent Serotonin Transporter and Receptor Pathways Regulate S100A4/Mts1, a Gene Associated With Pulmonary Vascular Disease. Circ. Res. 2005, 97, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, P.; Pimentel, D.R.; Murphy, M.P.; Colucci, W.S.; Parini, A. A new hypertrophic mechanism of serotonin in cardiac myocytes: Receptor-independent ROS generation. FASEB J. 2005, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, D.; Zhang, N.; Wang, Z.; Pang, L. Plasma serotonin level is a predictor for recurrence and poor prognosis in colorectal cancer patients. J. Clin. Lab. Anal. 2018, 32, e22263. [Google Scholar] [CrossRef]
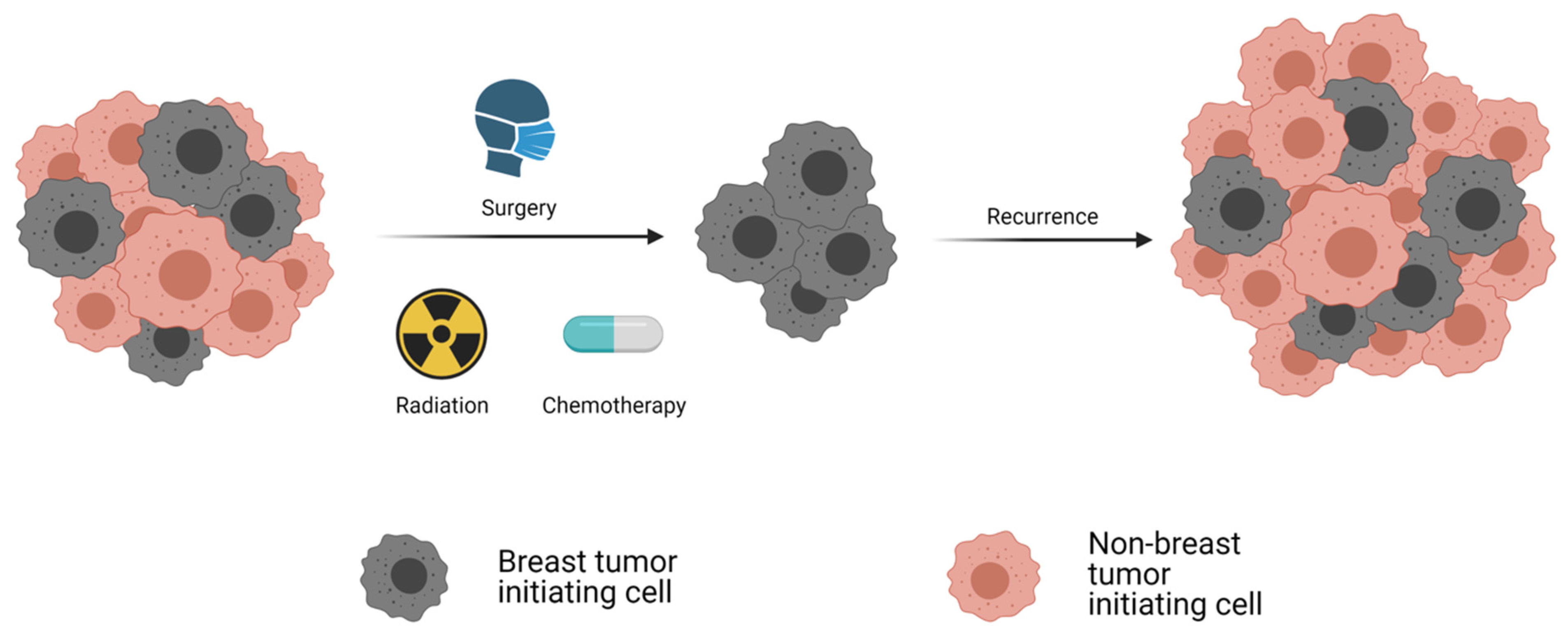
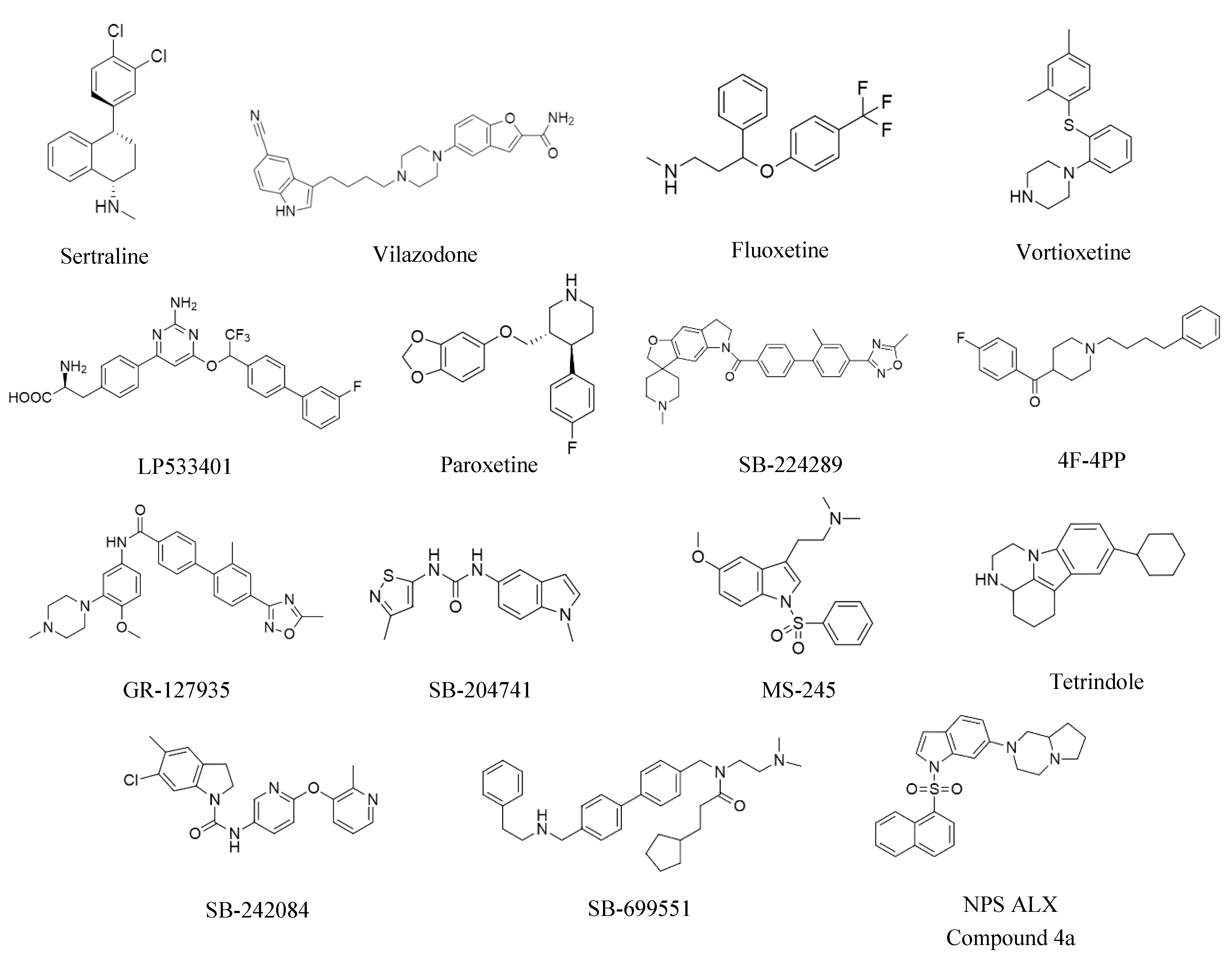

| Protein | Sample Type | Detection Method | Reference | |
|---|---|---|---|---|
| SERT | MMTV-Her2/Neu tumorspheres and tumors | IF | [22,43,56] | |
| Patient-derived breast tumors xenografts | IHC | |||
| Human breast tumor cell lines, tumorspheres and xenografts | WB, IF, RT-PCR | |||
| TPH1 | MMTV-Her2/Neu tumorspheres and tumors | IF | [22,43,55,56] | |
| Human breast tumor cell lines, tumorspheres and xenografts | IF | |||
| 5-HT | MMTV-Her2/Neu tumorspheres and tumors | IF | [22,43] | |
| Human breast tumor cell line tumorspheres and xenografts | IF | |||
| MAO-A | Human breast tumor cell lines and tumorspheres | NanoString, WB | [57,58,59] | |
| Chemically induced rat mammary tumors | Enzymatic | |||
| 5-HTR | 1A 1B 2A 2B 4 5A 7 | Human breast tumor cell lines | DNA microarray, RT-PCR, IF, WB | [52,53,54,55,56] |
| Molecule | FDA-Approved | Target | Sample Type | Species | Observed Effects |
|---|---|---|---|---|---|
| LP533401 | No | TPH1 | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22,43] |
| Breast tumor cell lines | Human | ||||
| Sertraline | Yes | SERT | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22] |
| Targets tumor-initiating cells [22] | |||||
| Induces tumor regression in combination with docetaxel [22] | |||||
| Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] | |||
| Targets tumor initiating cells [43] | |||||
| Paroxetine | Yes | SERT | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22,43] |
| Breast tumor cell lines | Human | ||||
| Fluoxetine | Yes | SERT | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22,43] |
| Breast tumor cell lines | Human | ||||
| Vortioxetine | Yes | SERT | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| Vilazodone | Yes | SERT, 5-HTR1A | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| Induces tumor regression in combination with docetaxel [43] | |||||
| SB-224289 | No | 5-HTR1B | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22,43] |
| Breast tumor cell lines | Human | ||||
| GR-127935 | No | 5-HTR1D | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| 4F-4PP | No | 5-HTR2A | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| SB-204741 | No | 5-HTR2B | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| SB-242084 | No | 5-HTR2C | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| SB-699551 | No | 5-HTR5A | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22,61] |
| Breast tumor cell lines Patient-dervied breast tumor xenografts | Human | Inhibits tumorsphere formation [43] | |||
| Targets tumor-initiating cells [61] | |||||
| Induces tumor regression in combination with docetaxel [61] | |||||
| MS-245 | No | 5-HTR6 | MMTV-Her2/Neu | Mouse | Inhibits tumorsphere formation [22] |
| NPS ALX Compound 4a | No | 5-HTR6 | Breast tumor cell lines | Human | Inhibits tumorsphere formation [43] |
| Tetrindole | No | MAOA | Breast tumor cell lines | Human | Inhibits tumorsphere formation [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwynne, W.D.; Shakeel, M.S.; Girgis-Gabardo, A.; Hassell, J.A. The Role of Serotonin in Breast Cancer Stem Cells. Molecules 2021, 26, 3171. https://doi.org/10.3390/molecules26113171
Gwynne WD, Shakeel MS, Girgis-Gabardo A, Hassell JA. The Role of Serotonin in Breast Cancer Stem Cells. Molecules. 2021; 26(11):3171. https://doi.org/10.3390/molecules26113171
Chicago/Turabian StyleGwynne, William D., Mirza S. Shakeel, Adele Girgis-Gabardo, and John A. Hassell. 2021. "The Role of Serotonin in Breast Cancer Stem Cells" Molecules 26, no. 11: 3171. https://doi.org/10.3390/molecules26113171
APA StyleGwynne, W. D., Shakeel, M. S., Girgis-Gabardo, A., & Hassell, J. A. (2021). The Role of Serotonin in Breast Cancer Stem Cells. Molecules, 26(11), 3171. https://doi.org/10.3390/molecules26113171







