An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors
Abstract
1. Introduction
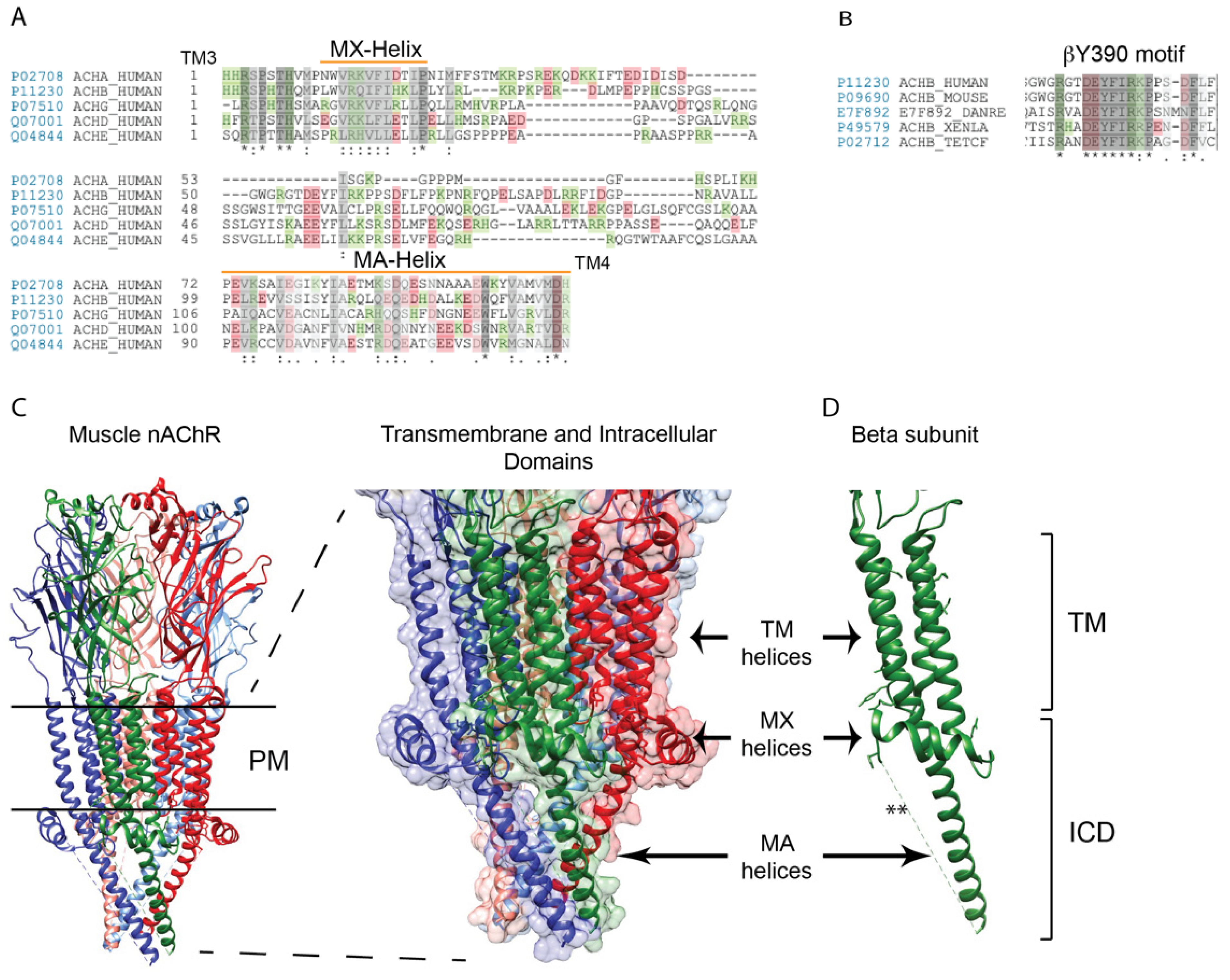
2. Structure of the nAChR Intracellular Domain
3. Postsynaptic Localization of the Muscle nAChR
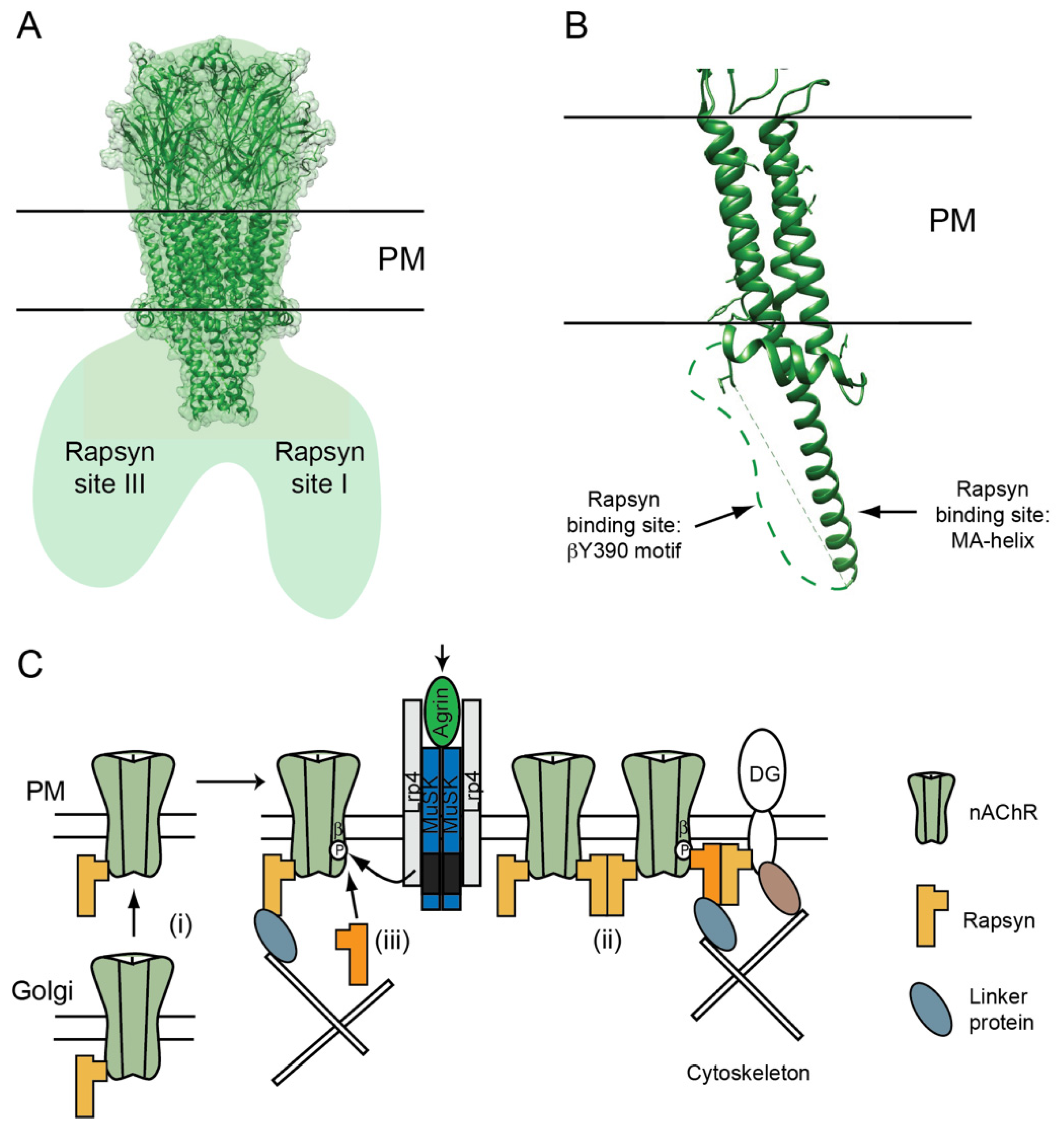
3.1. AChR Localization Is Mediated by Rapsyn
3.2. AChR-Rapsyn Stoichiometry
3.3. The nAChR-Rapsyn Binding Sites
3.4. Function of nAChR-Rapsyn Interactions
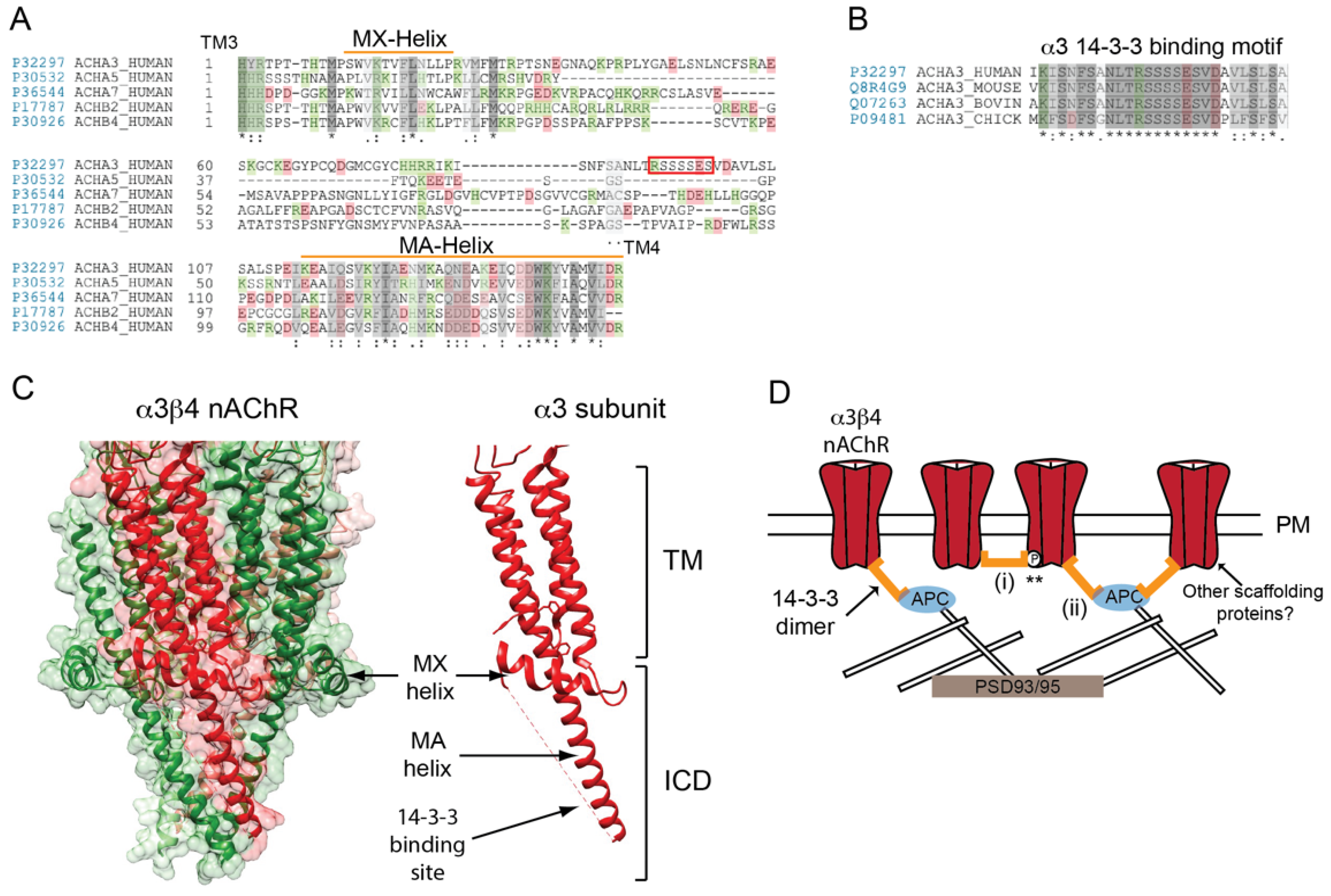
4. Postsynaptic Localization of Neuronal nAChRs
Determinants for Neuronal nAChR Localization
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | adenomatous polyposis coli |
| CNS | central nervous system |
| CMS | congenital myasthenic syndrome |
| ICD | intracellular domain |
| nAChR | nicotinic acetylcholine receptor |
| NMJ | neuromuscular junction |
| PNS | peripheral nervous system |
| PM | plasma membrane |
| TM | transmembrane |
References
- Sine, S.M. End-plate acetylcholine receptor: Structure, mechanism, pharmacology, and disease. Physiol. Rev. 2012, 92, 1189–1234. [Google Scholar] [CrossRef]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharm. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2002, 2, 797–804. [Google Scholar] [CrossRef]
- Engel, A.G.; Shen, X.M.; Selcen, D.; Sine, S.M. Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet. Neurol. 2015, 14, 461. [Google Scholar] [CrossRef]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Harkness, P.C. Assembly and trafficking of nicotinic acetylcholine receptors (Review). Mol. Membr. Biol. 2008, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.; Treinin, M.; Papke, R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharm. Sci. 2015, 36, 514–523. [Google Scholar] [CrossRef]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 2020, 106, 952–962.e955. [Google Scholar] [CrossRef]
- Miyazawa, A.; Fujiyoshi, Y.; Stowell, M.; Unwin, N. Nicotinic acetylcholine receptor at 4.6 A resolution: Transverse tunnels in the channel wall. J. Mol. Biol. 1999, 288, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the alpha7 nicotinic acetylcholine receptor. Cell 2021. [Google Scholar] [CrossRef]
- Gharpure, A.; Teng, J.; Zhuang, Y.; Noviello, C.M.; Walsh, R.M., Jr.; Cabuco, R.; Howard, R.J.; Zaveri, N.T.; Lindahl, E.; Hibbs, R.E. Agonist Selectivity and Ion Permeation in the alpha3beta4 Ganglionic Nicotinic Receptor. Neuron 2019, 104, 501–511.e506. [Google Scholar] [CrossRef]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human alpha4beta2 nicotinic receptor. Nature 2016, 538, 411–415. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef]
- Huang, X.; Chen, H.; Michelsen, K.; Schneider, S.; Shaffer, P.L. Crystal structure of human glycine receptor-alpha3 bound to antagonist strychnine. Nature 2015, 526, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Hassaine, G.; Deluz, C.; Grasso, L.; Wyss, R.; Tol, M.B.; Hovius, R.; Graff, A.; Stahlberg, H.; Tomizaki, T.; Desmyter, A.; et al. X-ray structure of the mouse serotonin 5-HT3 receptor. Nature 2014, 512, 276–281. [Google Scholar] [CrossRef]
- Gharpure, A.; Noviello, C.M.; Hibbs, R.E. Progress in nicotinic receptor structural biology. Neuropharmacology 2020, 171, 108086. [Google Scholar] [CrossRef]
- Wu, Z.S.; Cheng, H.; Jiang, Y.; Melcher, K.; Xu, H.E. Ion channels gated by acetylcholine and serotonin: Structures, biology, and drug discovery. Acta. Pharm. Sin. 2015, 36, 895–907. [Google Scholar] [CrossRef]
- Unwin, N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Q. Rev. Biophys. 2013, 46, 283–322. [Google Scholar] [CrossRef] [PubMed]
- Verschuuren, J.; Strijbos, E.; Vincent, A. Neuromuscular junction disorders. Handb. Clin. Neurol. 2016, 133, 447–466. [Google Scholar] [CrossRef]
- Engel, A.G. Genetic basis and phenotypic features of congenital myasthenic syndromes. Handb. Clin. Neurol. 2018, 148, 565–589. [Google Scholar] [CrossRef]
- Glass, D.J.; Bowen, D.C.; Stitt, T.N.; Radziejewski, C.; Bruno, J.; Ryan, T.E.; Gies, D.R.; Shah, S.; Mattsson, K.; Burden, S.J.; et al. Agrin acts via a MuSK receptor complex. Cell 1996, 85, 513–523. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. LRP4 serves as a coreceptor of agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef]
- Beeson, D.; Higuchi, O.; Palace, J.; Cossins, J.; Spearman, H.; Maxwell, S.; Newsom-Davis, J.; Burke, G.; Fawcett, P.; Motomura, M.; et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 2006, 313, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Inoue, A.; Okada, M.; Murata, Y.; Kakuta, S.; Jigami, T.; Kubo, S.; Shiraishi, H.; Eguchi, K.; Motomura, M.; et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006, 312, 1802–1805. [Google Scholar] [CrossRef]
- Linnoila, J.; Wang, Y.; Yao, Y.; Wang, Z.-Z. A Mammalian Homolog of Drosophila Tumorous Imaginal Discs, Tid1, Mediates Agrin Signaling at the Neuromuscular Junction. Neuron 2008, 60, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Hallock, P.T.; Xu, C.F.; Park, T.J.; Neubert, T.A.; Curran, T.; Burden, S.J. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010, 24, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.G.; Wang, Q.; Zhou, J.Z.; Wang, J.; Luo, Z.; Liu, M.; He, X.; Wynshaw-Boris, A.; Xiong, W.C.; Lu, B.; et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 2002, 35, 489–505. [Google Scholar] [CrossRef]
- Weston, C.; Yee, B.; Hod, E.; Prives, J. Agrin-induced acetylcholine receptor clustering is mediated by the small guanosine triphosphatases Rac and Cdc42. J. Cell Biol. 2000, 150, 205–212. [Google Scholar] [CrossRef]
- Weston, C.; Gordon, C.; Teressa, G.; Hod, E.; Ren, X.D.; Prives, J. Cooperative regulation by Rac and Rho of agrin-induced acetylcholine receptor clustering in muscle cells. J. Biol. Chem. 2003, 278, 6450–6455. [Google Scholar] [CrossRef]
- Gautam, M.; Noakes, P.G.; Moscoso, L.; Rupp, F.; Scheller, R.H.; Merlie, J.P.; Sanes, J.R. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 1996, 85, 525–535. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996, 85, 501–512. [Google Scholar] [CrossRef]
- Weatherbee, S.D.; Anderson, K.V.; Niswander, L.A. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 2006, 133, 4993–5000. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Dominguez, B.; Yang, J.; Aryal, P.; Brandon, E.P.; Gage, F.H.; Lee, K.F. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 2005, 46, 569–579. [Google Scholar] [CrossRef]
- Misgeld, T.; Kummer, T.T.; Lichtman, J.W.; Sanes, J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA 2005, 102, 11088–11093. [Google Scholar] [CrossRef]
- Fu, A.K.; Ip, F.C.; Fu, W.Y.; Cheung, J.; Wang, J.H.; Yung, W.H.; Ip, N.Y. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc. Natl. Acad. Sci. USA 2005, 102, 15224–15229. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dominguez, B.; de Winter, F.; Gould, T.W.; Eriksson, J.E.; Lee, K.F. Nestin negatively regulates postsynaptic differentiation of the neuromuscular synapse. Nat. Neurosci. 2011, 14, 324–330. [Google Scholar] [CrossRef]
- Mohseni, P.; Sung, H.-K.; Murphy, A.J.; Laliberte, C.L.; Pallari, H.-M.; Henkelman, M.; Georgiou, J.; Xie, G.; Quaggin, S.E.; Thorner, P.S.; et al. Nestin Is Not Essential for Development of the CNS But Required for Dispersion of Acetylcholine Receptor Clusters at the Area of Neuromuscular Junctions. J. Neurosci. 2011, 31, 11547–11552. [Google Scholar] [CrossRef]
- Froehner, S.C.; Gulbrandsen, V.; Hyman, C.; Jeng, A.Y.; Neubig, R.R.; Cohen, J.B. Immunofluorescence localization at the mammalian neuromuscular junction of the Mr 43,000 protein of Torpedo postsynaptic membranes. Proc. Natl. Acad. Sci. USA 1981, 78, 5230–5234. [Google Scholar] [CrossRef]
- Burden, S.J. The subsynaptic 43-kDa protein is concentrated at developing nerve-muscle synapses in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 8270–8273. [Google Scholar] [CrossRef] [PubMed]
- Noakes, P.G.; Phillips, W.D.; Hanley, T.A.; Sanes, J.R.; Merlie, J.P. 43K protein and acetylcholine receptors colocalize during the initial stages of neuromuscular synapse formation in vivo. Dev. Biol. 1993, 155, 275–280. [Google Scholar] [CrossRef]
- LaRochelle, W.J.; Froehner, S.C. Determination of the tissue distributions and relative concentrations of the postsynaptic 43kDa protein and the acetylcholine receptor in Torpedo. J. Biol. Chem. 1986, 261, 5270–5274. [Google Scholar] [CrossRef]
- Burden, S.J.; DePalma, R.L.; Gottesman, G.S. Crosslinking of proteins in acetylcholine receptor-rich membranes: Association between the beta-subunit and the 43 kd subsynaptic protein. Cell 1983, 35, 687–692. [Google Scholar] [CrossRef]
- Froehner, S.C.; Luetje, C.W.; Scotland, P.B.; Patrick, J. The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron 1990, 5, 403–410. [Google Scholar] [CrossRef]
- Phillips, W.P.; Kopta, C.; Blount, P.; Gardner, P.D.; Steinbach, J.H.; Merlie, J.P. ACh receptor-rich membrane domains organized in fibroblasts by recombinant 43-kilodalton protein. Science 1991, 251, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Noakes, P.G.; Mudd, J.; Nichol, M.; Chu, G.C.; Sanes, J.R.; Merlie, J.P. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 1995, 377, 232–236. [Google Scholar] [CrossRef]
- Ohno, K.; Engel, A.G.; Shen, X.M.; Selcen, D.; Brengman, J.; Harper, C.M.; Tsujino, A.; Milone, M. Rapsyn Mutations in Humans Cause Endplate Acetylcholine-Receptor Deficiency and Myasthenic Syndrome. Am. J. Hum. Genet 2002, 70, 4. [Google Scholar] [CrossRef]
- Maselli, R.A.; Dunne, V.; Pascual-Pascual, S.I.; Bowe, C.; Agius, M.; Frank, R.; Wollmann, R.L. Rapsyn mutations in myasthenic syndrome due to impaired receptor clustering. Muscle Nerve 2003, 28, 293–301. [Google Scholar] [CrossRef]
- Ramarao, M.K.; Bianchetta, M.J.; Lanken, J.; Cohen, J.B. Role of rapsyn tetratricopeptide repeat and coiled-coil domains in self- association and nicotinic acetylcholine receptor clustering. J. Biol. Chem. 2001, 276, 7475–7483. [Google Scholar] [CrossRef]
- Ramarao, M.K.; Cohen, J.B. Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc. Natl. Acad. Sci. USA 1998, 95, 4007–4012. [Google Scholar] [CrossRef]
- Li, L.; Cao, Y.; Wu, H.; Ye, X.; Zhu, Z.; Xing, G.; Shen, C.; Barik, A.; Zhang, B.; Xie, X.; et al. Enzymatic Activity of the Scaffold Protein Rapsyn for Synapse Formation. Neuron 2016, 92, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.S.; Mihaylova, V.; Abicht, A.; Lochmuller, H. Congenital myasthenic syndromes: Spotlight on genetic defects of neuromuscular transmission. Expert Rev. Mol. Med. 2007, 9, 1–20. [Google Scholar] [CrossRef]
- Beeson, D.; Webster, R.; Cossins, J.; Lashley, D.; Spearman, H.; Maxwell, S.; Slater, C.R.; Newsom-Davis, J.; Palace, J.; Vincent, A. Congenital myasthenic syndromes and the formation of the neuromuscular junction. Ann. New York Acad. Sci. 2008, 1132, 99–103. [Google Scholar] [CrossRef]
- Apel, E.D.; Glass, D.J.; Moscoso, L.M.; Yancopoulos, G.D.; Sanes, J.R. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 1997, 18, 623–635. [Google Scholar] [CrossRef]
- Bartoli, M.; Ramarao, M.K.; Cohen, J.B. Interactions of the rapsyn RING-H2 domain with dystroglycan. J. Biol. Chem. 2001, 276, 24911–24917. [Google Scholar] [CrossRef]
- Cartaud, A.; Coutant, S.; Petrucci, T.C.; Cartaud, J. Evidence for in situ and in vitro association between beta-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J. Biol. Chem. 1998, 273, 11321–11326. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, G.C.; Luo, S.; Yang, Z.; Xiong, W.C.; Mei, L. alpha-Actinin interacts with rapsyn in agrin-stimulated AChR clustering. Mol. Brain 2008, 1, 18. [Google Scholar] [CrossRef]
- Oury, J.; Liu, Y.; Topf, A.; Todorovic, S.; Hoedt, E.; Preethish-Kumar, V.; Neubert, T.A.; Lin, W.; Lochmuller, H.; Burden, S.J. MACF1 links Rapsyn to microtubule- and actin-binding proteins to maintain neuromuscular synapses. J. Cell Biol. 2019, 218, 1686–1705. [Google Scholar] [CrossRef]
- Antolik, C.; Catino, D.H.; O’Neill, A.M.; Resneck, W.G.; Ursitti, J.A.; Bloch, R.J. The actin binding domain of ACF7 binds directly to the tetratricopeptide repeat domains of rapsyn. Neuroscience 2007, 145, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Mihailovska, E.; Raith, M.; Valencia, R.G.; Fischer, I.; Al Banchaabouchi, M.; Herbst, R.; Wiche, G. Neuromuscular synapse integrity requires linkage of acetylcholine receptors to postsynaptic intermediate filament networks via rapsyn-plectin 1f complexes. Mol. Biol. Cell. 2014, 25, 4130–4149. [Google Scholar] [CrossRef] [PubMed]
- Bignami, F.; Camus, G.; Marchand, S.; Bailly, L.; Stetzkowski-Marden, F.; Cartaud, J. Targeting of acetylcholine receptor and 43 kDa rapsyn to the postsynaptic membrane in Torpedo marmorata electrocyte. J. Physiol. Paris 1998, 92, 177–181. [Google Scholar] [CrossRef]
- Marchand, S.; Bignami, F.; Stetzkowski-Marden, F.; Cartaud, J. The myristoylated protein rapsyn is cotargeted with the nicotinic acetylcholine receptor to the postsynaptic membrane via the exocytic pathway. J. Neurosci. 2000, 20, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Devillers-Thiery, A.; Pons, S.; Changeux, J.P.; Cartaud, J. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J. Neurosci. 2002, 22, 8891–8901. [Google Scholar] [CrossRef]
- Park, J.Y.; Ikeda, H.; Ikenaga, T.; Ono, F. Acetylcholine receptors enable the transport of rapsyn from the Golgi complex to the plasma membrane. J. Neurosci. 2012, 32, 7356–7363. [Google Scholar] [CrossRef]
- Chen, P.J.; Martinez-Pena, Y.V.I.; Aittaleb, M.; Akaaboune, M. AChRs Are Essential for the Targeting of Rapsyn to the Postsynaptic Membrane of NMJs in Living Mice. J Neurosci 2016, 36, 5680–5685. [Google Scholar] [CrossRef]
- Moransard, M.; Borges, L.S.; Willmann, R.; Marangi, P.A.; Brenner, H.R.; Ferns, M.J.; Fuhrer, C. Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J. Biol. Chem. 2003, 278, 7350–7359. [Google Scholar] [CrossRef]
- Xing, G.; Xiong, W.C.; Mei, L. Rapsyn as a signaling and scaffolding molecule in neuromuscular junction formation and maintenance. Neurosci. Lett. 2020, 731, 135013. [Google Scholar] [CrossRef]
- Gervasio, O.L.; Armson, P.F.; Phillips, W.D. Developmental increase in the amount of rapsyn per acetylcholine receptor promotes postsynaptic receptor packing and stability. Dev. Biol. 2007, 305, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, J.; Cole, R.N.; Gervasio, O.L.; Ngo, S.T.; Noakes, P.G.; Phillips, W.D. Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse. Dev. Neurobiol. 2008, 68, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Gervasio, O.L.; Phillips, W.D. Increased ratio of rapsyn to ACh receptor stabilizes postsynaptic receptors at the mouse neuromuscular synapse. J. Physiol. 2005, 562, 673–685. [Google Scholar] [CrossRef]
- Maimone, M.M.; Merlie, J.P. Interaction of the 43 kd postsynaptic protein with all subunits of the muscle nicotinic acetylcholine receptor. Neuron 1993, 11, 53–66. [Google Scholar] [CrossRef]
- Huebsch, K.A.; Maimone, M.M. Rapsyn-mediated clustering of acetylcholine receptor subunits requires the major cytoplasmic loop of the receptor subunits. J. Neurobiol. 2003, 54, 486–501. [Google Scholar] [CrossRef]
- Lee, Y.; Rudell, J.; Ferns, M. Rapsyn interacts with the muscle acetylcholine receptor via alpha-helical domains in the alpha, beta, and epsilon subunit intracellular loops. Neuroscience 2009, 163, 222–232. [Google Scholar] [CrossRef]
- Borges, L.S.; Yechikhov, S.; Lee, Y.I.; Rudell, J.B.; Friese, M.B.; Burden, S.J.; Ferns, M.J. Identification of a motif in the acetylcholine receptor beta subunit whose phosphorylation regulates rapsyn association and postsynaptic receptor localization. J. Neurosci. 2008, 28, 11468–11476. [Google Scholar] [CrossRef]
- Koppel, N.; Friese, M.B.; Cardasis, H.L.; Neubert, T.A.; Burden, S.J. Vezatin is required for the maturation of the neuromuscular synapse. Mol. Biol. Cell 2019, 30, 2571–2583. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, B.; Dong, X.P.; Tao, Y.; Ting, A.; Zhou, Z.; Meixiong, J.; Luo, J.; Chiu, F.C.; Xiong, W.C.; et al. HSP90 beta regulates rapsyn turnover and subsequent AChR cluster formation and maintenance. Neuron 2008, 60, 97–110. [Google Scholar] [CrossRef]
- Zuber, B.; Unwin, N. Structure and superorganization of acetylcholine receptor-rapsyn complexes. Proc. Natl. Acad. Sci. USA 2013, 110, 10622–10627. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Heinemann, S.F. Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J. Neurosci. 2006, 26, 9780–9793. [Google Scholar] [CrossRef]
- Johnson, B.; Leek, A.N.; Sole, L.; Maverick, E.E.; Levine, T.P.; Tamkun, M.M. Kv2 potassium channels form endoplasmic reticulum/plasma membrane junctions via interaction with VAPA and VAPB. Proc. Natl. Acad. Sci. USA 2018, 115, E7331–E7340. [Google Scholar] [CrossRef]
- Feng, G.; Steinbach, J.H.; Sanes, J.R. Rapsyn clusters neuronal acetylcholine receptors but is inessential for formation of an interneuronal cholinergic synapse. J. Neurosci. 1998, 18, 4166–4176. [Google Scholar] [CrossRef]
- Kassner, P.D.; Conroy, W.G.; Berg, D.K. Organizing effects of rapsyn on neuronal nicotinic acetylcholine receptors. Mol. Cell. Neurosci. 1998, 10, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Armson, P.F.; Cha, J.; Phillips, W.D. Clustering Of Gaba(a) Receptors By Rapsyn/43kd Protein In Vitro. Mol. Cell. Neurosci. 1997, 8, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Quiram, P.A.; Ohno, K.; Milone, M.; Patterson, M.C.; Pruitt, N.J.; Brengman, J.M.; Sine, S.M.; Engel, A.G. Mutation causing congenital myasthenia reveals acetylcholine receptor beta/delta subunit interaction essential for assembly. J. Clin. Investig. 1999, 104, 1403–1410. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Jing, Z.; Zhang, L.; Zhou, G.; Braun, J.; Yao, Y.; Wang, Z.Z. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat. Neurosci. 2003, 6, 1017–1018. [Google Scholar] [CrossRef]
- Borges, L.S.; Ferns, M. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol. 2001, 153, 1–11. [Google Scholar] [CrossRef]
- Friese, M.B.; Blagden, C.S.; Burden, S.J. Synaptic differentiation is defective in mice lacking acetylcholine receptor beta-subunit tyrosine phosphorylation. Development 2007, 134, 4167–4176. [Google Scholar] [CrossRef]
- Mitra, A.K.; McCarthy, M.P.; Stroud, R.M. Three-dimensional structure of the nicotinic acetylcholine receptor and location of the major associated 43-kD cytoskeletal protein, determined at 22 A by low dose electron microscopy and x-ray diffraction to 12.5 A. J. Cell Biol. 1989, 109, 755–774. [Google Scholar] [CrossRef]
- Apel, E.D.; Roberds, S.L.; Campbell, K.P.; Merlie, J.P. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron 1995, 15, 115–126. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Mathias, A.; Gautam, M.; Hall, Z.W. Metabolic stabilization of muscle nicotinic acetylcholine receptor by rapsyn. J. Neurosci. 1999, 19, 1998–2007. [Google Scholar] [CrossRef]
- Rudell, J.B.; Ferns, M.J. Regulation of muscle acetylcholine receptor turnover by beta subunit tyrosine phosphorylation. Dev. Neurobiol. 2013, 73, 399–410. [Google Scholar] [CrossRef]
- Bruneau, E.; Akaaboune, M. The dynamics of the rapsyn scaffolding protein at individual acetylcholine receptor clusters. J. Biol. Chem. 2007, 282, 9932–9940. [Google Scholar] [CrossRef]
- Bruneau, E.G.; Akaaboune, M. Dynamics of the rapsyn scaffolding protein at the neuromuscular junction of live mice. J. Neurosci. 2010, 30, 614–619. [Google Scholar] [CrossRef]
- Nam, S.; Min, K.; Hwang, H.; Lee, H.O.; Lee, J.H.; Yoon, J.; Lee, H.; Park, S.; Lee, J. Control of rapsyn stability by the CUL-3-containing E3 ligase complex. J. Biol. Chem. 2009, 284, 8195–8206. [Google Scholar] [CrossRef]
- Wang, N.; Orr-Urtreger, A.; Korczyn, A.D. The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: Lessons from knockout mice. Prog. Neurobiol. 2002, 68, 341–360. [Google Scholar] [CrossRef]
- Xu, W.; Gelber, S.; Orr-Urtreger, A.; Armstrong, D.; Lewis, R.A.; Ou, C.N.; Patrick, J.; Role, L.; De Biasi, M.; Beaudet, A.L. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 5746–5751. [Google Scholar] [CrossRef]
- Xu, W.; Orr-Urtreger, A.; Nigro, F.; Gelber, S.; Sutcliffe, C.B.; Armstrong, D.; Patrick, J.W.; Role, L.W.; Beaudet, A.L.; De Biasi, M. Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1999, 19, 9298–9305. [Google Scholar] [CrossRef]
- Rassadi, S.; Krishnaswamy, A.; Pie, B.; McConnell, R.; Jacob, M.H.; Cooper, E. A null mutation for the alpha3 nicotinic acetylcholine (ACh) receptor gene abolishes fast synaptic activity in sympathetic ganglia and reveals that ACh output from developing preganglionic terminals is regulated in an activity-dependent retrograde manner. J. Neurosci. 2005, 25, 8555–8566. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Coggan, J.S.; Berg, D.K. Synaptic currents generated by neuronal acetylcholine receptors sensitive to alpha-bungarotoxin. Neuron 1996, 17, 1231–1240. [Google Scholar] [CrossRef][Green Version]
- Gingras, J.; Rassadi, S.; Cooper, E.; Ferns, M. Agrin plays an organizing role in the formation of sympathetic synapses. J. Cell Biol. 2002, 158, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Gingras, J.; Rassadi, S.; Cooper, E.; Ferns, M. Synaptic transmission is impaired at neuronal autonomic synapses in agrin-null mice. Dev. Neurobiol. 2007, 67, 521–534. [Google Scholar] [CrossRef]
- Martin, A.O.; Alonso, G.; Guerineau, N.C. Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J. Cell Biol. 2005, 169, 503–514. [Google Scholar] [CrossRef]
- Neff, R.A., 3rd; Gomez-Varela, D.; Fernandes, C.C.; Berg, D.K. Postsynaptic scaffolds for nicotinic receptors on neurons. Acta Pharm. Sin. 2009, 30, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Temburni, M.K.; Levey, M.S.; Bertrand, S.; Bertrand, D.; Jacob, M.H. The long internal loop of the alpha 3 subunit targets nAChRs to subdomains within individual synapses on neurons in vivo. Nat. Neurosci. 1998, 1, 557–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Temburni, M.K.; Blitzblau, R.C.; Jacob, M.H. Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo. J. Physiol. 2000, 525, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.M.; Yang, F.; Giovanni, M.; Mohn, J.L.; Temburni, M.K.; Jacob, M.H. Adenomatous polyposis coli plays a key role, in vivo, in coordinating assembly of the neuronal nicotinic postsynaptic complex. Mol. Cell. Neurosci. 2008, 38, 138–152. [Google Scholar] [CrossRef]
- Temburni, M.K.; Rosenberg, M.M.; Pathak, N.; McConnell, R.; Jacob, M.H. Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein. J. Neurosci. 2004, 24, 6776–6784. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.J.; Zhao, S.; Bredt, D.S.; Sanes, J.R.; Feng, G. PSD93 regulates synaptic stability at neuronal cholinergic synapses. J. Neurosci. 2004, 24, 378–388. [Google Scholar] [CrossRef]
- Conroy, W.G.; Liu, Z.; Nai, Q.; Coggan, J.S.; Berg, D.K. PDZ-containing proteins provide a functional postsynaptic scaffold for nicotinic receptors in neurons. Neuron 2003, 38, 759–771. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferns, M. An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors. Molecules 2021, 26, 3065. https://doi.org/10.3390/molecules26113065
Ferns M. An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors. Molecules. 2021; 26(11):3065. https://doi.org/10.3390/molecules26113065
Chicago/Turabian StyleFerns, Michael. 2021. "An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors" Molecules 26, no. 11: 3065. https://doi.org/10.3390/molecules26113065
APA StyleFerns, M. (2021). An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors. Molecules, 26(11), 3065. https://doi.org/10.3390/molecules26113065





